Role of minimally invasive surgery for rectal cancer
Kurt A Melstrom, Andreas M Kaiser
Abstract Rectal cancer is one of the most common malignancies worldwide. Surgical resection for rectal cancer usually requires a proctectomy with respective lymphadenectomy (total mesorectal excision). This has traditionally been performed transabdominally through an open incision. Over the last thirty years, minimally invasive surgery platforms have rapidly evolved with the goal to accomplish the same quality rectal resection through a less invasive approach. There are currently three resective modalities that complement the traditional open operation: (1) Laparoscopic surgery; (2) Robotic surgery; and (3) Transanal total mesorectal excision. In addition, there are several platforms to carry out transluminal local excisions (without lymphadenectomy). Evidence on the various modalities is of mixed to moderate quality. It is unreasonable to expect a randomized comparison of all options in a single trial. This review aims at reviewing in detail the various techniques in regard to intra-/perioperative benchmarks, recovery and complications, oncological and functional outcomes.
Key words: Rectal cancer; Minimally invasive surgery; Laparoscopic surgery; Robotic surgery; Transanal total mesorectal excision; Transanal minimally invasive surgery
INTRODUCTION
In the United States, there are approximately 44000 new cases of rectal cancer each year. Paired with colon cancer, colorectal cancer represents the third most common cancer both in incidence and in mortality[1], hence representing a significant healthcare burden. Several factors contribute to the higher complexity of rectal cancer as compared to colon cancer. The importance of proper surgical techniques for improved outcomes has been documented on multiple occasions and nearly all circumstances. Innovation in the treatment of rectal cancer has been unstoppable which on one hand continues to be desperately needed and represents progress but on the other hand renders structured research and long-term comparisons difficult. Advances were seen in the radiation and chemotherapeutic fields, diagnostics, as well as the realm of surgery. The constantly changing landscape with multiple variables adds additional complexity to rectal cancer’s intrinsic difficulty when treatment is to be measured not only by oncological outcome parameters but also functional and quality of life aspects (Table 1).
Until about 30 years ago, open surgical techniques represented the only modality available to remove a rectal tumor. Propagation of a specimen-oriented anatomical dissection technique, aka total mesorectal excision (TME), became a turning point in reducing local recurrence rates and became the gold standard[2]. Since then, there has not only been a technological revolution with development of several new and less invasive platforms but also an overall paradigm shift in the in the management of rectal cancer. Multimodality treatment has become the standard for all but the very early rectal cancers. Laparoscopic surgery was introduced to colorectal surgery in the 1990s, but initially excluded the rectum. Increased familiarity with the technique and development of more sophisticated minimally invasive surgery tools allowed for an increasingly robust advantage that triggered a slow but steady market penetration. Independent of that, the implementation of a major management shift happened with introduction of enhanced recovery protocols (ERAS), which themselves led to a measurable reduction of the length of stay and complication rates.
In 2020, there are four major platforms to perform an oncological resection and hybrid versions thereof. In addition, endoluminal surgeries have evolved from simple transanal local excisions to more sophisticated technology-dependent interventions (Table 2). This review aims at highlighting the role of these various minimally invasive platforms for rectal cancer as opposed to the conventional open resection.
BACKGROUND
Evaluation and adoption of minimally invasive surgery for colorectal cancer in general and specifically for rectal cancer has been a comparably slow process spanning the last three decades. In contrast to gallbladder and appendiceal surgeries, colorectal surgery for cancer was slower due to a combination of the more complex surgery as such spanning multiple quadrants and not corroborated early concerns about a higher incidence of port site recurrences. Prior to rectal cancer trials, the appropriateness of the laparoscopic technique was evaluated for colon cancer by numerous nonrandomized observational studies before properly designed trials were published in the mid-2000’s. It is important to note that rectal cancer was most commonly excluded. These randomized controlled trials (RCT) of laparoscopicvsopen surgery for colon cancer included the Barcelona, COST, COLOR, and CLASICC trials[3-6]. The results were largely similar and primarily showed at least equivalent oncologic outcomes, no difference in complications, and at best a modest reduction in length of stay and return of bowel function after laparoscopic surgery. Yet, as surgical history demonstrates, laparoscopic surgery continued to penetrate as routine resulted in more measurable benefits.
These landmark trials set the framework to expand research to laparoscopic rectal cancer surgery. The seminal clinical trials for minimally invasive rectal cancer surgery are listed in Table 3.
The first laparoscopicvsopen rectal cancer randomized controlled trial was part ofthe MRC CLASICC trial which included both colon and rectal cancer patients[6]. The rectal arm found a non-significant increase in positive circumferential resection margins (CRM), but the authors argued against the use of laparoscopic surgery for rectal cancer at that time. It took another five years for another major randomized trial to finish. The COREAN trial out of South Korea found similar short-term outcomes between the two modalities[7]. The COLOR II trial out of Europe found a quicker return of bowel function and significantly shorter lengths of stay in laparoscopic patients while the primary outcome of locoregional recurrence was identical with 5% in each group[8]. In 2015, two parallel major RCTs of equal design carried out in the USA and Australia, respectively, compared the immediate pathological outcomes after laparoscopicvsopen rectal surgery: The Z6051 and ALaCaRT trial[9,10]. Both studies, based on a composite of oncologic margins, failed to demonstrate noninferiority of the laparoscopic approach. While the long-term oncological outcomes were then not yet reported, these preliminary results were cause to significant concerns among surgeons as there were suddenly three published high-quality studies with trends toward inferior pathologic margins after laparoscopic rectal cancer surgery when compared with the standard open approach. Since the initial commotion, both trials have published their intermediate-term oncological outcomes spanning two years of followup: There were no differences in overall or disease-free survival which cast doubt on the relevance of the initial CRM composite index[11,12].
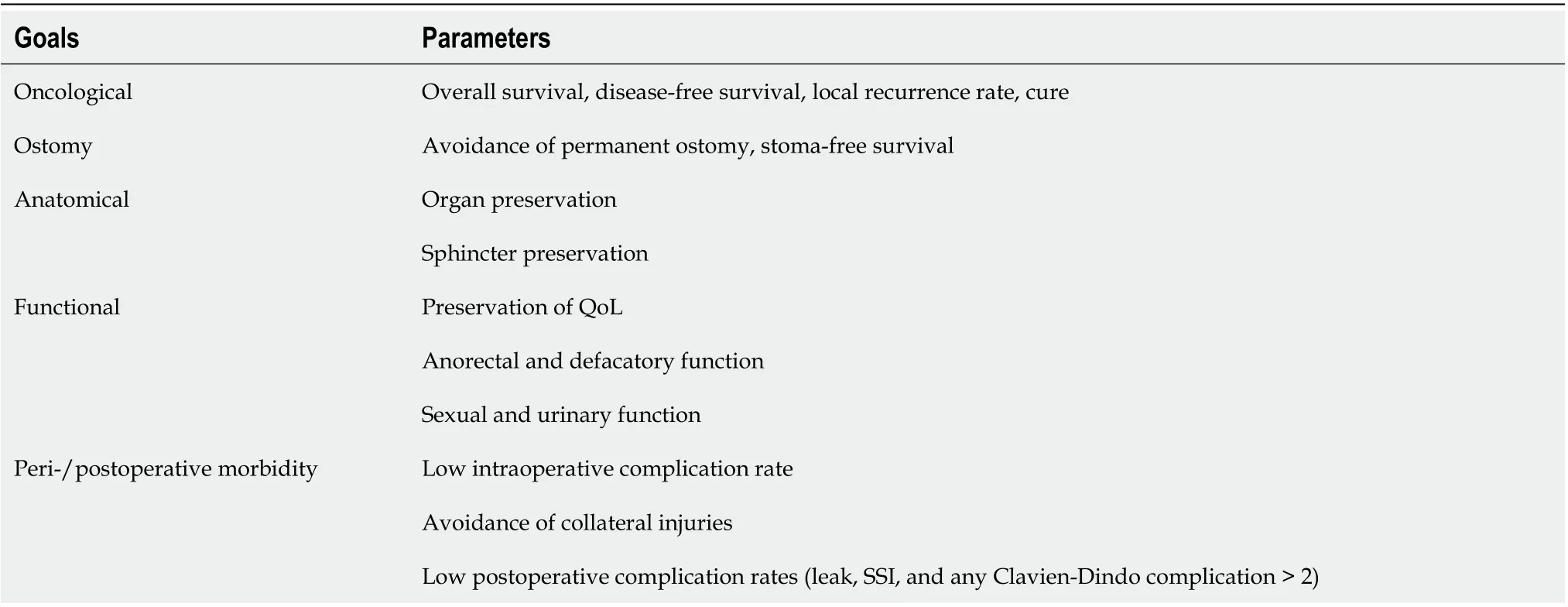
Table 1 Goals of care in rectal cancer patients

Table 2 Surgical platforms for rectal neoplasias
These studies mainly reported on conventional multi-port laparoscopic surgerywith an abdominal extraction site. However, in the mid-2010's some surgeons focused on experimenting with alternative approaches. Single-port surgery relies on one larger port where the camera and all working instruments are inserted. This can later be used for the extraction of the specimen. Numerous feasibility studies and small comparative studies did show promise[13], but no definitive advantage. Since that time, the use of single-port laparoscopic surgery has decreased - likely due to its technical challenges and the evolution of robotic surgery[14]. While strict natural orifice transluminal endoscopic surgery (NOTES) never reached full feasibility, natural orifice specimen extraction through the vagina or the open rectal stump was investigated and advocated by some.

Table 3 Major randomized controlled trials in minimally invasive surgery for rectal cancer
In the early 2000s, in the shadow of proving the value of laparoscopy and executing the above-mentioned trials, a third technical modality entered the arena: Robotic rectal cancer surgery. Robotic surgery rapidly gained traction, but similar arguments were voiced against the platform as had in the past at the onset of laparoscopy: Longer operative times, oncological inferiority, and higher cost. Again, evidence was first observational until in 2017, when the first prospective randomized multicenter trial was published. This ROLARR trial was a worldwide effort with a primary end point of conversion rates[15]. Disappointingly, there were – except in subgroup analysis - no significant differences between the two modalities. A subsequent smaller RCT out of Korea focusing on the quality of the TME specimen as primary endpoint again found similar results between the 2 groups[16].
Meanwhile, local transanal excision had taken its own its own technical evolution (see later) but was never considered equivalent to an oncological resection as it was found to be associated with unacceptably high local recurrence rates. However, as an offspring from natural orifice transluminal endoscopic surgery (NOTES), a transanal total mesorectal excision (TaTME) evolved as a bottom-up approach to carry out an oncological total mesorectal resection. This technique has been popularized since 2010 but the approach has remained under intense investigation and scrutiny. The major RCTs are still enrolling patients; but in 2014, an early single-center RCT, the Bordeaux trial, reported a lower CRM positivity rate for TaTME as compared to laparoscopic TME[17]. The TaTME was developed and marketed as an alternative to the transabdominal laparoscopic or robotic approaches but in reality required an abdominal support approach. The primary goal was to facilitate the most distal dissection in a narrow pelvis, especially in an increasingly obese patient population. In 2019, three large trials reported their initial outcomes from TaTME. The first detailed the nationwide Dutch experience with TaTME over a three-year period and compared it to laparoscopic TME[18]. The primary outcome of CRM positive rates was identical at 4% in both groups. The second trial was a worldwide matched comparison of TaTME and robotic TME. The primary outcome in this study was a composite score of pathologic margins. There were no differences overall, however, the distal resection margin positivity was higher in TaTME specimens (1.8%vs0.3%,P= 0.051)[19]. Finally, the Norway experience of TaTME compared to all other modalities showed higher anastomotic leak and local recurrences rates[20]. This resulted in a suspension of the procedure in that country[21].
Just as with the laparoscopic rectal cancer trials, these initial reports with TaTME uncovered relevant concerns. However, judgement is being held until two large RCTs on TaTME are completed and more information is available about complication and functional outcomes.
BENCHMARKS OF SURGICAL TECHNIQUE
The specimen-oriented TME has evolved as the standard of care in rectal cancer surgery since it was initially reported in the 1980s[22]. A complete TME with an with preservation of an intact mesorectal envelope on the specimen has been shown to be vital to minimizing local recurrences even without addition of radiation[2]. A major goal and benchmark for any alternative, such as a minimally invasive technique, is hence to preserve the quality of the TME. The other major goal is to minimize the surgical trauma and to achieve a quicker recovery and optimized functional outcome.
The main disadvantage of open rectal surgery lies in the required large incision with a possible extension into the epigastrium for mobilization of a challenging splenic flexure. In contrast, laparoscopic rectal surgery eliminates this incision and bowel exposure to the room air as it excels in its plasticity with flexible placement and number of trocars as needed. A supportive hand port is optional and may be used to facilitate the dissection and serve as extraction site for the specimen. The position of the operating table can be adapted to momentary needs to take full advantage of gravity, and the surgeon can move easily around the table. These aspects are relevant in a multi-quadrant operation that ranges from the primary target in the pelvis, the ligation of the mesenteric vessels, to the mobilization of the splenic flexure. The steps are further facilitated by the magnification of the laparoscope. However, laparoscopic rectal surgery is a labor-intensive procedure with a significant learning curve that is estimated to be around 40-90 cases[23]. The critical portion of the TME as such requires a high level of expertise. A challenge for laparoscopic instruments is that they are straight and may have difficulty at the pelvic inlet to navigate around the sacral promontory and reach the pelvic floor, particularly in a narrow and obese pelvis.
The robotic approach with stabilized 3D vision and higher degree of freedom for instrument motion and maneuverability was engineered to address some of those specific problems of laparoscopic surgery. The robotic TME has been standardized over time[24,25]. The instruments are touted as having seven ranges of motion and behave more like a normal human wrist. Many studies found the learning curve to be quicker at around 15-44 cases[23]. Furthermore, the robot offers an ergonomic advantage as the surgeon sits down for most of the procedure. Limitations for the robot are that the surgeon is not at the bedside which may impair the teaching of trainees and could prolong the time to execute an acute conversion to an open procedure. Maneuvering through other quadrants is more challenging than with laparoscopic surgery and on occasion may require to re-target or redock the robot prior to proceeding. While the standard table cannot be rotated without undocking the robot, newer models are available that are integrated with the robot to allow for continuous adjustment of the position[26].
Particularly in the current era of epidemic obesity, any of the previously mentioned approaches may encounter limitations to achieve an optimal exposure and reach the pelvic floor. Merging the concepts of NOTES and TAMIS, the TaTME technique was developed to address this concern[27]. The two main advantages of this approach are that (1) the bottom-up technique may proceed even in presence of substantial visceral obesity; and (2) that the distal margin can be visually chosen at the beginning of the dissection. With the other modalities, the distal margin is often approximated based on feel, tattoo, or experience; when a stapler is fired, there is always the potential for encroaching on the distal margin. There are several limitations with this technique as well. In contrast to the other techniques which pursue the replication of the open method, the TaTME is a radically different approach that may lead to disorientation, incorrect dissection planes with unusual complications, as well as a stretch injury of the sphincter complex. The learning curve and training are cumbersome and require 45-51 cases before achieving proficiency[28]. While this surgery can be performed completelyviathe transanal approach, most centers will work with two teams from the abdomen and transanally, duplicating the use of resources and team members at the same charge.
QUALITY OF DATA/ADOPTION
Due to the challenges, adoption of minimally invasive surgery techniques for rectal cancer has been slower than for colon resections. In 2005, about 90% of the proctectomies were done open[29]. Since then, there was a slow overall increase in laparoscopic and robotic techniques to 52% by 2016. Notably, however, robotic rectal surgery rose faster than laparoscopic rectal surgery with a 3.8-foldvs1.7-fold increase from 2010-2014, respectively[30]. The gain in popularity of these surgeries allowed for better quality of the related research. Publications on each of the minimally invasive surgery platforms have followed the natural investigative pattern starting with the initial pilot series of feasibility, subsequent single center observational studies, and ultimately multicenter RCTs.
Correlating with the time since introduction to rectal cancer, laparoscopic surgery with currently five nationwide RCTs has the highest number of publications (Table 3 and 4), all of which meanwhile have follow-up reports on long-term outcomes. Following about a decade behind, robotic surgery has only one large multicenter RCT, but at least several single-center RCTs along with a plethora of matched observational studies from national databases and single centers (Table 5). Most researchers focused on comparing laparoscopic and robotic surgery while there is only a small number of studies comparing open to robotic surgery. Even though strictly speaking not permissible, there was the biased assumption that earlier findings from the laparoscopicvsopen trials could be extrapolated onto robotic surgery since both were minimally invasive techniques. The body of evidence for TaTME as the newest surgical technique is still limited with only one modest sized RCT (Table 6). There are a several observational single-center studies starting in 2015 with a hybrid series of 140 patients[31], as well as retrospective studies comparing TaTME to laparoscopic or robotic surgery, respectively.
Looking at these four modalities of achieving an oncological resection as well as additional hybrid variations, the total number of possible individual comparisons becomes exhaustive, and a randomized 4-arm is highly unlikely. In the following section, surgical outcomes are presented based on the highest available level of evidence for each modality.
SHORT TERM OUTCOMES: OPERATIVE CHARACTERISTICS
Operative times
Intraoperative findings that define quality in surgery include operative time, blood loss, and rate of conversions to an open procedure. Laparoscopic times, with an average of 215 min, have been consistently and on average 34 min longer than open surgery times (Table 4). The clinical significance of this remains uncertain, particularly since this applied to several of the earlier trials when minimally invasive surgery was not yet routine. The difference was most pronounced in the COREAN and COLOR II trial where the operating time was 47 and 52 min longer for laparoscopic surgery, respectively[7,32].
Robotic times in most studies tend to be longer than laparoscopic times (Table 5), on average 250 min,i.e., 15 min longer. Reflected in this is also the extra time and expertise to dock the robot which initially inflated times. The difference between laparoscopic and robotic arms was most pronounced in the Kimet al[16]’s RCT with 227 minvs339 min, whereas the ROLARR trial did not find a significant difference with 261 minvs298 min, respectively[15].

Table 4 Selected laparoscopic vs open rectal cancer surgery studies

Table 5 Selected laparoscopic vs robotic rectal cancer surgery studies
Finally, TaTME times are on average about 250 min (Table 6). Two studies suggested a significant reduction in operating times when compared to open, laparoscopic, and robotic approaches[33,34]. In all fairness, it should be noted though that a large contributing factor lies in the resource-intense use of two teams that simultaneously work transabdominally and transanally.

Table 6 Selected transanal total mesorectal excision rectal cancer surgery studies
Intraoperative blood loss
This parameter is an estimated and self-reported variable that unsurprisingly has much more variability between studies than time. However, blood loss is generally and on average 87 mL larger in open surgery than in laparoscopic surgery. Whether this is clinically meaningful is doubtful. The largest variation was seen in the COLOR II trial with 200 mL seen in laparoscopicvs400 mL in the open group[32]. Blood loss was much less strictly reported in robotic and TaTME comparative studies. In the former, it averaged 131 mL with no major differences compared to laparoscopic surgery. For TaTME, the average in the three studies that reported blood loss was 66 mL, which was significantly less than the other modalities in two studies[33,34].
Conversion rates
Perhaps the most relevant operative variable is the conversion rate which has been associated with substantial incremental cost[35]. In the major RCTs, laparoscopic conversion rates average 11% with a range from (0)1-17%. Comparison between laparoscopic and robotic conversions was the primary end point in the ROLARR trial. In theory, the robotic approach was expected to reduce the number of conversions caused by obstructed vision and limited working space. However, the observed difference between conversions, which were 12%vs8% for laparoscopic and robotic, respectively, did not reach statistical significance[15]. In contrast, two retrospective studies noted lower conversion rates by robotic approach: The larger was a single center study with 200 patients per arm (2%vs10%), the smaller one with 29vs37 patients (0%vs20%)[36,37]. Four larger database studies found the robotic approach to reduce the number of conversions[38-41], which was associated with a reduced length of stay of 8 d and 15 d, respectively.
Conversions from a TaTME surgery are harder to define, especially when two teams are working and tackling the challenging sequences together. If difficulty is encountered from one direction that would warrant a conversion, the other team can finish without technically recording a conversion. As a result, the literature records a very small TaTME conversion rate averaging 2.7%. Two small matched studies comparing TaTME with laparoscopic surgeries showed zero conversions with TaTME had and 11% and 20% with the laparoscopic technique[33,42].
SHORT TERM OUTCOMES: POSTOPERATIVE CHARACTERISTICS
Standard variables reported for the postoperative phase of care include: Return of gastrointestinal function (e.g., time to food intake, flatus, and bowel movements), length of stay, postoperative complications, and mortality. It should be noted that a highly promising driver of quicker recovery,i.e., intracorporeal instead of extracorporeal anastomosis, has not been yet included in most publications. The robotic approach clearly facilitates that technique and there has been a significant push towards it in the last two years. But it may be too early to see the subjective impression of a benefit substantiated by objective solid evidence.
Return of GI function
Return of bowel function has been assessed in several different ways, including tolerance of liquid or solid diet, as well as time to flatus and bowel movements. That passive approach has in recent years been revisited by the proactive ERAS protocols that were nearly invariably recognized as preferred postoperative management regardless of the surgical approach.
Different studies used different metrics, and pooling of data is difficult. Several studies showed a modest but significant reduction with the laparoscopic compared to the open approach. Specifically in the COREAN trial, laparoscopic surgery was associated with significant reductions of the time to bowel movements, flatus, and the time to solid diet by 0.8 d, 0.9 d, and 0.4 d, respectively[7]. A smaller RCT from 2011 revealed nearly identical significant results in each category[43]. – The impact of robotic surgery on the return of bowel function has not yet been studied as extensively, with the most robust data coming from the Kimet al[16]’s RCT. There was no difference between the laparoscopic and the robotic groups with return of bowel function on day 2. – As to the TaTME trials, these data points appeared not to be in the focus of the studies and were not reported to any relevant degree. Only the Persiani study analyzed bowel function and noted a reduction in time to flatus and time to oral intake in the TaTME group compared to the laparoscopic group (1 dvs3 d, respectively for both metrics)[33].
Length of stay
The reduction in hospital stay and a quicker return of bowel function seen in the laparoscopicvsopen colon surgery trials was expected to carry over into laparoscopic rectal surgery. There was indeed a modest but real reduction in length of stay. The COREAN and COLOR II trials both saw a one day reduction in hospital stay while the CLASICC trial recorded a two day reduction[6,7,32]. The Z6051 trial had the shortest length of stay at 7 d and did not see a difference between the two groups[9]. Both the laparoscopic and robotic techniques share the same advantages of minimally invasive surgery with small incisions as one of the driving forces to faster recovery. Not unexpectedly, most quality studies did not show any difference in length of stay. The average robotic length of stay is 8.5 d, the shortest was 4 d as reported in an observational study[44]. One large database study found a shorter length of stay (4 d) in the laparoscopic group as opposed to 6 d in both the open and robotic group[45]. However, two other similar databases reported the shortest length of stay in the robotic group (6 d), followed by the laparoscopic (7 d) and open approach (8 d)[39,46].
The same holds true for the TaTME approach as well. The average stay is 6.8 d with only a 3-arm case-matched study from Denmark with 100 patients per approach showing a difference between the groups[33]. The TaTME group had an 8-d stay compared to 14 and 15 d for laparoscopic and open. However, it is difficult to draw conclusions as all groups had above-average length of stay compared to US studies.
Morbidity and mortality
Open and minimally invasive colorectal surgery are generally predictable and safe. Mortality for all is fortunately equally low at 0-3%. However, the complication rates are widely variable from study to study, likely the result of varying inclusion criteria. Rectal surgery has always carried a substantial risk for complications with the most common issues related to anastomotic leak, wound infections, ileus, and stoma related problems (e.g., dehydration from ileostomy). The major laparoscopic RCTs reported complication rates in the range of 19%-59%, however they did not significantly differ from open or robotic surgeries. Similar to others, the ROLARR trial found the robotic complication rate to be 33% as opposed to 31% in the laparoscopic group[15].
Finally, TaTME appeared to be associated with a higher rate as well as unusual type of complications. An early publication noted a difference between TaTME (32%) and laparoscopic surgery (44%) which did not reach statistical difference[17]. Later, however, the Norwegian trial reported a significantly higher anastomotic leak rate of 8.4% when compared to their overall national averages of 4.5%[20]. This, in part, led Norway to put a hold on the TaTME procedure. A large prospective, observational, multi-center audit study with 2579 patients found TaTME leak rates to be 10.4% when the abdominal part was done laparoscopically and 15.6% when done robotically. Both were significantly higher that the pure laparoscopic (6.7%), pure robotic (6.5%) or open (5.5%) approaches. However, the significance was lost when a mixed-effects model was applied[47].
SHORT TERM OUTCOMES: PATHOLOGIC CHARACTERISTICS
The complete removal of the rectum and lymph nodes within an intact mesorectal envelope represents the primary goal of successful rectal cancer surgery. Pathologic assessment of the specimen is arguably a core parameter and quality benchmark. Even if residual disease after surgery is microscopic only (R1 resection), the risk of overall failure is substantial with 56% distant recurrence and disease-free survival of only 41%[48]. There are four commonly reported elements of the pathologic results: Lymph node harvest, circumferential resection margin (CRM), distal resection margin (DRM), and quality of the TME specimen.
Lymph node harvest
A lymph node harvest of at least 12 lymph nodes is universally considered a surrogate for a complete TME, regardless of the technique. In the literature, in fact an average harvest of 15 lymph nodes is reported in all groups. No difference in nodal retrieval was noted in most studies except in the Kim RCT and a smaller trial where robotic surgery shifted the balance by three additional nodes[16,49].
Circumferential resection margin
A negative CRM of at least 1mm is another surrogate for a complete TME. Three major trials (CLASICC, ALaCaRT, ACOSOG Z6051) showed a higher rate of positive CRMs in the laparoscopic compared to the open group. The most striking difference was 12%vs6% in the CLASICC trial, even though it did not reach statistical significance[6]. The two other trials were designed but failed to demonstrate non-inferiority based on a composite pathology index[9,10]. Together, these trials at least early on red-flagged the use of laparoscopic surgery for rectal cancer. On long-term follow up, however, these data were less of concern as they did not translate into higher local or systemic failure rates. A recent large database audit found significantly different but overall small rates of positive CRM; 3.2% robotic, 4.1% laparoscopic, and 5.4% open[50]. The authors concluded though that the differences were real but too small to promote one technique over the other. The major roboticvslaparoscopic studies have shown the robot to maintain a low rate of CRM positivity of 0-8%, which in no study was statistically inferior to laparoscopy. The ROLARR trial found CRM positivity in 5.1% (laparoscopic) and 6.3% (robotic)[15].
In the Bordeaux trial, TaTME compared to laparoscopic specimens were associated with a much lower rate of CRM positivity of 4%vs18% (P< 0.05)[17]. Even though the laparoscopic comparison group had an unacceptably high recurrence rate, the authors prematurely claimed TaTME to be the new standard going forward. Also of note was that all these patients had low cancers (< 6 cm from the verge) which is the ideal setting for the TaTME. Subsequent studies could not corroborate such robust results. In fact, the large Dutch study and the recent paper by Lee both showed near identical rates of positive CRM[18,19].
Intactness of specimen mesorectal envelope
The integrity of the TME specimens is visually graded. As with the CRM, several major laparoscopic versus open trials found the completeness of the TME to be higher in the open group but not to a significant level. TME completeness in the ALaCaRT trial was high but statistically not significantly different in both the laparoscopic and the open group (87%vs92%, respectively)[10]. The robotic and TaTME technique appeared to produce equal quality of the TME even though at a lower range. The ROLARR trial reported identical TME completeness of 75% in each group[15]. There were no differences in TME completeness in TaTMEvsthe other groups except in a Danish study where TaTME completeness was abnormally low at 58% compared to 68% in lap and open groups[33].
AXIAL RESECTION MARGIN
The final pathology variable is the axial and particularly the distal resection margin. This is a more nebulous variable as it is inconsistently reported as a numeric value of the absolute distance or as a binary value of positive versus negative margin. If the tumor is high enough,i.e., in the mid to upper rectum, a margin of 5 cm should be targeted. For the tumors in the distal rectum, a shorter distance has generally been acceptable. Ideally, it should be at least 1-2 cm, but on occasion a negative margin of any length has been acceptable in the lowest cancers. There are no studies that show a significant difference in distal margin positivity between laparoscopic, robotic, or open techniques. However, the most recent matched trial found a higher distal margin positivity in TaTME than robotic cases of 1.8%vs0.3% (P= 0.051), respectively[19]. This finding is at variance with the claim that TaTME is the modality most suited to attaining a negative distal margin because the surgeon starts the dissection with this margin in mind and view usually at least 1 cm distal to the tumor. Paradoxically, the margin positivity rate was higher, but overall, the TaTME specimens had a longer DRM of 16.9 mmvs15.1 mm. Two other smaller TaTME studies found no difference in DRM positivity but the TaTME DRM distance in both was larger (2.5 cm) as compared to the laparoscopic technique (1.5 cm)[42,51].
LONG TERM OUTCOMES: SURVIVAL AND RECURRENCE
All previously discussed parameters are surrogate to the ultimate oncological longterm outcomes. The standard metrics include local recurrence rates (LRR), disease-free survival (DFS), and overall survival (OS). Most of the landmark RCTs have even published long-term data.
Four major laparoscopic trials have robust data of at least 24-36 mo follow-up comparing laparoscopicvsopen rectal cancer surgery. Reported local recurrences rates were comparably low in the range of 2%-6%. This is especially meaningful in the Z6051 trial where the CRM positivity was by 6% higher in the laparoscopic arm. The reported 2-year local recurrence rates, DFS and OS were nearly identical at 4.6%vs4.5%, 83%vs85%, and 85%vs86%, respectively[11]. The other RCTs reported similar results.
Regarding robotic data, long-term data are still awaited. The ROLARR trial has not yet reported its long-term outcomes. Current information is limited to several single center, propensity score matched studies that reported on these outcomes. Robotic surgery was a positive prognostic factor for OS at 36 mo[52], and at 60 mo was associated with increased OS of 90%vs78% compared to the laparoscopic group[53]. Whether these results can be corroborated by large RCT remains to be seen. Large database studies at least were not able to reproduce such a difference[40,50].
As previously noted, the Bordeaux trial analyzed the respective 5-year TaTME outcomes. Even though there was a difference by 12% of CRM positivity in favor of TaTME, the local recurrence rates did not differ and were around 4% in both groups, and DFS and OS were comparable[54]. In contrast, the Norwegian trial had not only a substantially higher leak rate, but also an alarmingly high local recurrence rate of 11.6% at 9 months as opposed to the reported average of 2.4% for all approaches in their national databases[20]. None of the other studies replicated this high local recurrence rate[31,55]. At five years, TaTME in 159 patients in the Netherlands resulted in a 3.8% LRR, 81% DFS, and 77% OS[56].
In summary, laparoscopic and open technique appear to achieve comparable results, robotic surgery is likely in the same range, and the TaTME awaits further analysis. The focal areas of differences are likely a product of underpowered studies that should be overcome by better evidence to reach a final verdict.
LONG TERM OUTCOMES: BOWEL, BLADDER AND SEXUAL FUNCTION
Quality of life parameters including postoperative bowel, bladder and sexual function are a big issue and from patient perspective just as important as the oncologic outcome. This is true at any time point in a patient’s journey but increasingly moves into the center of attention as the patients achieve cure from the cancer. Unfortunately, this topic has not been studied in similar detail as other outcomes for several reasons: Physicians may not assess those parameters systematically, patients may not always want to share their experiences, and the validated instruments to study these parameters entail lengthy and cumbersome questionnaires. Finally, the functional outcomes are affected by a multitude of individual factors, both independent and dependent on the treatment as such (surgery, chemotherapy, radiation).
Bowel function after rectal cancer surgery is mainly a product of the following components: The level of the anastomosis, the absence of an anastomotic leak, the reservoir function, and the sphincter function.
The reservoir function for the very low anastomoses may in the short run be improved with a colonic J-pouch, but the benefit may wane in the long run and turn into a disadvantage with stool clustering. The post-treatment sphincter function is a function of the preexisting condition, technical preservation, and inadvertent injury (e.g., stretch injury during transanal or TaTME dilation).
Sexual and urinary function are affected by multi-factorial impairment to visible and microscopic neurological networks: Hypogastric nerves, nervi erigentes, pelvic plexus, and cavernous nerves[57]. It has been postulated that minimally invasive techniques, in particular the robotic approach, may better visualize and preserve the identifiable structures and cause less trauma.
Most of the trials to include function are small single center comparative studies. Fortunately, several of the large RCTs also included this component as part of the trial but provide limited information about the technical details of surgery. The COREAN and COLOR II laparoscopicvsopen trials employed the EORTC quality of life questionnaires three months after surgery. Although this was early, there were fewer problems with urination and defecation in the laparoscopic group[7]. In 2015, a followup to the COLOR II trial specifically reported on genitourinary function up to two years after surgery[58]. There was no difference in urinary or sexual function between open and laparoscopic surgery at any time point between four weeks and two years follow-up. Sexual function, which also contains a psychological component, suffered a greater impact than urinary function but both improved with time.
There is decent body of evidence on this field in the roboticvslaparoscopic literature. The ROLARR study and two smaller single-center RCTs included sexual and urinary function as secondary end points. In the ROLARR trial, patients selfreported their bladder and sexual function using well-established scoring systems at baseline and at six months. The validated instruments included the international prostate scoring systems (IPSS), international index of erectile function (IIEF), and the female sexual function index (FSFI). The specific minimally invasive approach did not make a difference[15]. A 2015 Chinese single center RCT with urinary and male sexual function as primary end points noted worse IPSS/IIEF scores in 66 laparoscopic compared to 71 robotic patients. On multivariate analysis, only the laparoscopic approach was predictive of worse sexual function[59]. In contrast, another single-center RCT based on EORTC instruments showed no difference in quality of life, bowel or bladder function at 12 mo; however, sexual function was better in the robotic cohort[16]. Finally in 2018, a propensity score study comparing 130 matched pairs reported at six and twelve months worse sexual and urinary function in the laparoscopic group as compared to the robotic group[60]. Even though these trials suggest that the robotic approach may offer a more gentle approach to better preserve sexual and urinary function, it is hard to corroborate in the absence of a difference in the largest trial (ROLARR).
Finally, comparative studies have also looked at the impact of TaTME versus the laparoscopic technique on sexual and urinary function. Proponents suggest that the initial approach that immediately encroaches on the nerves in question might help to prevent nerve injury. Critics, on the other hand, pointed out the significant stretch injury to the pelvic floor and sphincter complex. The current data remain sparse. Based on EORTC questionnaires, two small prospective single center trials in 2019 found no difference in urinary or sexual function at six months after TaTME and laparoscopic surgery; however, fecal incontinence scores were worse in the TaTME group[61]. Using numerous questionnaires, TaTME and laparoscopic surgery had no difference in low anterior resection syndrome score or IPSS scores; on individual questions, though, the laparoscopic group fared better on diarrhea, clustering, and urgency scores at eight months, whereas the TaTME group had improved urinary status[62]. These trials were small and only compiled 54 and 85 patients in total. Larger studies and systematic before and after assessment will be needed before the functional concerns about TaTME are either found to be unsubstantiated or corroborated.
TRANSANAL MINIMALLY INVASIVE SURGERY
Radical oncological resection with removal of the rectum and its corresponding mesorectal envelope including lymph nodes is the appropriate and default intervention of choice for rectal cancer. However, a local excision may be appropriate for precancerous lesions and plays a clarifying role for lesions of uncertain behavior and for an organ-preserving watch and wait approach after chemoradiation[63]. Local excision may be appropriate but remains controversial for very early presentations of invasive rectal cancer. T1 lesions comprise about 15% of the total rectal lesions[50]. The concerns with local excision relate to the significant incidence of local recurrences, related to retained positive lymph nodes (7%-13%) and possibly the direct implantation of cancer cells into the excision site.
There are different surgical platforms to perform a transanal local excision: (1) Conventional transanal excision under direct vision for relatively low lesions; (2) Transanal endoscopic microsurgery (TEMS)[64]; or (3) Transanal minimally invasive surgery (TAMIS). For the latter, there is the standard laparoscopic instrumentarium (LTAMIS), and more recently, a robotic approach (R-TAMIS) has be utilized. These techniques offer improved visualization, reach and allow for a finer and better controlled dissection in the limited rectal space, which likely explains the trend to overall lower recurrence rates of 4%-6%, but still up to 24%[65,66].
Due to the longer history, data on TEMS are more robust even though there is no RCT. In 2018, a series of 70 cases over a ten year period reported a 16% complication, a 14% positive margin rare, and a local recurrence rate of 8% at 60 mo[67]. A recent metaanalysis of six such studies included a total of 927 local excisions[68]. TEMS was superior to standard local excision with less specimen fragmentation, less positive margins, and a lower recurrence rate. Local recurrence rates vary and in reviews have been in the summarized in the range of (0-)4-6%, but still up to 24%[65,66].
The TAMIS approach was originally described in 2010[69], subsequently grew in popularity, and with a 6% complication rate is considered a safe and effective option. The research remains in evolution; there are no RCTs to compare TEMS to TAMIS. The rate of positive margins is around 6%, and the local recurrence 4% at 18 mo[70]. Comparison of 53 TEMS and 68 TAMIS patients showed some differences in operative times but no difference in positive margins or fragmentation[71]. A larger study from 2017 comparing 247 TEMS with 181 TAMIS showed identical recurrence rates of 7% and similar five-year disease-free survival of 80%vs78%, respectively[72].
More recently, the robot has been used to perform a TAMIS robotically (R-TAMIS): The docked ports are placed through a TAMIS port to accomplish a local excision from the console. R-TAMIS in 58 patients achieved the excisions with a 1.7% fragmentation, 5.2% positive margin and a 5.5% local recurrence rate[73]. A recent paper compared LTAMIS and R-TAMIS in 40 patients which found no difference in outcome except an $880 increase in cost with the robot[74].
Regardless of the platform, local excision for proven invasive cancer carries a substantial risk of local recurrence. Addition of multimodality treatment for these stage I cancers (that by means of an oncological resection have a greater than 90% cure rate) is not justified for the sole purpose to avoid a proper operation. Local excisions should be limited to highly select circumstances where the downsides are outweighed by the expected benefits.
SYNOPSIS AND FUTURE DIRECTIONS
As we enter the fourth decade of minimally invasive surgery, its popularity for rectal cancer surgery continues to grow. Of the four surgical modalities to perform an oncological resection, there is no convincing evidence to rate one superior to any other as long as the set goals are met. More impact on outcomes than the choice of approach appears to have (1) whether or not to perform an oncological resection versus a local excision by any means; and (2) the selection, tailoring, and timing of non-surgical treatment modalities.
Unquestionably, the rates of minimally invasive surgery continue to increase worldwide each year despite initial skepticism and concerns about noninferiority and cost. The exact path and role each modality will take remains to be seen. The early driving force and support most prominently come from both patients’ and surgeons’ perceptions that patients tend to experience a smoother short- and long-term recovery while achieving comparable oncological outcomes. Unfortunately, this biased impression remains a major obstacle to systematic randomized research. Maintaining support in the longer run therefore mostly builds on at least equal if not superior outcomes, and the force of routine and familiarity of the surgical community that individually and collectively move past the learning curves. Except for the Norwegian moratorium on TaTME, there has been no large study that has shown significant inferiority of the minimally invasive approach to open surgery.
The main factors that will drive the future of rectal cancer surgery are selection, outcomes, access, technology, and standardization. It is highly unlikely that there will be a trial to compare all different modalities in a direct fashion. Further trials will continue to give us a patchy picture with select comparisons. For example in regard to defining the role of TaTME, there are currently two large RCTs underway comparing it to a laparoscopic TME. The first is the European COLOR III trial with the target enrollment of 1098 patients and the primary outcome the positive CRM[75,76]. The second is the smaller French GRECCAR 11 trial with similar setup[77].
The major factor defining the future of minimally invasive surgery for rectal cancer is access and costs. With each new modality, new instruments, tools and infrastructure are developed with respective associated costs. This was the case with the introduction of laparoscopic surgery and has equally been a major target of criticism in rolling out robotic surgery. The current robotic platform costs approximately $2.5 million in capital investment along with many add-ons, service contracts, and disposable instruments which average $80000-170000 annually[78]. Almost every early cost study pertaining to robotic surgery has shown significantly increased costs (1.5-2.4 times) when compared to laparoscopic surgery[79]. With limited healthcare resources, robotic surgery is at risk if costs continue to remain high unless there is a measurable benefit beyond the operating room. In particular, the push to perform an anastomosis intracorporeally has been facilitated by the robotic platform and appears to be associated with an accelerated recovery.
Minimally invasive surgery is not equally available to the entire population with a significant urban-rural and potentially socio-economic difference. A recent study has shown a significant proportion of patients who received robotic rectal cancer surgery were white, male, privately insured, and in a metropolitan area[80].
Probably the most crucial factor affecting the future of minimally invasive surgery is the technology itself. Currently, there is a dominance of the DaVinci robot systems who initiated the revolution and carry an advantage of almost two decades over their competitors. Nonetheless, competition is forming and expected to advance the technology and to stimulate a reduction in price. And as new systems come online, there likely will be numerous comparative studies. Areas of desired improvements include haptic feedback, better and multilevel internal articulation, less external arm movements and collisions, as well as force reduction across the abdominal wall, decrease of instrument diameters, increased versatility through multiple quadrant surgeries, and 3D vision for all team members. New optics and robotic-endoscopic instrumentation will be crucial to advance the field of endoluminal surgical interventions (ELSI). Transanal work for local excision or TaTME will likely benefit from the newly introduced da Vinci SP (single port) robot. And finally, artificial intelligence with advance planning capabilities, fusion of imaging with the surgical field, and highlighting of key structures (ureters, lymph nodes, blood vessels) are exciting features that will contribute to increased safety of complex procedures.
CONCLUSION
The role of minimally invasive surgery for rectal cancer is constantly changing and advancing. There are currently four main modalities for minimally invasive rectal cancer surgery. Laparoscopic and robotic approaches do require a longer operative time. Conversion data is conflicted but there does appear to be a trend toward reduced times with robotic surgery. There are no differences in morbidity and mortality between any group. In addition, all short- and long-term oncologic outcomes appear to be similar. Urinary and sexual function also appear to have better recovery with robotic surgery. Finally, there are three minimally invasive approaches to local excision. They all are comparable to one another and the conventional local excision at this point. In conclusion, minimally invasive surgery for rectal cancer has evolved to an accepted and well researched practice over the last thirty years. However, there are still many questions to be answered about superiority of any modality over another. In addition, future technologies are likely to challenge the current platforms already in place.
 World Journal of Gastroenterology2020年30期
World Journal of Gastroenterology2020年30期
- World Journal of Gastroenterology的其它文章
- Comment on pediatric living donor liver transplantation decade progress in Shanghai: Characteristics and risks factors of mortality
- Risk prediction platform for pancreatic fistula after pancreatoduodenectomy using artificial intelligence
- Predictive model for acute abdominal pain after transarterial chemoembolization for liver cancer
- Vedolizumab for ulcerative colitis: Real world outcomes from a multicenter observational cohort of Australia and Oxford
- Accelerating the elimination of hepatitis C in Kuwait: An expert opinion
- Differential diagnosis of diarrhoea in patients with neuroendocrine tumours: A systematic review
