Chinese Expert Proposal on the Diagnosis and Management of Pemphigus Vulgaris (2020)#
Dermatology Branch of China, International Exchange and Promotion Association for Medical and Healthcare; Ya-Gang Zuo, Li Li, Jin-Bo Chen, Liu-Qing Chen, Su-Ying Feng, Wei Li,Xiao-Qun Luo, Meng Pan, Gang Wang, Ting Xiao, Bao-Qi Yang, Kang Zeng0,Gui-Ying Zhang, Hong-Zhong Jin,∗
1Department of Dermatology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China; 2Department of Dermatology, Wuhan No. 1 Hospital, Wuhan, Hubei 430022, China;3Department of Dermatology, Hospital for Skin Diseases (Institute of Dermatology), Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, Jiangsu 210042, China; 4Department of Dermatology, West China Hospital of Sichuan University, Chengdu, Sichuan 610041, China; 5Department of Dermatology, Huashan Hospital Affiliated to Fudan University,Shanghai 200040, China; 6Department of Dermatology, Ruijin Hospital Affiliated to Shanghai Jiaotong University Medical College,Shanghai 310000, China; 7Department of Dermatology, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi 710032,China; 8Department of Dermatology, The First Hospital of China Medical University, Shenyang, Liaoning 110001, China;9Department of Dermatology, Shandong Provincial Dermatology Hospital, Jinan, Shandong 250022, China; 10Department of Dermatology, Southern Hospital of Southern Medical University, Guangzhou, Guangdong 510515, China; 11Department of Dermatology, Xiangya Second Hospital of Central South University, Changsha, Hunan 410011, China.
Abstract Pemphigus vulgaris(PV)is a life-threatening autoimmune bullous disease that causes blisters and erosions on the skin and mucous membranes to standardize the diagnosis and treatment of PV,Chinese experts in this field were invited to make recommendations which are presented in this article.Pemphigus vulgaris can be divided into cutaneous mucous type, cutaneous type and mucous mucous dominant type according to the different clinical manifestations. The pathological manifestations of PV are acantholysis above the basal layer.The diagnosis of PV and the assessment of the severity of the disease are made in accordance with the clinical manifestations, histopathological features,immunofluorescence assay results, and detection of pathogenic serum antibodies. The first-line treatment of PV comprises systemic glucocorticoids.Early combination with immunosuppressive agents or rituximab is recommended for moderate and severe PV. Intravenous immunoglobulin administration is recommended for patients being treated with rituximab. Plasma exchange and stem cell transplantation can be performed if necessary. During the course of therapy,the disease activity should be closely monitored,and actions should be taken to prevent adverse reactions.
Keywords: pemphigus, proposal, diagnosis, management#The Chinese version of the Proposal has been published on the Chinese Journal of Dermatology, 2020, 53(1):1-7.
Introduction
Pemphigus vulgaris(PV)is the most common and severe type of pemphigus, and the primary purpose of the therapeutic management is to control the disease,reduce recurrence, and minimize the glucocorticoid dosage and adverse reactions. To standardize the diagnosis and management of PV,the“Expert Recommendations for the Diagnosis and Management of Pemphigus Vulgaris”were formulated in 2016.1With the constant deepening of the understanding of PV and the continuous development of treatment methods,these original expert recommendations need revising. Based on the original guidelines, this 2020 version was developed by reviewing the newly published guidelines, consensus, or diagnosis and treatment recommendations from different countries and regions,combined with knowledge of the situation in China and deep communication among Chinese experts.
The evidence levels used in this article were based on the internationally recognized quantitative systematic evaluation evidence grading tool: the Grading of Recommendations, Assessment, Development, and Evaluation. The recommendations come from the opinions of experts involved in the discussion.
Epidemiology and pathogenesis
PV is the most common pemphigus subtype in Japan and the United States.2-3The incidence of pemphigus in the Mediterranean region is two to three times higher than that in other countries,and ranges from 0.76 new cases per million per year in Finland to 16.1 cases per million per year in Jerusalem.4PV is also the most common type of pemphigus in China.5
Currently, it is believed that the pathogenesis of pemphigus is the destruction of the desmosomes between epidermal cells caused by anti-desmoglein (Dsg) antibodies, which leads to blisters and bullae formation clinically.The target antigens are Dsg 3 and Dsg 1,which are cell-cell adhesion molecules found in desmosomes.4
Clinical findings
The first symptom in most patients with PV is oral ulceration. This ulceration most frequently affects the buccal mucosa and pharynx, which significantly affects eating. The next most commonly affected sites are the genital mucosa and eye conjunctiva, characterized by persistent, painful erosions or ulcers. The typical skin lesions are flaccid blisters or bullae that mainly develop on healthy skin. The blisters are easily broken, forming an expanding erosion surface with painful ulceration without pruritus in most patients.The Nikolsky sign is positive.The predilection sites of these lesions are the chest, back, face,head,axillae,groin,and buttocks.1,4In accordance with the clinical manifestations, PV is divided into three types:mucosal dominant, mucocutaneous,and cutaneous dominant.Patients with the mucocutaneous type have extensive skin lesions and mucosal damage,with a poor prognosis.6Patients with the mucosal dominant type have severe mucosal lesions, but the skin lesions are usually mild and localized, or even absent. The cutaneous dominant type mainly manifests as skin damage without mucosal damage,although some patients develop mucosal damage later.1,7
Diagnostic investigations
Histopathology
It is recommended to choose a newly erupted (within 24 hours) small blister as the sampling site. The histopathological features of PV are intraepidermal blister formation with acantholysis above the basal layer and loss of intercellular attachments in the epidermis.7
Direct immunofluorescence
It is recommended to choose normal-appearing perilesional skin or mucosa (located up to 1cm away from a fresh lesion)as the sampling site.PV is characterized by the deposition of IgG and/or complement C3 at the surface of epidermal keratinocytes or the stratified squamous epithelial cells of mucosal epithelium.7
Indirect immunofluorescence
Indirect immunofluorescence(IIF)shows green fluorescence in agrid-likeorfishnet-likedepositionpattern,whichiscausedby circulating intercellular antibodies in the serum binding to the epidermis of monkey esophagus or human skin.7
ELISA
ELISA is used to detect anti-Dsg 3 and/or 1 antibodies.The positive rate of IgG autoantibodies of PV patients by ELISA is more than 90%.7In general, the ELISA index correlates with the activity of the disease.
Immunoblot
Dsg3 (130kDa) protein is contained in patients with mucosal dominant PV,Dsg1(160kDa)protein and Dsg3 protein in patients with mucocutaneous PV, and Dsg1 protein in patients with cutaneous dominant PV.1
Diagnostic criteria
PV is diagnosed in patients with at least one of the following clinical manifestations plus at least one of the histopathological and immunological indicators,and also in those with at least two of the clinical manifestations plus two of the immunological indicators.3
Clinical manifestations
(1) Flaccid blisters and bullae on the skin that are easily broken.(2)Refractory erosions that occur after the blisters and bullae rupture. (3) Blisters or erosions in the visible mucosal area. (4) Positive Nikolsky sign.
Histopathology
Acantholysis occurs between epidermal cells or epithelial cells, resulting in blisters and bullae.
Immunological indicators
(1) Direct immunofluorescence on normal-appearing perilesional skin or mucosa shows IgG and/or complement C3 deposition at the surface of epidermal keratinocytes or the stratified squamous epithelial cells of mucosal epithelium. (2) IIF shows anti-epidermal intercellular antibodies in the serum. (3) ELISA shows anti-Dsg antibodies in the serum.4
Assessment indicators of disease severity
Clinical indicators
Several evaluation systems are currently used to assess the severity of PV. The most commonly used evaluation system is the pemphigus disease area index(PDAI),which is the internationally recognized method of pemphigus evaluation.8The PDAI is used to classify PV as mild(0–8),moderate(9–24),and severe(more than 25).The PDAI is a reliable tool for evaluating the severity of PV,but its use is relatively complicated.
Chinese scholars have proposed a PV grading method based on the affected percentage of the body surface area(BSA) as mild (<10% BSA), moderate (10%–50% BSA),and severe(>50%BSA).9However,these standards have not been assessed for reliability and validity.
The Japanese pemphigus disease severity score is simple,practical, and convenient for rapid assessment.10
Laboratory indicators
The level of anti-Dsg antibodies in the serum partially reflects the severity and activity of the disease. Although the anti-Dsg antibody levels decrease after symptom resolution in most patients, more than 50% of patients remain positive for anti-Dsg3 and anti-Dsg1 antibodies.11However, the anti-Dsg antibody level is used as a disease evaluation indicator for patients at different stages of treatment. An increase in the antibody level during treatment may indicate that the condition is likely to recur or worsen, and so close follow-up is required. In addition, persistently high levels of anti-Dsg1 antibodies suggest a greater likelihood of skin lesion recurrence, but sustained high levels of anti-Dsg3 antibodies do not predict recurrence of mucosal damage.6-7We suggest that antibody levels should be assessed at the beginning of treatment, every 3 months during the stable period, and when relapse occurs. If the condition is stable for more than 1 year and the dose of glucocorticoids is maintained below 10mg/d of prednisone, the interval between antibody testing can be extended to 6 months. If ELISA cannot be performed, IIF can be used instead.7,12
Timepoints of disease assessment and definitions of treatment response
The definitions of various stages of treatment developed by the International Pemphigus Commission in 2008 are still widely accepted and applied.8
Early timepoints
Baseline
The day that therapy is started by a physician.
Control of the condition
The time at which new lesions cease to form and established lesions begin to heal.This is also the beginning of consolidation treatment.
Endpoint of consolidation treatment
The time at which no new lesions have developed for a minimum of 2 weeks and the majority (approximately 80%) of established lesions have healed. Most clinicians begin to taper the glucocorticoid dosage at this timepoint.
Late observation timepoints
Complete remission off therapy
The absence of new skin lesions and/or established lesions while the patient has been off all systemic therapy for at least 2 months.
Complete remission on treatment
No new skin lesions and/or established lesions while the patient is receiving minimal therapy.
Partial remission off therapy
The presence of transient new lesions that heal within 1 week without treatment while the patient is off all systemic therapy for at least 2 months.
Partial remission on minimal therapy
The presence of transient new lesions that heal within 1 week while the patient is receiving minimal therapy,including topical steroids.
Minimum therapy
Prednisone ≤10mg/d and/or minimum immunosuppressant treatment for at least 2 months.
Relapse
The appearance of three or more new skin lesions per month that do not heal spontaneously within 1 week, or the extension of established skin lesions.
Fluctuations
The appearance of fewer than three new skin lesions per month that do not heal spontaneously within 1 week.
Treatment failure
The failure to control disease activity with full doses of systemic glucocorticoids for 3 weeks with or without the administration of any of the following agents for 12 weeks:cyclophosphamide (CTX) 2mg/(kg·d), azathioprine 2.5 mg/(kg·d), methotrexate (MTX) 20mg/week, or mycophenolate mofetil (MMF) 3g/d.
Treatment
PV treatment comprises initial and maintenance treatment phases.Detailed examinations should be performed before and after treatment(Table 1).The diagnosis of PV should be made as early as possible and the importance of initial treatment should be recognized. Most patients with PV require treatment for 3 years or more.13The PV treatment flowchart is shown in Figure 1.
Glucocorticoids (level of evidence, high;recommendation level, A)
Systemic glucocorticoids are the first-line treatment option for PV.After the introduction of glucocorticoid therapy in the early 1950s, the 1-year mortality rate of pemphigus dropped from 75% to less than 30%.4

Table 1 Recommended pre- and post-treatment examination items for patients with pemphigus vulgaris.
Initial treatment
The initial prednisone dose is 0.5mg/(kg·d) for mild PV(PDAI 0–8) and 1.0mg/(kg·d) for moderate PV (PDAI 9–24). If the condition is not controlled within 1 week, the dose of glucocorticoids is increased to 1.5mg/(kg·d). For severe PV, an initial prednisone dose of 1.5mg/(kg·d) is administered.12The dose of prednisone is not further increased, except for pulse therapy, and immunosuppressive agents should be administered at the same time. For patients treated with prednisone combined with rituximab,the recommended doses of prednisone are 0.5mg/(kg·d)for moderate PV and 1.0mg/(kg·d)for severe PV.14
Consolidation and maintenance therapy
Once remission is induced and the disease is controlled,the prednisone dose is tapered. The strategies for prednisone tapering differ between countries.It is recommended that the initial oral prednisone dose is administered for 2 weeks and then gradually tapered. The prednisone dose should be tapered by 10% biweekly at 60–90mg/d, followed by tapering of 5mg per week at 40–60mg/d,and then tapering of 5mg per month at 20–40mg/d.When the prednisone dose is tapered to 20mg/d, tapering of 2.5mg every 3 months is recommended. A maintenance prednisone dose of 0.2mg/(kg·d)or 10mg/d should be administered long-term.Only a few patients can be maintained at a prednisone dose lowerthan0.2mg/(kg·d)or10mg/d.Theprednisonetapering periodmustbelengthenedorshortenedinaccordancewiththe individual situation. The level of anti-Dsg antibody must be checked regularly. If the antibody titer rises, the prednisone tapering should be done more slowly or stopped. Most patients require prednisone treatment for 3 years or more.4,13Wang et al. recommend a tapering schedule in which the glucocorticoid dose is reduced by 50%per year.15
Methylprednisolone pulse therapy
Methylprednisolone pulse therapy can be considered in patients with treatment failure of the recommended dose of prednisone combined with immunosuppressive agents.Pulse therapy comprises the administration of intravenous methylprednisolone at a dosage of 500 or 1,000mg/d for 3 days, and then a return to the prior treatment regimen.6For patients with no response,the pulse therapy is repeated once every 2 to 3 weeks. Remission is generally achieved after two cycles of pulse therapy.Immunosuppressants do not need to be discontinued during pulse therapy.If relapse occurs after lesion improvement achieved by pulse therapy, the pulse therapy is repeated.6
Immunosuppressants
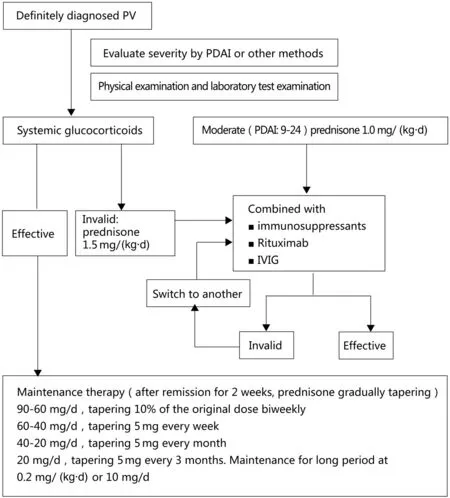
Figure 1. Flowchart of pemphigus vulgaris (PV) treatment. IVIG: intravenous immunoglobulins, PDAI: pemphigus disease area index.
Immunosuppressants should be administered early as glucocorticoid-sparing agents for patients with moderate to severe PV, especially those with diabetes mellitus,hypertension,and osteoporosis.Immunosuppressant therapy should be combined with prednisone in these patients.The combined administration of immunosuppressants can shorten the time required for prednisone tapering, reduce the amount of prednisone required,and prevent recurrence during prednisone tapering. Adverse effects of immunosuppressants include increased risks of tumors, infection,hematopoietic dysfunction,liver and kidney damage,and urogenital complications.
MMF (level of evidence, high; recommendation level, B)
The optimal dose of MMF is weight dependent, with a recommended dose of 2g/d for patients weighing less than 75kg,and 3g/d for patients weighing more than 75kg.To reduce gastrointestinal adverse events, an initial MMF dose of 500mg/d is proposed, with a progressive dose increase by 500mg per week until the final dose.12It is recommended to administer MMF in combination with prednisone.16MMF has fewer adverse effects and better tolerance than azathioprine.3However, MMF is contraindicated in patients with active gastrointestinal ulcers,and patients must be monitored for pulmonary infection during treatment.
MTX(level of evidence,medium;recommended level,B)
Few controlled clinical trials have assessed the efficacy of MTX for PV. An initial MTX dose of 7.5mg per week is proposed,with a progressive dose increase to 15 or 20mg per week, orally or intravenously.3,7,12On the day after MTX administration,it is recommended to administer 5–15mg of folic acid orally. Common adverse reactions of MTX are gastrointestinal symptoms, pancytopenia, liver damage, and liver fibrosis. Folic acid supplementation reduces or prevents these adverse reactions.17Regardless of the presence/absence of risk factors for liver disease,patients should be checked for liver fibrosis by evaluating the amino terminal peptide of type III procollagen when the cumulative dose of MTX reaches 1.5 g.18
Azathioprine (level of evidence, high; recommendation level, C1)
The thiopurine methyltransferase(TPMT)activity should be assessed before azathioprine administration, and patients with lower TPMT activity should receive a maintenance azathioprine dose of 0.5–1.5mg/(kg·d).Patients with regular enzyme activity should be started on a minimum azathioprine dose of 50mg/d to avoid causing severe bone marrow suppression.19Weekly routine blood testing is recommended. If no idiosyncratic reactions occur, the azathioprine dose is increased by 50 mg/d each week. The maximum azathioprine dosage for patients with PV is 3mg/(kg·d).It usually takes 6 weeks for azathioprine to take effect.6If myelosuppression occurs,azathioprine should be discontinued immediately. In China, as most of the hospitals do not perform TPMT activity testing, azathioprine is not recommended as the first immunosuppressant. Patients undergoing TPMT monitoring also need to be checked for NUDT15 mutation.20
CTX(level of evidence,medium;recommended level,C1)
CTX is administered either as a 500–1000mg(usually 750 mg)intravenous infusion once per month or as 2mg/(kg·d)orally.3As CTX is associated with risks of hemorrhagic cystitis, cancer, and infertility, it should be discontinued after 3–6 months of CTX treatment, or when the cumulative dose reaches 15g.21In view of the toxicity of CTX, it is recommended to take it in the morning and drink more water to reduce bladder toxicity.CTX should only be administered to patients with refractory or severe PV. Oral administration of 2mg/(kg·d) of CTX is an alternative treatment to azathioprine or MMF.
Cyclosporine(level of evidence,medium;recommendation level, C1)
The common cyclosporine dosage is 2.5–3mg/(kg·d).The most common adverse reactions to cyclosporine are nephrotoxicity and hypertension, especially in patients receiving long-term and substantial doses.As cyclosporine is greatly affected by food and other medications,and the effective concentration is very close to the toxic concentration,it is necessary to monitor the blood concentration of cyclosporine during treatment.22
Rituximab (level of evidence, high; recommendation level, B)
Rituximab is a chimeric human-mouse monoclonal antibody that targets the B lymphocyte-specific antigen CD20.Rituximab is usually administered in combination with systemic glucocorticoids, intravenous immunoglobulins (IVIG), or immunoadsorption.6Rituximab is recommended as the first-line treatment option for moderate to severe pemphigus.6-7In a study that compared the outcomes of a short-term regimen (3–6 months) of prednisone combined with rituximab versus prednisone alone in the treatment of moderate to severe PV,complete remission was achieved after 2 years of treatment by 90%of the patients in the combined treatment group and 28%in the prednisone alone group.14
The regimen of rituximab is either 1,000mg intravenously every 2 weeks two times or 375mg/m2every week intravenously four times;the efficacy of the two schemes is similar, but the first scheme is preferred.6During the maintenance period, 500mg of rituximab is administered intravenously at the 12thmonth, and subsequent administrations are performed every 6 months or as needed based on clinical evaluation.If the disease recurs,1000mg of intravenous rituximab is given, and the dose of glucocorticoid and/or immunosuppressant is adjusted in accordance with the clinical evaluation. Rituximab is usually administered no earlier than 16 weeks after the previous treatment.14
The common acute adverse events associated with rituximab are infusion reactions and allergic reactions.Long-term adverse reactions are mainly infections. The B lymphocyte levels in peripheral blood should be monitored during rituximab administration. Rituximab may cause hepatitis B virus (HBV) reactivation. If the patient is carrying the HBV, a hepatitis monitoring and HBV treatment plan should be developed in conjunction with an infectious physician before and during the treatment.
IVIG (level of evidence, high; recommendation level, B)
IVIG are mostly used in patients with moderate to severe refractory PV and/or in patients with contraindications to glucocorticoids or immunosuppressants, especially patients at risk of infection.3The usual dose of IVIG is 400mg/(kg·d),which is administered over 3–5 consecutive days once a month.If the condition is not controlled after one cycle of IVIG,the cycle is repeated until the disease is under control.6IVIG are mostly administered in combination with glucocorticoids and immunosuppressants.IVIG in combination with rituximab achieves a better effect than IVIG administration alone.18Patients with migraine may develop aseptic meningitis with IVIG administration, while patients with IgA deficiency are prone to severe allergic reactions to IVIG.19
Plasma exchange (level of evidence, medium;recommended level, C2)
Plasma exchange is achieved by separating the plasma and blood cells from the patient’s peripheral blood,selectively removing pathogenic antibodies from the plasma, and replenishing it with colloidal substances such as fresh frozen plasma or human albumin. There is currently no standardized protocol for the number and volume of plasma exchanges. The existing evidence suggests that plasma exchange should be performed two or three times within 7–10 days, with 1 to 1.5 times the plasma volume exchanged each time.19
Immunoadsorption (level of evidence, medium;recommended level, C2)
Immunoadsorption may be considered for refractory PV cases that cannot be improved with conventional therapies. The immunoadsorption method uses an immunoadsorption column to remove pathogenic antibodies in the blood. The most widely used immunoadsorption column in clinical practice is staphylococcal protein A. The Fcbinding region at the amino terminus of the single-chain polypeptide structural protein A on the staphylococcal cell wall specifically binds to the autoantibodies (mainly IgG type) and the Fc segment of the circulating immune complex, thereby eliminating pathogenic autoantibodies.23Immunoadsorption can be combined with the administrations of rituximab and immunosuppressants to reduce circulating pathogenic antibody levels.23Contraindications to immunoadsorption include severe systemic infection,severe cardiovascular disease,hypersensitivity to the components of the immunoadsorption column, the need for angiotensin-converting enzyme inhibitor treatment, and generalized bleeding tendencies.12
Both plasma exchange and immunoadsorption only remove pathogenic antibodies from plasma,and need to be combined with the administration of glucocorticoids and/or immunosuppressants and/or biological agents to inhibit the production of antibodies and prevent disease rebound.
Stem cell transplantation (level of evidence, medium;recommended level, C2)
Stem cell transplantation may be considered for patients with poor clinical outcome, intolerable adverse reactions,or repeated exacerbations with poor prognosis after 1 year of PV treatment. Stem cell transplantation may enable some patients with PV to obtain a good outcome and even long-term clinical remission.24–26
Management of relapse
Relapse of PV is common during the regular reduction process. If there are fewer than three new blisters per month, topical potent glucocorticoids are recommended first.If the disease is not controlled within 1 week and there are still one to three new blisters per month, the dose of oral glucocorticoids should be increased to the last effective dose. If there are more than three new blisters,the dose should be reincreased to two steps back in the previous dose until control of the lesions is achieved.19An immunosuppressive agent is recommended for relapsed patients. If an immunosuppressant agent has previously been administered, this should be switched to a different one.19
Relapse may occur after the sudden withdrawal of high doses of glucocorticoids.In this case,patients usually have a large area of skin involved.A comprehensive evaluation of the condition is recommended to determine the most appropriate treatment regimen.
Monitoring and treatment of adverse reactions
The adverse reactions related to glucocorticoids mainly include diabetes mellitus, hypertension, cardiac insufficiency, myopathy, osteoporosis, gastrointestinal inflammation or ulceration (severe bleeding, perforation),infection, electrolyte disorder, ischemic osteonecrosis,compressive fracture, glaucoma, cataracts, and coagulation disorder.7Baseline screening for and prevention of osteoporosis and ophthalmic evaluation are recommended.7Potassium, calcium, and vitamin D should be supplemented at the beginning of glucocorticoid treatment. The treatment regimen of patients at risk of osteoporosis should be selected in accordance with the relevant guidelines.
Long-term use of glucocorticoids and immunosuppressants increases the risk of infection, and the respiratory tract is most commonly affected. Therefore, patients should be evaluated before and during PV treatment for the endocrine, cardiovascular, and/or respiratory system,tumors, and infection (Table 1). PV can cause secondary bacterial,fungal,and viral infections owing to the damage of the skin and mucous membranes. Therefore, topical cream should be applied to prevent infection. The appropriate antibiotics for local or systemic treatment should be selected based on the culture and sensitivity results of pathogenic microorganisms collected from secretions at the skin ulcer site.
β-D-glucan and cytomegalovirus antibody and virus load should be monitored regularly from the initial treatment stage to the early maintenance stage. Special attention should be paid to the detection of pneumocystis pneumonia or other fungal infections(such as Aspergillus).Antibiotics can be used to control infection if necessary(with evidence of bacterial infection).Owing to the risk of HBV reactivation in patients treated with rituximab,patients should be screened for HBV before rituximab treatment.If a patient has a history of tuberculosis or has been recently exposed to a patient with tuberculosis,HBV,and/or other severe infection, the treatment regimen should be selected in consensus with an infectious physician. Live vaccines are prohibited during immunosuppressant and rituximab treatment.7–12
Conclusions
The treatment options for PV are diverse.An appropriate PV treatment regimen must be selected in accordance with the severity of the disease, concomitant diseases, and physical conditions. This proposal will help dermatologists achieve an early diagnosis of PV, which will enable the initiation of early treatment for PV and the achievement of long-term remission as much as possible.Adverse reactions must be managed during PV treatment.
- 国际皮肤性病学杂志的其它文章
- Epithelioid Blue Nevus
- Dermoscopy Features of Trichofolliculoma:A Case Report
- A Novel Small Deletion in the ATP2A2 Gene in a Patient with Sporadic Darier’s Disease and Concomitant Depression: A Case Report
- Instructions for Authors
- A Case Report of Pterygium Inversum Unguis Associated with Systemic Lupus Erythematosus
- Conversion of Adipose-Derived Stem Cells into Sweat Gland-Like Cells: An In Vitro Phenotypic Study

