Achyranthes bidentata polypeptides prevent apoptosis by inhibiting the glutamate current in cultured hippocampal neurons
Rong-Lu Pan , Wen-Qing Hu , Jie Pan Li Huang Cheng-Cheng Luan Hong-Mei Shen
1 Key Laboratory of Neuroregeneration of Jiangsu and Ministry of Education and Institute of Nautical Medicine, Co-Innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
2 Department of Neurobiology, Physiology and Behavior, College of Biological Science, Davis, CA, USA
3 Affiliated Mental Health Center of Nantong University, Nantong Brain Hospital, Nantong, Jiangsu Province, China
Abstract Glutamate-induced excitotoxicity plays a critical role in the neurological impairment caused by middle cerebral artery occlusion. Achyranthes bidentata polypeptides have been shown to protect against neurological functional damage caused by middle cerebral artery occlusion,but the underlying neuroprotective mechanisms and the relationship to glutamate-induced excitotoxicity remain unclear. Therefore, in the current study, we investigated the protective effects of Achyranthes bidentata polypeptides against glutamate-induced excitotoxicity in cultured hippocampal neurons. Hippocampal neurons were treated with Mg2+-free extracellular solution containing glutamate (300 μM)for 3 hours as a model of glutamate-mediated excitotoxicity (glutamate group). In the normal group, hippocampal neurons were incubated in Mg2+-free extracellular solution. In the Achyranthes bidentata polypeptide group, hippocampal neurons were incubated in Mg2+-free extracellular solution containing glutamate (300 μM) and Achyranthes bidentata polypeptide at different concentrations. At 24 hours after exposure to the agents, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and Hoechst 33258 staining were used to assess neuronal viability and nuclear morphology, respectively. Caspase-3 expression and activity were evaluated using western blot assay and colorimetric enzymatic assay, respectively. At various time points after glutamate treatment, reactive oxygen species in cells were detected by H2DCF-DA, and mitochondrial membrane potential was detected by rhodamine 123 staining. To examine the effect of Achyranthes bidentata polypeptides on glutamate receptors, electrophysiological recording was used to measure the glutamate-induced inward current in cultured hippocampal neurons. Achyranthes bidentata polypeptide decreased the percentage of apoptotic cells and reduced the changes in caspase-3 expression and activity induced by glutamate. In addition, Achyranthes bidentata polypeptide attenuated the amplitude of the glutamate-induced current. Furthermore, the glutamate-induced increase in intracellular reactive oxygen species and reduction in mitochondrial membrane potential were attenuated by Achyranthes bidentata polypeptide treatment. These findings collectively suggest that Achyranthes bidentata polypeptides exert a neuroprotective effect in cultured hippocampal neurons by suppressing the overactivation of glutamate receptors and inhibiting the caspase-3-dependent mitochondrial apoptotic pathway. All animal studies were approved by the Animal Care and Use Committee, Nantong University, China (approval No. 20120216-001) on February 16, 2012.
Key Words: Achyranthes bidentata polypeptides; apoptosis; caspase-3; excitotoxicity; glutamate receptors; mitochondrial dysfunction; mitochondrial membrane potential; neuroprotection; reactive oxygen species; staurosporine
Introduction
Achyranthes bidentata (A. bidentata) Blume is an amaranthaceous species of perennial herb, and its roots are used as a traditional Chinese medicine because it promotes blood circulation and menstruation for the treatment of various diseases, including amenorrhea, dysmenorrhea, lumbago,gonalgia, paraplegia, and edema (He et al., 2017). The extract of A. bidentata Blume roots contains polysaccharides,saponins, sterols, coumarins, alkaloids and polypeptides. A.bidentata saponins reduce inflammation and apoptosis in interleukin-1β-treated chondrocytes (Xu et al., 2017). In our previous studies, we isolated polypeptides from the extract of A. bidentata Blume roots by ammonium sulfate precipitation. These A. bidentata polypeptides (ABPPs) have the ability to enhance functional rehabilitation, including motor,sensory, coordination and cognitive functions, in rats that survive experimental acute ischemic stroke produced by middle cerebral artery occlusion (Shen et al., 2013; Cheng et al., 2019).
Ischemic stroke is initiated by the interruption of the blood supply in the central nervous system (He et al., 2019;Simon et al., 2019), and many studies have clarified the mechanisms of cell death in ischemic stroke (Evans et al.,2018; Long et al., 2019). To maintain the fidelity of synaptic transmission, the glutamate concentration is less than 4 μM in vivo (Lerma et al., 1986; Baker et al., 2002; Nyitrai et al.,2006; Pal, 2018). The accumulation of extracellular glutamate plays a critical role in the initiation of apoptosis after ischemic stroke, particularly the overstimulation of N-methyl-D-aspartate (NMDA) receptors (Griffin, 1976; Burnashev et al., 1995; Garaschuk et al., 1996; Rodriguez-Munoz et al.,2018; Tameh et al., 2018). Accordingly, numerous NMDA receptor antagonists have been developed in the past few decades (Lai et al., 2014; Seyedsaadat and Kallmes, 2019).Unfortunately, most of these antagonists have met failure in clinical trials, including MK-801.
Numerous studies have shown that both the localization and subunit composition of the NMDA receptor modulate its function (Zhou and Baudry, 2006; Liu et al., 2007; Hansen et al., 2017). NR2A is predominantly localized to the synaptic region, and has a pro-survival function, while NR2B is localized at extrasynaptic regions and mediates excitotoxicity (Zhou and Baudry, 2006; Liu et al., 2007; Shi et al., 2017;Shah et al., 2019). Using calcium imaging, we previously showed that ABPPs not only suppress the hyperactivation of NR2B-containing NMDA receptors, but also stimulate NR2A-containing receptors (Shen et al., 2008). Glutamate is the endogenous ligand of the NMDA receptor, and the excessive accumulation of glutamate in the central nervous system is involved in ischemic stroke and chronic neurodegenerative diseases, such as Huntington’s chorea, Alzheimer’s disease and Parkinsonism (Blandini et al., 1996; Lai et al.,2014; Chamorro et al., 2016). Therefore, in the present study,we used high-concentration glutamate-mediated excitotoxicity to evaluate the neuroprotective effect of ABPPs and to clarify their mechanism of action.
Materials and Methods
Preparation of the A. bidentata Blume root decoction and ABPPs
A. bidentata Blume root was purchased from a Chinese medicine grocery, and identified by Dr. Haoru Zhao, and its powder was soaked in 80°C ultrapure water. ABPPs were subsequently prepared by the Key Laboratory of Neuroregeneration of Jiangsu and Ministry of Education as described by Shen et al. (2008). The ammonium sulfate precipitate was desalted using a 1000 MW cutoff tubing with ultrapure water. The dialysate was freeze-dried to obtain a powder of ABPPs, which is soluble in water. The ABPPs were characterized by high performance liquid chromatography (Waters,Milford, MA, USA).
Primary neuron culture
All animal studies were approved by the Animal Care and Use Committee, Nantong University, China (approval No.20120216-001) on February 16, 2012. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals(NIH Publication No. 85-23, revised 1996).
The experiments were performed on primary cultures of rat hippocampal neurons. Tissues were taken from 37 specific-pathogen-free female Sprague-Dawley rat [license No. SYXK (Su) 2012-0031] embryos at 18 days of gestation from Nantong University, and were prepared as described previously (Zhang et al., 2007). Briefly, the fetal brains were dissected, and the hippocampi were isolated and digested with trypsin (0.25% for 5 minutes at 37°C; Gibco). Neurons were cultured in Dulbecco’s modified Eagle’s medium (Gibco), supplemented with 10% fetal bovine serum (Gibco),and plated at a density of 6-8 × 104cells/cm2onto poly-D-lysine-coated multi-well plates or 8 mm glass coverslips in dishes. After 24 hours, the medium was replaced with Neurobasal medium (Gibco) containing 2% B27 supplement(Gibco) and glutamine (0.5 mM, Gibco). Half of the medium was replaced with fresh medium every 3 days.
Glutamate-mediated excitotoxicity model and ABPP treatment groups
Neurotoxicity was evaluated by exposing hippocampal neurons to high-concentration glutamate (Sigma-Aldrich,St. Louis, MO, USA). Because of the blockade by Mg2+of NMDA receptors, hippocampal cultures, at 7 days in vitro,were washed with Mg2+-free extracellular solution (CaCl22 mM, NaCl 140 mM, KCl 3 mM, HEPES 10 mM, glucose 10 mM, pH 7.2-7.3 [290 ± 5 mOsmol/L]). After washing, glutamate in Mg2+-free extracellular solution was added to the wells to induce excitotoxicity. After glutamate stimulation,cultures were washed and returned to the previous medium. Hippocampal neurons were treated with glutamate to establish the glutamate-mediated excitotoxicity model (glutamate group). Hippocampal neurons incubated in Mg2+-free extracellular solution were designated the normal group.In the ABPP treatment group, ABPP powder was dissolved in culture medium to the desired concentration and then added to primary hippocampal neurons 12 hours before glutamate-induced injury. All experiments were performed in triplicate.
Measurement of neuronal cell viability
Cellular viability assessments were performed after 24 hours of exposure to the agents using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) assay. The detailed procedures were described previously by Shen et al. (2008). Data were normalized to the control cultures using the percentage value (Elx 800, Bio-TEK Instruments Inc., VT, USA).
Assessment of caspase-3 activity
At 24 hours after glutamate treatment, the medium in 6-well plates was rinsed twice with 0.01 M phosphate-buffered saline, and the hippocampal neurons were scraped off. The caspase-3 activity assay was performed according to the manufacturer’s instructions in the caspase-3/CPP32 colorimetric assay kit (Bio Vision, Mountain View, CA, USA).
Determination of reactive oxygen species (ROS) levels
The effect of ABPPs on intracellular ROS levels was measured quantitatively using the oxidative stress-sensitive probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA;Sigma-Aldrich) (Russo et al., 2005). At 7 days in vitro, the original medium was rinsed twice with Mg2+-free extracellular solution, and the cultures were incubated with 20 μM H2DCF-DA at 37°C for 30 minutes in the dark with gentle shaking. The cultures were then washed twice with Mg2+-free extracellular solution and returned to the original culture medium for 20 minutes. The fluorescence (A488nm/520nm) intensity of the samples was then measured with a microplate reader (Vector multilabel counter; Perkin Elmer Life Sciences). The ROS changes were calculated after treatment (normal medium, glutamate, ABPPs) every 5 minutes by comparison with the fluorescence before treatment.
Assessment of mitochondrial membrane potential
Mitochondrial membrane potential (ΔΨm) was assessed with rhodamine 123 (ThermoFisher, Shanghai, China), a fluorescent probe (Juan et al., 1994). The method was similar to that for measuring intracellular ROS levels, with 2 μM rhodamine 123 in place of H2DCF-DA. The fluorescence(A488nm/520nm) intensity of the samples was measured with a microplate reader (Vector multilabel counter; Perkin Elmer Life Sciences) every 5 minutes. Data were normalized to the fluorescence intensity before treatment.
Identification of apoptotic cells by Hoechst staining
Apoptosis was assessed 24 hours after excitotoxic injury with Hoechst 33258 (Molecular Probes; 10 μg/mL). Cells on 8 mm glass coverslips were washed with extracellular solution,and then exposed to the dye for 15 minutes. Afterwards,cultures were washed three times with extracellular solution and fixed with 4% paraformaldehyde for 20 minutes. For each coverslip, five fields were randomly selected, and each field had more than 100 cells, as assessed by the Leica Imagine System (20×; Q550 IW, Leica, Germany). The nuclei of apoptotic cells were round or irregular in shape and bright blue or uneven in color, indicating high chromatin condensation. The data were expressed as the percentage of apoptotic cells in the fields.
Electrophysiological recording
Receptors for glutamate that are ligand-gated ion channels play a key role in glutamate-induced neuronal apoptosis(Xing et al., 2012; Lewerenz and Maher, 2015; DeGregorio-Rocasolano et al., 2019). To test whether ABPPs affect the function of glutamate-gated ion channels, the glutamate-induced current was examined by whole-cell patch clamp recording of hippocampal neurons. All currents were measured in Mg2+-free solution containing Ca2+(2 mM),with 2-minute bath perfusion after the glutamate current was recorded (Shen et al., 2013). All responses were elicited at an ambient temperature of 23-25°C.
Staurosporine-induced neuronal injury model
Staurosporine, widely used as a protein kinase C inhibitor with a broad spectrum of activity, is a microbial alkaloid isolated from the culture broth of Streptomyces staurospores. Staurosporine has been shown to induce apoptosis by activating caspase-3 and glutamate receptors (Chae et al.,2000; Zhang et al., 2016; Malsy et al., 2019). We examined the effect of ABPPs on glutamate receptor overactivation and the caspase-3-dependent mitochondrial apoptotic pathway.Hippocampal neurons were treated with staurosporine for 24 hours to produce the staurosporine-induced neuronal injury model (staurosporine group). ABPP powder was directly dissolved in culture medium at the desired concentration(ABPP treatment group).
Statistical analysis
Data were analyzed using SigmaPlot 13.0 software (Systat software, San Jose, CA, USA). Significance was analyzed by one-way analysis of variance, and differences between two groups were assessed by Student-Newman-Keuls post hoc test.A value of P < 0.05 was considered statistically significant.
Results
ABPPs protect against glutamate-induced neuronal injury
Cells were exposed to increasing concentrations of glutamate(30-1000 μM) for 3 hours or to 300 μM glutamate for different periods (1-5 hours), as shown in Figure 1. Cell viability decreased as the glutamate concentration or exposure time increased (Figure 1A and B). Based on the data, exposure to 300 μM glutamate for 3 hours was selected for subsequent experiments.
As the ABPP concentration increased, the cell viability of hippocampal neurons increased gradually, compared with the glutamate group (Figure 1C). These results indicate that ABPPs protect against glutamate-induced neuronal injury.In vitro and in vivo studies provide evidence for both apoptotic and necrotic neuronal cell death following exposure to high-concentration glutamate (Ankarcrona et al., 1995,Santos-Carvalho et al., 2013, Anilkumar et al., 2017). In our experiment, exposure to glutamate (300 μM) for 3 hours induced apoptosis, detected by Hoechst 33528 staining, in rat cultured hippocampal neurons (Figure 2). In the glutamate group, approximately 35% of cells underwent programmed cell death, as assessed by their nuclear morphological features, while 8% of neurons in the control group underwent programmed cell death (Figure 2A and B). Apoptosis of hippocampal neurons decreased to 12% in the ABPP treatment group (Figure 2A and B). Therefore, these data suggest that ABPPs protect against glutamate-induced apoptotic cell death.
Effects of ABPPs on the glutamate-evoked current
ABPPs (3 μg/mL) did not evoke a significant current (Figure 3A). However, the peak amplitude of the 300 μM glutamate-induced current was 400.00 ± 57.61 pA, and the amplitude of this current was inhibited by ABPP (3 μg/mL)by 50% (Figure 3B and C). These results suggest that ABPP prevents the overactivation of glutamate receptors induced by high-concentration glutamate.
Effects of ABPPs on the change in caspase-3 induced by glutamate
In cultured hippocampal neurons, a 3-fold change in caspase-3 activity was observed 24 hours after exposure to glutamate (300 μM, 3 hours; Figure 4A). ABPPs (3, 10 μg/mL) attenuated this glutamate-induced change in caspase-3 activity (Figure 4A). Moreover, the expression of activated caspase-3 protein increased by approximately 200%, 24 hours after glutamate(300 μM) exposure in hippocampal cultures. After pre-treatment with ABPPs (1, 3, 10 μg/mL), the change in activated caspase-3 expression was restored to baseline in hippocampal cultures exposed to glutamate (Figure 4B).
Effects of ABPPs on the glutamate-induced increase in ROS levels
Intracellular ROS levels were detected using DCF (Figure 5A). The fluorescence intensity of DCF was measured before and after exposure to glutamate (300 μM). Glutamate significantly increased intracellular ROS levels, and ABPPs markedly attenuated this effect of glutamate in cultured hippocampal neurons (Figure 5A).
Effects of ABPPs on glutamate-induced mitochondrial dysfunction
As shown in Figure 5B, the mitochondrial membrane potential was evaluated using rhodamine 123. We examined the intensity of rhodamine 123 fluorescence before and after treatment with glutamate (300 μM). This revealed that glutamate significantly affected the mitochondrial membrane potential.ABPPs attenuated this effect of glutamate (Figure 5B).
Effects of ABPPs on staurosporine-induced neurotoxicity
Exposure to staurosporine (10-1,000 nM) for 24 hours reduced the viability of hippocampal neurons in a concentration-dependent manner, as measured by MTT assay (Figure 6A). As shown in Figure 6B, exposure to 300 nM staurosporine for 24 hours decreased the cell viability of hippocampal neurons, and pre-treatment with ABPPs abrogated this effect of staurosporine (Figure 6B). These data further demonstrate that ABPPs exert a neuroprotective effect by inhibiting the caspase-3-dependent mitochondrial apoptotic pathway.
Discussion
Glutamate is the main excitatory neurotransmitter in the central nervous system, contributing to normal neural transmission, development, differentiation and plasticity (Zhou and Danbolt, 2014; Dupuis and Groc, 2019). However, under pathological conditions, such as neurodegenerative diseases and ischemic stroke, high-concentration extracellular glutamate leads to uncontrolled, continuous depolarization of cells that leads to cellular injury in a process termed excitotoxicity (Xing et al., 2012; Lewerenz and Maher, 2015; De-Gregorio-Rocasolano et al., 2019). In the current study, we evaluated the neuroprotective effect of ABPPs, a differential modulator of NR2A- and NR2B-containing NMDA receptors, on high-concentration glutamate-induced excitotoxicity in primary cultured hippocampal neurons. At 24 hours after exposure to high-concentration glutamate, MTT assay as well as morphological observation showed that glutamate induced cellular viability loss, and that ABPPs prevented glutamate-induced neuronal apoptosis. Furthermore, our data indicate that ABPPs confer neuroprotection by inhibiting the glutamate-induced current and the caspase-3-dependent mitochondrial pathway.
Ischemic stroke results from a transient or permanent decrease in cerebral blood flow, which is, in most events,induced by the occlusion of a major brain artery, either by an embolus or by local thrombosis (Sommer, 2017). A key characteristic of ischemic stroke is excessive pathological release of endogenous glutamate, which leads to neuronal cell injury, in a process termed excitotoxicity (Taoufik and Probert, 2008; Fern and Matute, 2019). In our previous studies, ABPPs helped restore motor, sensory and cognitive functions in an animal model of ischemic stroke by reducing cellular damage in the central nervous system (Shen et al.,2013). However, it remained unknown whether ABPPs confer neuroprotection by reducing glutamate excitotoxicity.
Primary hippocampal cultures offer an excellent experimental model to investigate neuroprotection against glutamate-induced excitotoxicity in vitro because cell viability gradually diminishes with increasing glutamate concentration. Glutamate-induced excitotoxicity is related to the hyperactivation of glutamate receptors, which is followed by both necrotic and apoptotic cell death (Ientile et al., 2001;Mark et al., 2001; Kritis et al., 2015; Fern and Matute, 2019).Whether glutamate-evoked excitotoxicity leads to apoptotic neuronal death is dependent on the concentration of glutamate in the extracellular solution and the expression level of glutamate receptors in cultured neurons. In the present study, exposure to glutamate (300 μM) for 3 hours resulted in apoptotic cell death. ABPPs counteracted the reduction in cell viability in a dose-dependent manner. Furthermore,ABPPs (3 μg/mL) reduced the apoptosis of hippocampal neurons. In glutamate-induced neuronal apoptosis, the critical event is hyperactivation of glutamate receptors, leading to the overactivation of proteases and kinases, and the overproduction of free radicals, which ultimately result in mitochondrial dysfunction (Wong et al., 2002; Mehta et al.,2013). Ionotropic glutamate receptors, which are ligand-gated ion channels, can be examined using the whole cell patch clamp recording technique. Glutamate (300 μM) elicited a substantial inward current, which was inhibited in an ABPP concentration-dependent manner. The electrophysiological results suggest that ABPPs protect against glutamate excitotoxicity by reducing the glutamate-evoked current.

Figure 1 ABPPs protect against glutamate-induced excitotoxicity in cultured hippocampal neurons.
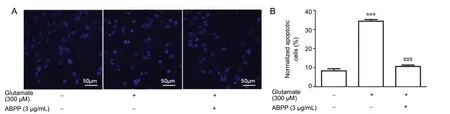
Figure 2 ABPPs prevent glutamate-induced karyopyknosis 24 hours after glutamate stimulation in cultured hippocampal neurons.

Figure 3 ABPPs inhibit the glutamate-induced current in cultured hippocampal neurons.

Figure 4 ABPPs reduce the overactivity and overexpression of caspase-3 evoked by glutamate in cultured hippocampal neurons.

Figure 5 ABPPs prevent the change in ROS and MMP evoked by glutamate.
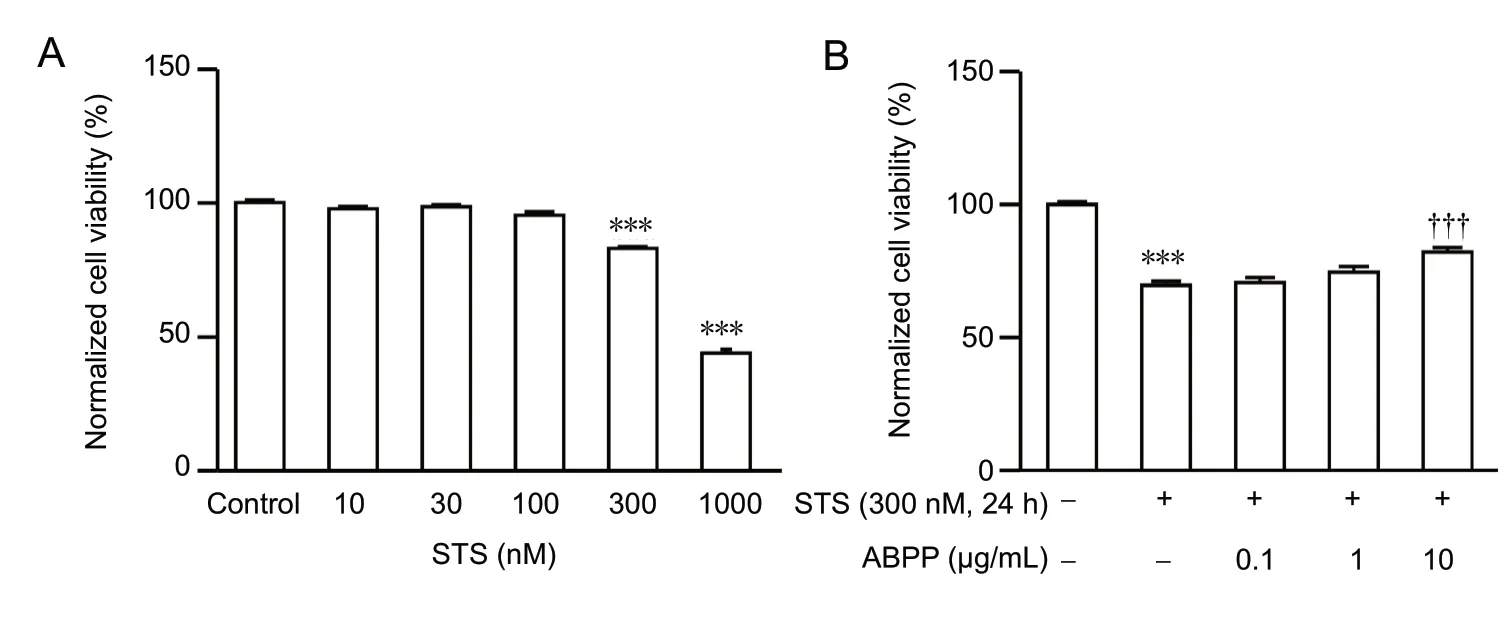
Figure 6 ABPPs protect against staurosporine-induced neuronal injury.
Glutamate induces apoptosis by shifting the balance between pro- and anti-apoptotic factors (Landshamer et al.,2008; Brunelle and Letai, 2009). Caspase-3 is a key mediator of programmed cell death, and extrinsic and intrinsic pathways involve caspase-3-dependent apoptosis. The active caspase-3 cleaves intracellular substrates, and it translocates from the cytoplasm to the nucleus, resulting in nuclear morphological changes, such as chromatin condensation(Kamada et al., 2005; Elmore, 2007). In the present study,ABPPs (3 μg/mL) reduced the percentage of apoptotic hippocampal neurons. To address whether the anti-apoptotic effect of ABPPs is caspase-3-dependent, its activity and expression were measured. Glutamate significantly elevated caspase-3 activity and expression. Furthermore, ABPPs suppressed the changes in caspase-3 activity and expression induced by high-concentration glutamate. Thus, the results might indicate that the inhibition of ABPPs on overstimulation of glutamate receptors might decrease the promotion of caspase-3 activity and its expression induced by glutamate excitotoxicity.
Mitochondria are at the core of intracellular energy metabolism. Glutamate-induced excitotoxicity is associated with the impairment of intracellular Ca2+homeostasis and the excessive production of ROS, which result in the collapse of mitochondrial membrane potential (Pereira and Oliveira,2000; Zorov et al., 2014; Nita and Grzybowski, 2016). We found that ABPPs reversed the glutamate-induced increase in intracellular ROS levels in a concentration-dependent manner. In addition, ABPPs ameliorated the loss of mitochondrial membrane potential elicited by glutamate in a concentration-dependent manner.
Staurosporine, a protein kinase inhibitor, induces mitochondria-dependent apoptosis via the activation of caspase-3(Chae et al., 2000; Zhang et al., 2016). AcDEVDCHO, a specific inhibitor of caspase-3, completely abolishes the effect of staurosporine on caspase-3 activity (Jantas-Skotniczna et al.,2006). Staurosporine also activates plasma membrane calcium channels, such as voltage-dependent calcium channels,and NMDA receptors in a protein kinase-independent manner (Zhaleh et al., 2012). Memantine, an NMDA receptor antagonist, attenuates staurosporine-induced apoptosis in hippocampal cultures (Jantas-Skotniczna et al., 2006). Therefore, staurosporine induces apoptosis in a caspase-3-dependent manner via the activation of glutamate receptors. In this study, ABPP treatment reversed the change in cell viability induced by staurosporine. This result provides further evidence that the neuroprotective effects of ABPPs are associated with the inhibition of glutamate receptor hyperactivation and the caspase-3-dependent mitochondrial pathway.
In summary, our findings suggest that ABPPs, a differential modulator of NR2A- and NR2B-containing NMDA receptors,protect against glutamate-induced excitotoxicity in primary cultured hippocampal neurons by reducing the overactivation of glutamate receptors and by ameliorating mitochondrial dysfunction. However, further studies are needed to identify the key bioactive components of ABPPs and to elucidate their molecular interactions with glutamate receptors.
Author contributions:Study design: HMS; experimental implementation: RLP, JP, WQH and CCL; data collection and analysis: WQH and LH; paper writing: WQH and HMS. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This work was financially supported by the National Natural Science Foundation of China, No. 81073079 (to HMS); the Natural Science Foundation of the Jiangsu Higher Education Institutes of China, No. 18KJA180009 (to HMS); the Science Foundation of Nantong City of China, No. MS12018043 (to HMS).The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:All animal procedures followed the Nantong University guidelines for Using Animals in Intramural Research and were approved by the Animal Care and Use Committee of Nantong University of China (approval No. 20120216-001) on February 16, 2012.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Shan Ping Yu, Emory University School of Medicine, USA; Mohammad Iqbal Hossain H Bhuiyan, University of Pittsburgh, USA.
Additional files:Open peer review report 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Astrocytic modulation of potassium under seizures
- Type XIX collagen: a promising biomarker from the basement membranes
- Adult neurogenesis from reprogrammed astrocytes
- Heterogeneity in the regenerative abilities of central nervous system axons within species: why do some neurons regenerate better than others?
- Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease
- Stroke gets in your eyes: stroke-induced retinal ischemia and the potential of stem cell therapy