Effects of Different Weaning Protocols on Survival,Growth and Nutritional Composition of Pharaoh Cuttlefish (Sepia pharaonis) Juvenile
JIANG Maowang, HAN Ziru, SHENG Peng, PENG Ruibing, HAN Qingxi,and JIANG Xiamin
Effects of Different Weaning Protocols on Survival,Growth and Nutritional Composition of Pharaoh Cuttlefish () Juvenile
JIANG Maowang, HAN Ziru, SHENG Peng, PENG Ruibing, HAN Qingxi,and JIANG Xiamin*
,,,315211,
The aim of this study was to determine the optimal weaning protocols for rearing juvenile cuttlefish, as well as to understand the feeding habits of cuttlefish and develop effective aqua-cultural practices. The effects of four experimental weaning protocols (., food type, cuttlefish size, food ration(bwd−1) and feeding frequency (mealsd−1)) on the growth, survival and muscle proximate composition of cuttlefish juvenile were studied in quintuplicate. The weaning period lasted 2 weeks. The results showed that cuttlefish fed with frozen white shrimp () displayed a significantly higher survival, growth parameters, ingestion rate, food conversion rate, and muscle protein content when compared with the other prepared frozen foods. The larger individuals revealed better growth performance than smaller individuals, especially in terms of survival and specific growth rate, indicating that larger individuals were more receptive to frozen prey.Furthermore, size grading should be conducted to ensure better growth during weaning. As food rations increased, the survival, growth and muscle protein content of juveniles were promoted, whereas the most appropriate food ration for cuttlefish juvenile was 35% bwd−1. Similarly, higher feeding frequency could stimulate the appetite of animals reared on weaning, and enhance survival, growth, and protein deposition compared to low feeding frequency.These results suggest that the optimal feeding protocols of juvenile weaning is as follows: the ideal prepared food is frozen shrimp, the weight of juveniles should be 4.0g (32 days old) or more, the food ration should be 35% (bwd−1), and the optimum feeding frequency is 4 meals day-1. This study offers a promising starting point to successfully breed this candidate aquaculture species.
weaning protocols;cuttlefish; survival; growth performance; proximate composition
1 Introduction
Knowledge of the biology of cuttlefish during the early life-history has expanded during the last decade as researchers have implemented laboratory tests to ascertain the critical processes that occur during the hatchling and juvenile life stages. Numerous laboratory studies have been directed toward evaluating the fertilized egg development (Samuel and Patterson, 2015), survival (Le., 2014), growth (Ramasamy., 2013), feeding, behavior (Anil., 2005) and nutritional requirements (Peng., 2015) of cuttlefish. To establish appropriate rear- ing protocols, research efforts have focused on the effects of food type (Jiang., 2018), feeding frequency (Zhu., 2018), size differences (Jiang., 2020), and en- vironmental factors, including temperature (Le., 2014), salinity (Peng., 2016), ammonia nitrogen (Peng., 2017), light intensity and photoperiods (Zhou., 2018), on the growth and survival of juvenile cuttlefish.
Low growth and high mortality are caused by a diet of frozen grass shrimp during early hatchling development (Sykes., 2013). Previous investigations have shown that during the early stages of hatchling.development, cuttlefish must be fed live prey, usually arte- mia and mysid (Richard, 1975; Domingues., 2001; Sykes., 2006). After that, they will accept dead food, such as frozen fish, shrimp, or crabs (Forsythe., 1994; Domingues., 2001). Several researchers have culturedsuccessfully with this transition to dead food (Ani., 2005; Ghazvineh., 2012), while others have fedwith live preys throughout the whole life cycle (Minton., 2001). However, only sub-adult and adult cuttlefish have been cultured successfully using alternative diets (Forsythe., 1994; Domingues., 2001; Koueta., 2002).
The first reports on the use of surimi and pelleted diets in cuttlefish demonstrated poor results in terms of growth and survival (DeRusha., 1989; Lee., 1991; Castro., 1993;Domingues., 2004). Sykes. (2013) demonstrated the possibility of rearing cuttlefish with pre- pared frozen prey in the early hatchling stage (1-day post- hatching (DPH) and 5 DPH); nevertheless, the results show- ed lower growth rates and higher mortality than those cut- tlefish fed live food preys. The weaning of marine ani- mals’ hatchling is a critical step in hatchling rearing. The successful transition from live food to aprepared feed depends on the quality of the feed supply (., palatabi- lity, digestibility, nutrients) and the hatchling themselves (., size, feeding habits, development of the digestive en- zyme). Koueta. (2002) initially demonstrated the de- mand of cuttlefish juvenile for polyunsaturated fatty acids (PUFA) in diet, which was confirmed by Domingues. (2004), and indicated which prey is beneficial to the sur- vival and growth of the individual. The investigations of Richard (1975) on cuttlefish food intake have shown that animals provided with sufficient food were indifferent to moving prey and the growth depends on the quantity of consummated prey. When animals are fed insufficiently or excessively, their growth or feed efficiency may decrease, resulting in increased production cost, and a source of de- terioration of water quality, especially when groups are overfed (Jiang., 2018). Therefore, the optimum food ration assists in minimizing food wastage, reduce size va- riation. Feeding animals in an effective way and increasing feed utilization are very important for large-scale farming, which usually can be realized by the control of timing and frequency of feed delivery. Koueta andBoucaud (2003) have shown that increasing feeding frequency can increase the survival and growth of juvenile cuttlefish by improving food intake, though feeding protocols were not optimized in these studies. Cuttlefish juvenile is large enough to accept frozen food well; however, presently there is no published data on optimal transition regimen for cuttlefish, especially for the weaning of juvenile.
In our pilot rearing trials,juvenile accept- ed frozen food and performed well around dorsal mantle length 3cm, at which stage high survival and growth rates were observed (unpublished observations). Therefore, the objectives of the present study were to determine the op- timal feeding protocols for cuttlefish juveniles from live prey to frozen food by analyzing the effects of food types (fish fry, white shrimp, and fish fry+white shrimp); cut- tlefish size (A, weight of 2.8g±0.2g; B, weight of 4.0g±0.2g;C, weight of 6.0g±0.1g;and mixed group (the ra- tio of A:B:C: was 1:1:1)), food ration(25%, 30%, 35%, and 40% bwd−1), and feeding frequency (2, 3, 4 or 5mealsd−1) on the survival, growth and muscle composition of cuttlefish juveniles.
2 Materials and Methods
2.1 Hatchling Rearing and Experimental Conditions
Experiments were carried out at the Marine Fisheries Research Institute of Zhoushan (Zhejiang Province, China). Fertilized eggs ofwere from the second generation (F2) of our research facility. Before the expe- rimental period, all the cuttlefish were reared according to what has been described for the species by Jiang. (2018). In short, newly hatched hatchling was fed with en-riched live()during the first 3 daysof post-hatching, and then with live mysids () twice a day (8:00 and 16:00) randomly.
All systems used in this study were composed of fiberglass cylindrical tanks (diameter, 1m; water depth, 80cm; and volume, 600L) as described in Jiang. (2020). Each tank had soothing aeration that was provided by an air stone and one airlift. Natural seawater was filtered through a filter bed and ultraviolet sterilizers before it was pump- ed into the tank. The water conditions were as follows: salinity at 30±0.5, the temperature at 25.7℃±0.8℃, pH at 7.76±0.49, ammonia nitrogen controlled at (0.06±0.02)mgL−1, and dissolved oxygen at (6.28±0.53)mgL−1. The temperature, salinity and dissolved oxygen were measureddaily with a YSI Pro DSS instrument (YSI; www.ysi.com), and 30% of the seawater was renewed every day under the same conditions. A natural photoperiod of 12h:12h day- night was maintained during the experiment with an intensity under 600lx. This low light intensity was adopted to maintain low-stress levels (Koueta andBoucaud, 2003). All procedures with live animals included in this study have been approved by the Animal Research Ethics Com- mittee of the Chinese Academy of Fishery Sciences. Au- thors are aware of the general principles stated by the Directive for the use of live cephalopod mollusks in sci- entific research as pointed out in several studies (Smith., 2013; Fiorito., 2015).
2.2 Weaning Protocols
Four experimental weaning protocols were performed in quintuplicate, with 60 individuals assigned to each of the replicates. To evaluate the effects of feeding protocols on survival, growth and muscle proximate composition, dif- ferent weaning protocols were carried out to test: i) food type (experiment 1), ii) cuttlefish size (experiment 2), iii) food ration (experiment 3), iv) feeding frequency (expe- riment 4).In all cases, the proceduresin 2.1 were follow- ed with paying special attention to the following points: a) the frozen prey should be completely thawed to ensure cut- tlefish accept the prepared food; b) the optimum results of each trial are used in the next test to obtain the optimum weaning protocol; c) the weaning feed training period was 2 weeks, and all cuttlefish in each tank counted and mea- sured at the end of the experiment to calculate the survi- val and growth parameters.
Experiment 1: The frozenprey used in this experiment included: 1) fish fry (sp.), 2) white shrimp (), and (3) fish fry+white shrimp (sp.+;the ratio is 1:1). The ave- rage weight and length of the fish fry and white shrimp fed to the cuttlefish were 32.64±1.63mg and 2.84±0.23cm(=50), and 30.75±2.86g and 2.91±0.12cm (=50), respectively. A total of 900 juveniles (36 days old, the weight of 6.06±0.18g; dorsal mantle length of 3.02±0.14cm) were used for this experiment.Domingues. (2001, 2004) indicated that feeding quantity for juveniles was set at 40% body weight (bw) per day, which is considered to be an appropriate ration. Based on a previous study (Jiang., 2018), all cuttlefish were fed randomly three times per day (7 AM, 11 AM and 4 PM) for this trial. Uneaten food was collected an hour after serving, which was weighed daily to calculate the statistical ingestion rate and food con- version rate. One hour before each feeding, tanks were si- phoned to remove feces and dead individuals.
Experiment 2:The effects of the cuttlefish size on the growth and survival of cuttlefish juveniles were evaluated during weaning. To reduce the costs of feeding live my- sids, 3 groups with different sizes (A: 26 days old, the weight of 2.85±0.12g, dorsal mantle length of2.41±0.23cm; B: 32 days old, the weight of 4.07±0.18g, dorsal man- tle length of 2.82±0.13cm; C: 37 days old, the weight of 6.13±0.21g, dorsal mantle length of 3.23±0.21cm)and a mixed group (the ratio of A:B:C is 20:20:20) were select- ed in this experiment. A total of 1200 juveniles met the size requirements for testing, and no significant differences (>0.05) in weight were found among the 5 replicates of groups A, B, and C at the beginning of the experiment. However, significant differences (<0.05) in weight were observed among the mixed group at the beginning of the experiment. As the remaining feed cannot be seperated and analyzed statistically in the mixed group, ingestion rate and food conversion rate were not detected in this expe- riment. Uneaten food was collected an hour after serving. One hour before each feeding, tanks were siphoned to re- move feces and dead individuals. Feeding methods were the same as those described in the above test.
Experiment 3: The effects of the four-food ration (25%, 30%, 35%, and 40% bwd−1, the amount of food given was based on the total biomass) on the survival, growth and muscle composition of cuttlefish juveniles were evaluated. Every 5 days, the total weight of the animals in each group wasreweighed to adjust the food supply.A total of 1200 juveniles were used for thistrial. Similarly, uneaten food was collected an hour after serving, which was weighed daily to calculate the ingestion rate and food conversion rate. One hour before each feeding, tanks were siphoned to remove feces and dead individuals. Feeding methods were the same as those described for the above test.
Experiment 4: Four feeding frequencies (2, 3, 4 or 5 mealsd−1) were applied to evaluate their effects on the survival, growth and muscle composition of cuttlefish ju- veniles during weaning.All cuttlefish were fed regularly and the feeding frequency (2, 3, 4 or 5 mealsd−1) were scheduled as follows: 7 AM and 3 PM; 7 AM, 12 AM and 4 PM; 7 AM, 10 AM, 2 PM and 5 PM; and 7 AM, 10 AM, 12 AM, 3 PM and 5 PM, respectively. The optimally pre- pared prey, cuttlefish size, and food rationwere identified in the first three trials used for this experiment. A total of 1200 juveniles were used for the feeding frequency trial. The ingestion rate and food conversion rate were not ana- lyzed in this experiment because the high feeding frequen- cy easily leads to the statistical deviation of the remaining feed, whileother conditions (culture conditions and feed- ing methods) were identical to those described above.
2.3 Weaning Basis, Growth Measurements,Sampling and Biochemical Analysis
Cephalopods are sensitive to starvation because of high metabolic rates and growth rates, which is related to their ‘live fast, die young’ life history. Speers-Roesch. (2016) showed that short-term starvation (3–5d) had few effects on enzymatic capacities; however, during long-term starvation (12d), glycolytic capacity for glucose use is decreased in cuttlefish tissues, while the capacities for use of lipid-based fuels (fatty acids and ketone bodies) and amino acid fuels are increased. Similarly, Le. (2016) indicated that 6 days of starvation would be the point of no return for the cuttlefish juveniles, without any compensatory effect after starvation and re-feeding. Therefore, to evaluate the short-term weaning protocols and the growth performance, the training period of weaning feed was 2 weeks for this study.On the first and fifth days of the ex- periment, the total weight of the animals in each group was reweighed to adjust the prey supply. The criterion for weaning is to put enough prey into the feeding area, and each animal can catch its own. They were considered sa- tiated when their foraging behavior ceased and juveniles assembled at the bottom of the tank.
The parameters in this study were calculated as follows:

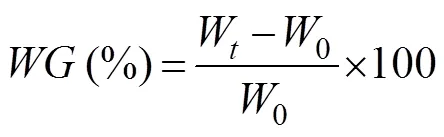
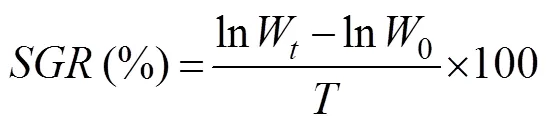

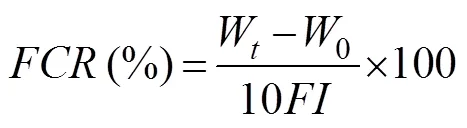
In these equations,is weight gain to body weight;is specific growth rate daily;is ingestion rate daily;is food conversion rate; Wis the final body weight (g);0is the initial body weight (g);Nis the number of cuttlefish at the end of culturing;0is the number of cuttlefish at the start of culturing;is the average wet weight of food ingested on a daily basis;ais the average wet weight (g) of the cuttlefish during the time period (days); andis the experimental time (days).
Cuttlefish muscle and the supplied prepared frozen food were analyzed for their proximate composition, and all sam- ples were stored at −20℃ until use. The moisture, protein, ash, and lipid were analyzed by the Association of Official Analytical Chemists (AOAC, 1995). The moisture le- vel was determined by drying the sample at 105℃ until a constant weight was obtained; the crude protein (N×6.25) was measured by the Kjeldahl method after acid digestion with an Autodigester (FOSS, Tecator, Höganäs, Sweden); and the ash was determined by incineration in a muffle furnace at 550℃ for 8h (Model1281, Parr, Moline, IL, USA). Fat extractions from the cuttlefish muscle and the frozen food were performed according to Bligh and Dyer (1959), using methanol and chloroform as solvents.
2.4 Statistical Analysis
All values were expressed as the mean±S.E (standard error).The survival percentages were arcsine square-root transformed before being submitted to ANOVA. Those pa- rameters (,,,, and proximate composi- tions) were tested with one-wayANOVA using the tank mean value as the observation unit. Post hoc comparisons between sample means were tested by Tukey’s test and 5% was taken as the level of significance. Unpaired Student’s-test (U-S-T) was used to compare the mean values between two groups (the single group Athe mixed group A, the same method applied to group B and group C).All statistical analyses were performed using the SPSS 20.0 statistical package (SPSS, Chicago, IL, USA).
3 Results
3.1 Effects of Food Type on Growth, Survival and Proximate Composition of Juvenile Cuttlefish During Weaning
After 2 weeks of weaning,data on the final weight,weight gain(, %bw), specific growth rate (, %d−1), ingestion rate (, %), food conversion rate (, %) and survival of juveniles weresignificantly affected by feed type (Table 1). White shrimp treatment displayed high- er,,,than fish fry andfish fry+white shrimp treatments. The cuttlefish fed with white shrimp had the highest(110%±7.6%) and(7.42%±0.55%) at the end of the experiment. Similarly, the highest(30.79%±1.48%) and(36.09%±0.81%) were also ob- served in the cuttlefish fed with white shrimp.During the trial, no events of massive mortality were observed in any food type.The survival was significantly higher in thewhite shrimp treatment group (91.78%±2.04%) in the groups fed fish fry (71.06%±3.41%) orfish fry+white shrimp (83.26%±2.34%).

Table 1 Effects of frozen food on growth, survival, ingestion rate, and food conversion rates of cuttlefish during weaning
Notes: Data are presented as the mean±S.E (=5). Within the same row, values with different superscript letters are significantly different (<0.05).
The proximate compositions of cuttlefish muscle and frozen food are presented in Table 2 and Table 3. The pro- ximate compositions of cuttlefish muscle distribution fromthree prepared frozen foods were different, with higher pro- tein content and lower levels of ash and moisture in cuttlefish fed white shrimp than in cuttlefish fed with fish fry or fish fry+white shrimp (<0.05). Furthermore, protein content in the white shrimp treatment (18.42%±0.17%) was similar to fish fry+white shrimp treatment (17.85%±0.32%). Table 3 shown a higher content in moisture in the prepared fish fry, compared to the other 2 preys, particularly the white shrimp.Protein content in the white shrimp was similar when compared to fish fry+white shrimp. Also, moisture and protein content levels in the cuttlefish were much closer to the ones found in the white shrimp and very different from the levels found in the fish fry. Com- paring the growth and proximate composition of frozen food on juveniles, we propose that the ideal prepared food is frozen white shrimp for cuttlefish juveniles weaning.

Table 2 Proximate composition (% dry weight) of reared S. pharaonis muscle
Notes: Data are presented as the mean±S.E (=5). Within the same column, values with different superscripts are significantly different (<0.05).
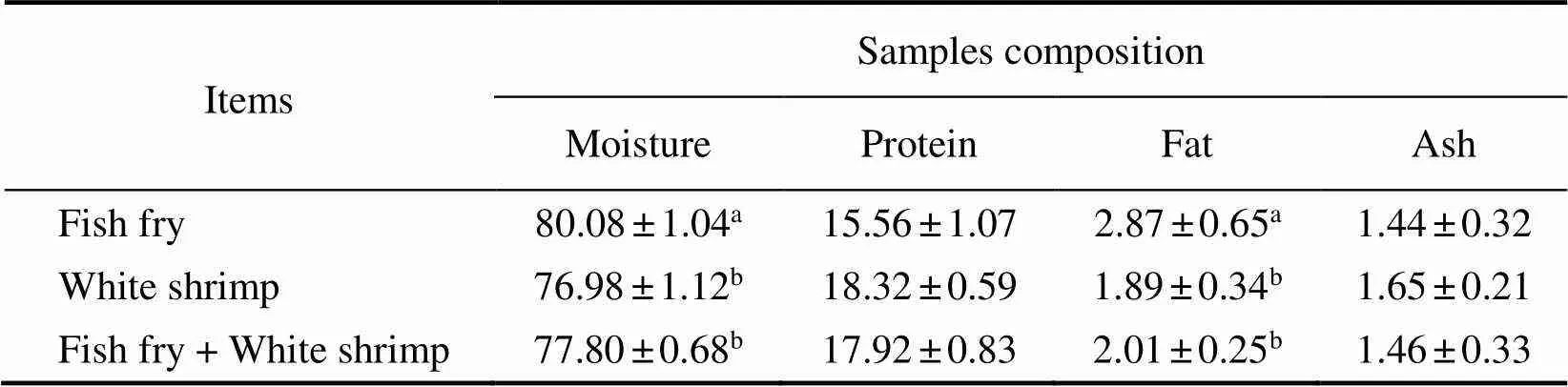
Table 3 Proximate composition (% dry weight) of frozen prey
Notes: Data are presented as the mean±S.E (=3). Within the same column, values with dif- ferent superscripts are significantly different (<0.05).
3.2 Effects of Size Difference on Growth and Survival of Juvenile Cuttlefish During Weaning
The growth performance and survival of cuttlefish in groups A (weight of 2.85g±0.12g), B (weight of 4.07g±0.18g), C (6.13g±0.21g) and the mixed groupsare presented in Table 4 and Table 5. The juveniles from group C displayed significantly higher weight increases than in other groups.The juveniles from the mixed group C exhibited a similar growth increase, whereas in the mixed group A, it was the lowest, and the difference was significant.

Table 4 Initial and final weight data for groups A, B, C and mixed group
Note: Data are presented as a mean±S.E (=5).

Table 5 Effects of size difference on growth and survival of cuttlefish during weaning
Notes: Data are presented as the mean±S.E (=5). Within the same column, mean values in the same group with different lowercase letters are significantly different (one-way ANOVA,<0.05); mean values between the two groups (single group and mixed group) with different uppercase letters are significantly different (Unpaired Student’s-test,<0.05).
Furthermore, it has been observed that the growth performance of juveniles differs between single treatment A and the mixed treatment A. The(66.46%±2.49%) (U-S-T,=9.422,=8,<0.0001) and(5.12%±0.07%) (U-S-T,=16.342,=8,<0.0001) of the single treatment A were significantly greater than the(46.17% ±4.12%) and(3.78%±0.16%) of the mixed treat- ment A. However, there was no difference in survival be- tween the single group A (62.6%±4.3%) and the mixed group A (54.0%±5.5%) (U-S-T,=2.211,=8,=0.058).On the other hand, the growth parameters of group A were different between the single group and the mixed group, but there was no difference between groups B and C be- tween single treatment and mixed treatment.This implies that large individuals were more receptive to frozen prey, and the growth performance of small individuals in the mixed treatment was not as good as that in the single treat- ment.The findings of this trial suggest that cuttlefish weight should 4g (32 days old) or more, grading should be done to ensure better growth during weaning.
3.3 Effects of Food Ration on Growth, Survival and Proximate Composition of Juvenile Cuttlefish During Weaning
The growth performance and survival of cuttlefish fed with different food rations (25%, 30%, 35%, and 40% bwd−1) are presented in Table 6. The final weight,,,,, and survival were significantly affected by the food rations(<0.05). These parameters were significantly higher in the 35% and 40% food rationtreatments than in the 25% and 30% food rationtreatments, but there was no significant difference (>0.05) between the 35% and 40% food ration treatments. This result suggests that high food ration can improve the survival and growth of juveniles during weaning, but food ration did not induce any change of growth and survival of juvenile cuttlefish at food ration of 40% (bwd−1) compared to 35% (bwd−1).

Table 6 Effects of food ration (bwd−1) on growth, survival, ingestion rate, and food conversion rates of cuttlefish during weaning
Notes: Data are presented as a mean±S.E (=5). Within the same row, values with different superscripts are significantly different (<0.05).
The proximate compositions of cuttlefish muscle between different food ration groups are shown in Table 7. The protein content was significantly lower (<0.05) with the food rations of 25% (16.52%±0.08%) and 30% (17.17%±0.13%)than those with the food rations of 35% (18.61%±0.17%) and 40% (18.56%±0.32%). The food ration of 25%showed the highest level of moisture content (81.21%±0.51%), and the 35% and 40% food rations exhibited the lowest levels that were 79.02%±0.27% and 78.81%±0.34%, respectively. The fat and ash contents were not significantly different among the groups.Based on growth performance and protein content, the optimum food ration for about 4g juvenile weaning was 35%bwd−1.

Table 7 Effects of food ration on proximate compositions (% dry weight) of cuttlefish S. pharaonis muscle
Notes: Data are presented as the mean±S.E (=5). Within the same column, values with different superscripts are significantly different (<0.05).
3.4 Effects of Feeding Frequency on Growth,Survival and Proximate Composition of Juvenile Cuttlefish During Weaning
A comparison of the performance data (., final weight, survival,, and) among the groups treated with different feeding frequencies are shown in Table 8. The final weight, survival,, andwere significantly dif- ferent in the various feeding frequencies (<0.05), and these variables in the cuttlefish fed frozen white shrimp 4 and 5mealsd−1were significantly higher than those in the cuttlefish fed frozen white shrimp 2 and 3mealsd−1; how- ever, there was not a significant difference (>0.05) between the feeding frequencies 4 and 5 mealsd−1.
The proximate analysis of the cuttlefish muscle at different feeding frequencies is shown in Table 9.As feeding frequency increased, the protein content of muscle in- creased. The protein content was significantly higher with feeding frequencies 4 mealsd−1(18.65%±0.15%) and 5 mealsd−1(18.14%±0.37%) than those with feeding fre- quency 2 mealsd−1(15.35%±0.28%). Conversely, higher moisture contentwas observed in feeding frequency 2 mealsd−1(82.04%±0.24%), and feeding frequencies 4 and 5mealsd−1displayed mean moisture content values of78.76%±0.27% and 78.42%±0.21%, respectively. There- fore, juvenile cuttlefish were weaning fed frozen white shrimp to apparent satiation 4 mealsd−1. To improve the growth, survival and protein content of juvenile during weaning, the optimum feeding frequency was 4 mealsd−1.

Table 8 Effects of feeding frequency on growth, and survival of cuttlefish during weaning
Notes: Data are presented as a mean±S.E (=5). Within the same row, values with different superscripts are significantly different (<0.05).

Table 9 Effects of feeding frequency (mealsd−1) on proximate compositions (% dry weight) of cuttlefish S. pharaonis muscle
Notes: Data are presented as the mean±S.E (=5). Within the same column, values with different superscripts are significantly different (<0.05).
4 Discussion
In this study, the goal was to determine the optimal wean- ing protocol for cuttlefish juvenile, which include the fro- zen prey type, cuttlefish size, food ration, and feeding fre- quency.
From the perspective of growth and nutritional needs, mysids promoted better cuttlefish survival and growth, es- pecially in the early hatchling stages (Richard, 1975; Do- mingues., 2004; Peng., 2015). The cuttlefish do accept dead food, such as frozen fish, shrimp or crabs (Forsythe., 1994; Domingues., 2001), but the ideal prepared food can help the development of juvenile cuttlefish through the weaning period. Growth performance and survival were significantly higher (<0.05) when juvenile cuttlefish were fed with white shrimp, compared to juveniles fed with fish fry and fish fry+white shrimp. Similar results were found in the result of Domingues. (2004), as the cuttlefish with an age of 30 or 60 days fed grass shrimp had the best growth rates than those fed fish fry. This indicated that fish fry was not suitable for cuttlefish juveniles weaning, which supported by Blanc. (1998), who showed that the diet of juvenile cuttlefish cap- tured from the wild was composed of crustaceans (89%), while fish only represented 4.6%. Not only did different growth patterns be found among cuttlefish fed different foods, but it was also related to the difference in muscle proximate composition. Cephalopod has a protein-domi- nant metabolism, making it essential for prepared diets to contain high protein to promote growth (Lee, 1994). If the food intake cannot meet its growth and development needs during weaning, it will inevitably lead to the degradation of protein in the body, and thus is unable to maintain nor- mal physiological functions. Therefore, the muscle proxi- mal composition can be used as an effective indicator of their response to short-term changes in environmental stresses. In the present experiments, the high protein content of the frozen prey was reflected by the muscle composition of cuttlefish fed with white shrimp. Thus, juveniles were able to satisfactorily digest and absorb dietary protein from white shrimp. According to Sinanoglou and Miniadis-Meimaroglou (1998), cephalopod muscle is cha- racterized by a small content of fat. It is noteworthy the low contents of fat in the muscle composition of both fish fry fed (0.76%±0.15%) and white shrimp fed (0.73%±0.06%) cuttlefish, despite the higher level of this lipid class in the fish fry food (2.87%±0.65%).
One of the key factors is to meet the nutritional needs of cuttlefish juveniles. Domingues. (2004, 2005) in- dicated that cephalopod hatchling and juvenile most like- ly need prey rich in polyunsaturated fatty acids (PUFA). Domingues. (2004), as well as Jiang. (2018),have shown that shrimps are rich in PUFA (48.89%), com- pared to fish fry (32.94%). Furthermore, Peng. (2015) reported that mysids () (45.58%) and white shrimp (48.89%) have similar PUFA content. As a result, shrimps may be more able to meet the nutritional needs of cuttlefish juvenile during weaning. The morphological as- pect of the pre-fed live mysids () and frozen white shrimps are similar in structure, which makes the cuttlefish to easily adapt to white shrimp as feed.
Choosing the right size cuttlefish for weaning not only improves survival but also shortens the transition period, which in turn, shortens the entire culture cycle. When cut- tlefish were fed frozen white shrimp in this experiment, it was noticed that the juveniles in group C (larger) had the highest survival and growth performance compared to the juveniles in the other treatments. In this study, the results indicated that larger individuals exhibited a better growth performance than smaller individuals, meaning that the larger individuals readily accepted frozen prey. The species, with an average weight of 5.0g, was fed live shrimp for one month, and the specific growth rate (SGR) was 5.7%±0.4% (Domingues., 2004). These two close relatives with the same sizehave similar growth rates.In this study, thewere 5.12%±0.07%, 6.89%±0.17% and 7.46%±0.11%, corresponding to an average weight of 2.85g±0.12g, 4.07g±0.18g, and 6.13g±0.21g, respectively. It can be found that the growth condition of cuttlefish is similar with or without weaning treatments, indicating that the best weaning protocol provided in this study was reliable.Furthermore, there are differences in the diet acceptance for cuttlefish of different sizes. Group C can be fully converted in 6 days, and group B takes 8days; however, group A did not perform well during wean- ing. With the increase in individual size, the acceptance and utilization of frozen prey increased.
Individual differences in growth contribute to the development of stress in social groups, which can reduce the fitness of individual members (Pottinger and Carrick, 1999; Buston, 2003). In the context of social hierarchy, the metabolism, internal physiological environment, and immune response pathways of subordinate individuals are inhi- bited (Elofsson., 2000; Sloman., 2000; Pottinger., 2001), which leads to the formation of feeding hierarchies and decreases the growth of subordinate individuals. This scenario will exacerbate growth differences between dominant and subordinate individuals (Jiang., 2020). This might be the cause of food grabbing in mixed groups. The results showed that the survival of cuttlefish with the same size in the mixed treatment was significantly lower than that in the single treatment, especially ingroup A. Similarly, theof cuttlefish of the size A in the mixed treatment was significantly lower than that in the single treatment. However, thedisplayed no significant difference in single group C and mixed group C. Therefore, grading should be applied to ensure better growth and survival of juvenile during weaning.
Cephalopods are unique because they have high growth rates (., between 3% and 10% bwd−1, on average) (Lee, 1994), which can be higher than 20% bwd−1during the early stages of life (Domingues., 2001). Additionally, they have high food conversion rates (Domingues., 2003) and incorporate between 20% and 50% of ingested food (Boucher-Rodoni., 1987). However, there are few reports on food ration, most of which are in the range of 20%–50% (bwd−1) or fed randomly. Moreover, the only related trials of live mysid diets during early stages used feed rates of 21%–35% (bwd−1) (Koueta and Boucaud- Camou, 1999; Domingues., 2005). The conversion from live prey to a chilled or artificial diet is a critical period for continuous testing or farming because it directly affects the survival and growth of the cultured species. In fact, food supply was directly related to cumulative mor- tality at different food rations. More competition for food occur when juveniles are placed together and weaker ani- mals tended to die out (Koueta and Boucaud-Camou, 1999). The diet transition is a critical period of food competition, causing them to jet ink and bump against the tank walls, which can explain the increased mortality at the end of the experiment. The increase in feeding has indeed contributed to the growth and protein accumulation of cuttlefish. How- ever, in terms of ingestion rate and food conversion rate, no significant difference was found between the feeding quan- tity of 35% and 40%, that is, the excessive feeding did not improve the ingestion rate and the food conversion rate.
Feeding frequency is one important factor that affects aquaculture, and an unreasonable feeding frequency will lead to the slow growth rates, causing different specifications among individuals (Sveier and Lied, 1998). Thus, the quantity of feed provided each time or the feeding frequen- cy may influence diet utilization. For cephalopods, an in- crease infeeding activity and utilization was also observed when food was distributed in several doses instead of a single dose (Iglesias., 2006). The frequency of feeding did induce changes in growth performance and survival of juvenile cuttlefish fed at a ratio of 35% of their body weight/meal. In this study, because the total daily amount of feed supply was constant with the various feeding frequencies, the survival,, andincreased in cuttlefish juveniles occurred in the higher feeding frequency treatments. Lee. (1991) in- dicated that an increase of feeding frequency (the daily ration was provided between three and five times per day) stimulated the intake of artificial diet in juvenile cuttlefish. Similar to the results of this experiment, high feeding frequency can induce more food intake, and the accumulation of protein content was significant. Considering the effects of various feeding frequencies on the cuttlefishbody composition, the protein content in cuttlefish remain- ed at a comparatively stable level. The conversion of diet from a live diet to a chilled diet requires multiple attempts for the cuttlefish to catch prey and start eating. Additionally, it will take several attempts before the cuttlefish com- pletely accept the chilled diet. During the early two days of weaning, only a small amount of feed can be accepted at a time. Thus, it is necessary to increase the feeding fre- quency to improve the food intake. From these results, we identified that a high feeding frequency results in high dai- ly food intake and higher feeding frequencies may be the most adequate approach for juveniles weaning.
In conclusion, this study demonstrates that the optimal weaning protocols for cuttlefish juvenile are as follows: the ideal prepared diet is frozen shrimp; the weight of juveniles should be 4.0g or more; the food ration is 35% (bwd−1); and the optimum feeding frequency is 4 times per day. This optimum feeding protocol can lead to shortened weaning time, increased food intake, and higher feed con- version rates, which are important for the higher survival and well growth performance of cuttlefish juvenile during weaning.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 41806186) and the Ning- bo Science and Technology Bureau (No. 2014C11001). This research was also sponsored by the K. C. Wong Magna Fund at Ningbo University. The authors are grateful to the Marine Fisheries Research Institute of Zhoushan for supplying the experimentcuttlefish hatchlings and providing logistical support throughout the experiment.
Anil, M. K., Andrews, J., and Unnikrishnan, C., 2005. Growth, behavior, and mating of pharaoh cuttlefish (Ehrenberg) in captivity.,57: 25-31.
Association of Official Analytical Chemists (AOAC), 1995.. 16th edition. Association of Official Analytical Chemists, Arlington, VA, 1899pp.
Blanc, A., Pinczon du Sel, G., and Daguzan, J., 1998. Habitat and diet of early stages ofL. (Cephalopoda) in Morbihan Bay, France., 64: 263- 274.
Bligh, E. G., and Dyer, W. J., 1959. A rapid method of total li- pid extraction and purification., 37: 911917.
Boucher-Rodoni, R., Boucaud-Camou, E., and Mangold, K., 1987.Feeding and digestion. In:,. Boyle, P. R., ed., Academic Press, London, 85-108.
Buston, P., 2003. Size and growth modification in clownfish., 424: 145-146.
Castro, B. G., DiMarco, F. P., DeRusha, R., and Lee, P. G., 1993. The effects of surimi and pelleted diets on the laboratory survival, growth and feeding rate of the cuttlefish., 170: 241-252.
DeRusha, R. H., Forsythe, J. H., DiMarco, F. P., and Hanlon, R. T., 1989. Alternative diets for maintaining and rearing cephalopods in captivity., 4: 306-312.
Domingues, P. M., Dimarco, P. F., Andrade, J. P., and Lee, P. G., 2005. Effect of artificial diets on growth, survival and condition of adult cuttlefish,, Linnaeus, 1758. Aqua- culture International,13: 423-440.
Domingues, P. M., Kingston, T., Sykes, A., and Andrade, J. P., 2001. Growth of young cuttlefish,(Linnaeus, 1758) at the upper end of the biological distribution temperature range., 32: 923-930.
Domingues, P., Sykes, A., Sommerfield, A., Almansa, E., Loren- zo, A., and Andrade, P., 2004. Growth and survival of cuttlefish,(Linnaeus, 1758) of different ages fed crustaceans and fish. Effects of frozen and live prey., 229: 239-254.
Domingues, P., Sykes, A., Sommerfield, A., and Andrade, P., 2003. The effects of feeding live or frozen prey on growth, survival and the life cycle of the cuttlefish,(Linnaeus, 1758).,11: 397-410.
Elofsson, U. O., Mayer, I., Damsgård, B., and Winberg, S., 2000. Intermale competition in sexually mature arctic charr: Effects on brain monoamines, endocrine stress responses, sex hormone levels, and behavior., 118: 450-460.
Fiorito, G., Affuso, A., Basil, J., Cole, A., de Girolamo, P., D’an- gelo, L., and Mark, F., 2015. Guidelines for the care and welfare of cephalopods in research–A consensus based on an initiative by CephRes, FELASA and the Boyd Group., 49: 1-90.
Forsythe, J., Derusha, R., and Hanlon, R., 1994. Growth, reproduc- tion and life span of(Cephalopoda: Mollusca) cultured through seven consecutive generations., 233: 175-192.
Ghazvineh, L., Valinassab, T., Savari, A., and Ghobadiyan, F., 2012. Reproductive biology of the pharaoh cuttlein the Persian Gulf., 4: 313-319.
Iglesias, J., Fuentes, L., Sánchez, J., Otero, J. J., Moxica, C., and Lago, M. J., 2006. First feeding ofCuvier, 1797 paralarvae using artemia: Effect of prey size, prey density and feeding frequency., 261: 817-822.
Jiang, M. W., Peng, R. B., Wang, S. J., Peng, R. B., Han, Q. X., and Jiang, X. M., 2018. Growth performance and nutritional composition ofunder artificial culturing con- ditions., 49: 2788-2798.
Jiang, M. W., Peng, R., Peng, R. B., Han, Q. X., and Jiang, X. M., 2020. Effects of size dominance on the survival, growth and physiological activities of juvenile pharaoh cuttlefish ().,525: 318-327.
Koueta, N., and Boucaud, E., 2003. Combined effects of photoperiod and feeding frequency on survival and growth of juvenile cuttlefishL. in experimental rearing.,296: 215- 226.
Koueta, N., and Boucaud-Camou, E., 1999. Food intake and growth in reared early juvenile cuttlefish, l. (Mollusca Cephalopoda).,240: 93-109.
Koueta, N., Boucaud-Camou, E., and Noel, B., 2002. Effect of enriched natural diet on survival and growth of juvenile cuttlefishL., 203: 293-310.
Le, K. X., Jiang, X. M., Peng, R. B., Luo, J., Tang, F., and Wang, C. L., 2014. Effects of four ecological factors on the growth and survival oflarvae.,31: 33-37 (in Chinese with English abstract).
Le, K. X., Wang, Y., Peng, R. B., Liang, J. J., Han, Q. X., and Jiang, X. M., 2016. Effects of starvation and re-feeding on survival rate, growth and digestive enzyme activities of juvenile, 27: 2002-2008 (in Chinese with English abstract).
Lee, P. G., 1994. Nutrition of cephalopods: Fueling the system., 25: 35-51.
Lee, P. G., Forsythe, J. W., DiMarco, F. P., DeRusha, R. H., and Hanlon, R. T., 1991. Initial palatability and growth trials on pelleted diets for cephalopods., 49: 362-372.
Minton, J. W., Walsh, L. S., Lee, P. G., and Forsythe, J. W., 2001. First multi-generation culture of the tropical cuttlefishEhrenberg, 1831.,9: 379- 392.
Peng, R. B., Le, K. X., Jiang, X. M., Wang, Y., and Han, Q. X., 2015. Effects of different diets on the growth, survival, and nutritional composition of juvenile cuttlefish,.,46: 650-664.
Peng, R. B., Le, K. X., Wang, P. S., Han, Q. X., and Jiang, X. M., 2017. Detoxification pathways in response to environmental ammonia exposure of the cuttlefish,: Glutamine and urea formation.,48: 342-352.
Peng, R. B., Wang, P. S., Jiang, M. W., Ruan, P., Han, Q. X., and Jiang, X. M., 2016. Effect of salinity on embryonic development of the cuttlefish., 48: 666-675.
Pottinger, T. G., and Carrick, T. R., 1999. A comparison of plas- ma glucose and plasma cortisol as selection markers for high and low stress-responsiveness in female rainbow trout., 175: 351-363.
Pottinger, T. G., and Carrick, T. R., 2001. Stress responsiveness affects dominant-subordinate relationships in rainbow trout., 40: 419-427.
Ramasamy, P., Subhapradha, N., Seedevi, P., Madeswaran, P., Vai- ramani, S., and Shanmugam, A., 2013. Length-weight and allometry relationship of a cuttlefish., 7: 309-314.
Richard, A., 1975. L’élevage de la seiche (L., mollusque céphalopode). 10th European Symposium on Ma- rine Biology, Ostend, Belgium, 1, 359-380.
Samuel, V. D., and Patterson, J., 2015. Studies on the embryonic development of pharaoh’s cuttlefishEhrenberg, 1831 under laboratory conditions., 44: 519-527.
Sinanoglou, V. J., and Miniadis-Meimaroglou, S., 1998. Fatty acid of neutral and polar lipids of (edible) Mediterranean cephalopods., 31: 467-473.
Sloman, K. A., Gilmour, K. M., Taylor, A. C., and Metcalfe, N. B., 2000. Physiological effects of dominance hierarchies within groups of brown trout,, held under simulated na- tural conditions., 22: 11-20.
Smith, J. A., Andrews, P. L., Hawkins, P., Louhimies, S., Ponte, G., and Dickel, L., 2013. Cephalopod research and EU Directive 2010/63/EU: Requirements, impacts and ethical review.,447: 31- 45.
Speers-Roesch, B., Callaghan, N. I., MacCormack, T. J., Lama- rre, S. G., Sykes, A. V., and Driedzic, W. R., 2016. Enzymatic capacities of metabolic fuel use in cuttlefish () and responses to food deprivation: Insight into the metabolic organization and starvation survival strategy of cephalopods., 186: 711- 725.
Sveier, H., and Lied, E., 1998. The effect of feeding regime on growth, feed utilisation and weight dispersion in large Atlantic salmon () reared in seawater., 165: 333-345.
Sykes, A. V., Domingues, P. M., and Andrade, J. P., 2006. Effects of using live grass shrimp () as the only source of food for the culture of cuttlefish,(Linnaeus, 1758)., 14: 551-568.
Sykes, A. V., Rui, A. G., and Andrade, J. P., 2013. Early weaning of cuttlefish (, L.) with frozen grass shrimp () from the first day after hatching., 44: 1815-1823.
Zhou, S. N., Lyu, T. T., Chen, Q. C., Peng, R. B., Han, Q. X., and Jiang, X. M., 2018. Effects of light intensity and photoperiod on the embryonic development of., 29: 2059-2067 (in Chinese with English abstract).
Zhu, T. T., Li, C. C., Lu, Y., Jin, M., Wang, X. X., Luo, J. X., and Zhou, Q. C., 2018. Effects of feeding frequency on growth performance, body composition, digestive enzyme activities, tissue fatty acid and amino acid compositions of., 30: 3581-3592 (in Chinese with English abstract).
. E-mail: jiangxiamin@nbu.edu.cn
February 10, 2020;
April 28, 2020;
June 12, 2020
(Edited by Qiu Yantao)
 Journal of Ocean University of China2020年6期
Journal of Ocean University of China2020年6期
- Journal of Ocean University of China的其它文章
- Numerical Simulation and Risk Analysis of Coastal Inundation in Land Reclamation Areas: A Case Study of the Pearl River Estuary
- Variation of Yellow River Runoff and Its Influence on Salinity in Laizhou Bay
- Cold Water in the Lee of the Batanes Islands in the Luzon Strait
- Preliminary Design of a Submerged Support Structure for Floating Wind Turbines
- Inversion of Oceanic Parameters Represented by CTD Utilizing Seismic Multi-Attributes Based on Convolutional Neural Network
- Trace-Norm Regularized Multi-Task Learning for Sea State Bias Estimation
