Conversion of Adipose-Derived Stem Cells into Sweat Gland-Like Cells: An In Vitro Phenotypic Study
Shuai Qiang, Xiao-Meng Wang, Cheng-Kun Zhang, Ying Liu, Zhi-Bo Xiao,∗, Qiang Li,Feng-Yong Li, Yu Zhou
1Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100144, China,2Department of Plastic and Aesthetic, The Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang 150086,China.
Abstract
Keywords: adipose-derived stem cells, human sweat gland-like cells, differentiation
Introduction
The sweat gland is an important accessory structure of the skin and has essential roles in dermatological metabolism and regulation of body temperature. Sweat glands can lower the body temperature by producing a film of water on the surface of the skin,allowing for evaporative cooling when the environmental temperature increases;this is one of the few methods of heat dissipation in humans.1In patients with deep burns,the injury may reach the muscle tissues and damage the sweat glands. Large areas of burned skin are restored by formation of a hypertrophic scar without regeneration of the sweat glands,resulting in loss of perspiration function.2In most cases of severe,extensive burns, the stem cells or progenitor cells within the injured sweat gland cannot regenerate.3-4
Research is showing that Adipose-derived stem cells(ADSCs)may provide new avenues for the repair of largearea burns and trauma. ADSCs are being given increasingly more attention in the fields of tissue engineering,regenerative medicine,and developmental biology because ADSCs have the ability to stably proliferate and differentiate into various cell types.5Our previous studies have suggested that ADSCs are a feasible source for skin regeneration. In this study, ADSCs were purified and induced by EGF to explore the possibility of inducing ADSCs to differentiate into hSG-like cells in vitro.
Materials and methods
Subjects
The normal human skin samples were obtained from patients undergoing rhytidectomy in Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.The study was approvaled by Institutional Review Board of Plastic Surgery Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College and informed consents were all signed.
Isolation, culture, and identification of ADSCs
IsolationoftheADSCswasperformedasfollows.Thefatwas washed with phosphate-buffered saline to remove the red blood cells. Visible vessels were excised with microsurgical scissors and cut into pieces.The fat was then enzymatically dissociated for 40minutes at 37°C using 0.2% collagenase type I.The partially digested tissue was neutralized by adding growth medium composed of low-glucose Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum(FBS)and centrifuged at 360g for 10minutes.After removal of the supernatant and resuspension in phosphate-buffered saline, the samples were filtered using a 200-mesh filter and centrifuged at 110g for 5minutes to harvest the sediment. The sediment was evenly mixed with low-glucose DMEM medium containing 10% FBS and cultured in a 37°C incubator with 5%carbon dioxide.After 24hours,the medium was changed for the first time,and the liquor was thereafter changed once every 3 days. After reaching >90% confluency, the cells were passaged using 0.25% trypsin containing 0.1% ethylenediaminetetraacetic acid solution and then passaged at a ratio of 1:3.ADSCs were nourished in low-glucose DMEM supplemented with 10%FBS and antibiotics (penicillin and streptomycin). At the exponential phase of growth,the cell number was adjusted to 1×105/mL after trypsinization. The cells were then seeded onto six-well plates(2-mL cell suspension in each well)and cultured as described by Liu et al.6
Identification of the ADSCs was performed as follows.We observed the morphology of the cells with an inverted microscope and used a digital camera to take photographs.When the cells had reached 80%–90% confluency, we separately added adipogenic induction medium(10%FBS,DMEM,0.5mmol/L 3-isobutyl-1-methylxanthine[IBMX],1μmol/L dexamethasone, 10μmol/L insulin, and 200 μmol/L indomethacin) and osteogenic induction medium(10%FBS,DMEM,0.1μmol/L dexamethasone,50μmol/L vitamin C,and 10mmol/L β-glycerophosphate).
To test the cells’ability of multidirectional differentiation,we performed Oil Red O staining after 12 days of culture and Alizarin Red S staining after 3 weeks of culture.
Isolation, culture, and identification of hSG-like cells
The skin samples were stored in Hank’s Balanced Salt Solution at 4°C.First,the subcutaneous fat was removed and the skin was chopped into 1-mm3pieces using sharp scissors. The skin pieces were then treated with dispase for 18hours to separate the epidermis and dermis,after which the dermis was treated with collagenase type IV for 1hour at 37°C. Finally, the sweat glands were dissociated, incubated, and cultured as described by Hongpaisan and Roomans.7hSG-like cells were identified by their morphologic characteristics,and biomarkers were detected by flow cytometry.
ADSCs treated with epidermal growth factor or coculture with hSG cells
A basal medium was prepared with DMEM supplemented with 100U/mL penicillin,100μg/mL streptomycin,insulintransferrin-sodium selenite solution (1mL/100mL),2nmol/L triiodothyronine,0.4mg/mL hydrocortisone hemisuccinate, and 10% FBS with the pH adjusted to 7.0.Primary ADSC were obtained by enzymatic digestion and cultured in L-DMEM supplemented with 10%FBS at 37°C with 5%carbon dioxide.The cells were passaged by 0.2%trypsinization, and devided into EGF 20ng/mL, EGF 200ng/mL,400ng/mL,hSG co-culture,and Control groups.
After 24hours culture,cytokeratin 7(CK7),CK14,and CK18 were then measured every day for 1 week. The ADSCs (1×106) were preplaced in the lower chambers and inserted into the Transwell device after the ADSCs had attached to the well;hSG cells(1×106)were then added to the upper chamber. The Transwell device prevents direct contact between the ADSCs and hSG cells but allows soluble cytokines produced by these cells to interact with each other, sharing the culture medium. The ADSCs and hSG cells were separately seeded onto glass slides in culture dishes, and the cultures were maintained under the same conditions as above described for 7 days.The cell cultures were then washed and fixed for immunohistochemistry.
Immunohistochemistry
Firstly,1×106cells wasadded in pertube,with PBS 1.0mL,251.5g for 5minutes centrifuged,and the supernatant was discarded. And then 250μL BD Cytofix Fixation buffer(554655)per tube was added,the tube was placed at 4°C for 30minutes and centrifugated at 251.5g for 5minutes,and supernatant was discarded.Secondly,the tube was add with BD Phosflow Perm/Wash Buffer type I 1.0mL centrifuged at 251.5g for 5 minutes, and supernatant was discarded.Thirdly, 100μL buffer type I was added to each tube,corresponding fluorescent antibodies were added tube by tube,incubated at 4 °C for 15minutes.Finally the surface markers were detected by flow cytometry.
Results
Identification of ADSCs
After 24hours inoculation, a little of large cell begins adherence were observed,and 48hours later,about 3.6×105of adherent cells were obtained,with a fibroblast-like morphology mostly.Round and polygonal cells were also found, and the number decreased with passage. After 2 weeks of induction with line age-specific media,the cells were positive for oil Red O staining,and after 3 weeks,the cells were positive for Alizarin Red S staining.The cultured cells were positive for the markers CD73, CD90, and CD105 and negative for CD44 (Fig. 1).

Figure 1. Isolation and characterization of ADSCs. (A) After inoculation for 48hours, most of the cells display a fibroblast-like morphology(×200).(B)After 2 weeks of induction with lineage-specific media,the cells are positive for oil Red O staining(×200).(C)After 3 weeks of culture,the cells are positive for Alizarin Red S staining(×200).(D)Representative flow cytometry histogram shows that ADSCs are positive for CD73,CD90, and CD105 and negative for CD44.
hSG cells
After digestion, the sweat glands were dissociated. When the cells had been attached for 3 to 4 days, the adherent sweat gland tissue tended to show hSG cells.After 14 days,examination under an inverted microscope showed that the cultivated cells were polygonal and displayed a typical cobblestone-like morphology after fusion to the monolayer with contact inhibition (Fig. 2).
Differentiation of ADSCs into hSG-like cells in vitro
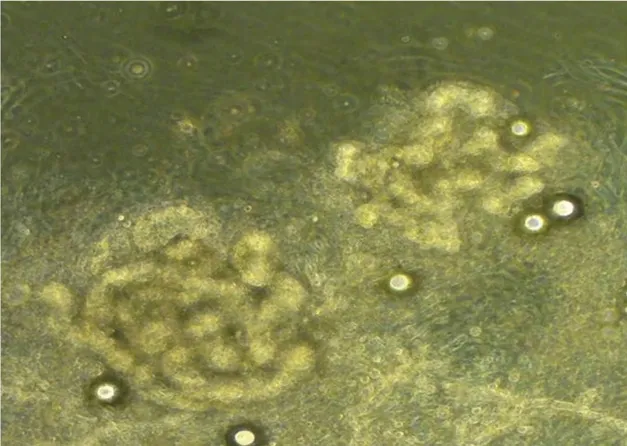
Figure 2. Morphology of cultivated sweat glands cells.After 14 days,the cultivated sweat glands cells were polygonal and displayed a typical cobblestone-like morphology after fusion to the monolayer with contact inhibition (×200).
During the differentiation process, the ADSCs exhibited changes in their morphology from mesenchymal-like cells to epithelioid cells.We used immunofluorescent staining to confirm the similarities between the ADSCs and hSG cells.Sweat gland cells cultured in vitro expressed CK7,CK14,CK18 normally. And the ADSCs of the control group showed weak expression of CK7, CK14, CK18. But ADSCs-hSG cultured highly expressed CK7,CK14,CK18,after induced by different concentrations of EGF, and its expression ability gradually improved with the increase of EGF concentration (40ng, 200ng, 400ng) (Fig. 3).
Discussion
In this study, we differentiated ADSCs into hSG-like cells and cultured the ADSCs with EGF or cocultured them with hSG cells.The ADSCs–hSG cells were biologically similar to the hSG cells, because they both expressed the hSGrelated markers CK7, CK14, and CK18.
ADSCs can be easily obtained from various sources with minimal pain without ethical issues for their use, and exhibit rapid proliferation. These characteristics make ADSCs ideal sources of stem cells for bioengineering research. ADSCs can be harvested from human adipose tissue, and the cultured cells in vitro exhibit a fibroblast morphology with characteristic features of a stable multiplication rate and apoptosis rate. In recent years,ADSCs have demonstrated potential advantages in tissue engineering. In one study, BMSCs and ADSCs from the same patient showed no significant differences in the yield of adherent stromal cells,cell senescence,growth kinetics,or multilineage differentiation capacity.8However, other studies have indicated that some characteristics of ADSCs are superior to those of BMSCs, such as the ability to maintain proliferation and colony frequency in culture.9-10
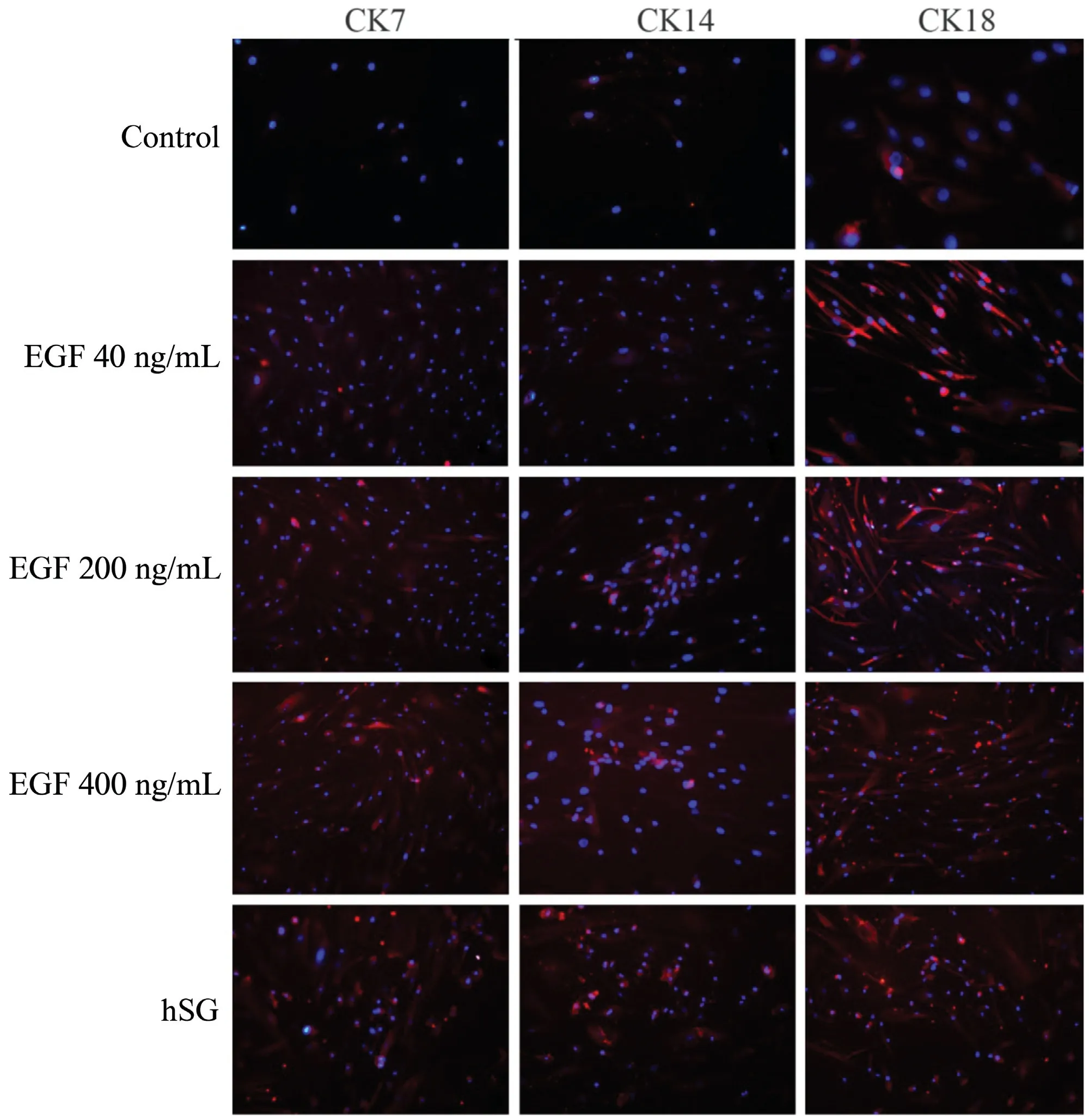
Figure 3. Biomarkers of cultured ADSCs(magnification,×200).hSG cells cultured in vitro express CK7,CK14,and CK18 normally,and the ADSCs of the control group show weak expression of CK7,CK14,CK18.But ADSCs-hSG highly expresses CK7,CK14,CK18,after induced by different concentrations of EGF,and its expression ability gradually improved with the increase of EGF concentration(40ng,200ng,400ng).
Numerous studies have implied that ADSCs are multipotent, i.e., have the potential to differentiate into fat,bone,cartilage,tendon,skeletal muscle,endothelium,and macrophages when cultivated under lineage-specific conditions.5,9,11–14This has led to the exploration of ADSCs as a promising new treatment for tissue regeneration.Although clinical trials and treatments using ADSCs have provided encouraging results,15more detailed and confirmatory studies are necessary, and conclusions from these preliminary data must be drawn very cautiously before speculating on the future clinical implications.
In patients with deep burns, the injury can damage the sweat glands, resulting in sweat gland dysfunction and a decline in quality of life. Stem cell therapy has been developed as a new tool with which to improve the therapeutic effect of burn wounds and may not only repair the destroyed skin structure and injured sweat glands but also reestablish the perspiration function. A recent study by Liang et al.16showed that human amniotic fluid stem cells can differentiate into hSG-like cells in vitro. Their study further indicated that human amniotic fluid stem cells can express the hSG-related genes encoding ectodysplasin-A,ectodysplasin-A receptor,and keratin 8 and that EGF enhances the efficiency of differentiation; these outcomes are identical to ours. Zhao et al.17introduced the nuclear factor kappa B gene (NF-kB) and lymphoid enhancer-binding factor 1 gene(LEF1)into fibroblasts and found that the transfected fibroblasts expressed specific markers of sweat glands,including CK7,CK14(as in our study), and CK19. Moreover, during regeneration, the authors noted increased expression of SHH (which encodes sonic hedgehog) and CCND1 (which encodes cyclin D1),both of which are genes downstream of NF-kB and LEF1 gene. The authors also carried out animal experiments and corresponding results were obtained.
The mechanism of sweat gland regeneration with mesenchymal stem cells is still under investigation.However,there are many indications that various cytokines and signaling pathways are involved in the regeneration and development of sweat glands, such as the extracellular signal-regulated kinase(ERK)signaling pathway,ectodysplasin-A1/ectodysplasin-A1 receptor signaling pathway,NF-kB signaling pathway, and Wnt/β-catenin signaling pathway. Complicated crosstalk exists among different signaling pathways. EGF and some other important regulatory cytokines can active certain extracellular signal-regulated kinase signaling pathway proteins that are also important members of the mitogen-activated protein kinase family, which controls many cellular and physiological processes(i.e.,cell growth,development,and apoptosis). Once EGF activates Ras, a cascade reaction occurs.The expression of EGF increases with the formation of sweat gland buds during embryonic development.18Blecher et al.19suggested that EGF can induce the development of dermal ridges and functional sweat glands in Ta/Y hemizygotes,indicating that EGF is involved in the morphogenesis of skin and sweat glands.
Encouragingly, our study proved that the plasticity of ADSCs may facilitate their conversion into hSG cells.Although our results are promising, several questions remain unanswered.The present report describes only the preliminary results of our study and does not directly prove that ADSCs can be grafted and secrete sweat in vivo.Because the findings were obtained only in vitro, the limitations of our data cannot be ignored. The in vitro environment may affect cell behavior; thus, in vivo evaluation of the original specimens will strengthen the findings.Despite the limitations of this study,our findings still have important significance in current research.To the best of our knowledge,this is the first report to describe the relationship between ADSCs and sweat gland regeneration.
There were a few limitations in this initial study.Although we preliminarily demonstrated ADSCs possessed the possibilities to convert into sweat gland-like cells in vitro,the most appropriate concentration of EGF is still unclear. Altered phenotype of ADSCs was observed,however,cell morphological changes cannot be confirmed.Further studies on above aims are required.
Overall, our results revealed that ADSCs possess the ability to differentiate into hSG-like cells in vitro and that their phenotype changes accordingly.These initial findings are promising for the study of sweat gland regeneration.Although the gap between in vitro research and clinical application is still wide, this study provides valuable information for further developments in this field.
Source of funding
This study was supported by the National Natural Science Fund of China (Nos. 81271711 and 81471796).
- 国际皮肤性病学杂志的其它文章
- Chinese Expert Consensus on the Diagnosis and Management of Food Allergy in Children With Atopic Dermatitis#
- Chinese Expert Consensus on Clinical Application of Patch Test (Revised 2020)#
- SOX4 Mediates BRAF Inhibitor Resistance in Melanoma through Regulation of IGF-1R Signaling: In Vitro Study
- A Case Report of Pterygium Inversum Unguis Associated with Systemic Lupus Erythematosus
- Instructions for Authors
- A Novel Small Deletion in the ATP2A2 Gene in a Patient with Sporadic Darier’s Disease and Concomitant Depression: A Case Report

