大斯托克斯位移远红光至近红外荧光探针用于检测肝损伤过程中过氧化亚硝酸盐的动态变化
吴 倩,程 丹,吕 芸,袁 林,张晓兵
(湖南大学化学化工学院,化学生物传感与计量学国家重点实验室,长沙410082)
1 Introduction
As one of the most significant organs of the human body,the liver plays vital roles in metabolism.Abnormal liver function can cause various diseases,such as hepatitis[1,2],fatty liver[3,4],and cirrhosis[5].Among them,drug-induced liver injury(DILI)is considered to be the primary clinical issues.Its emergence is regarded as the main reason of acute liver failure and withdrawal of marketed drugs[6].Currently,many studies have efforted to represent that the mechanism of DILI is closely related to the production of reactive nitrogen and reactive oxygen species,such as peroxynitrite(ONOO—)[7—13].Therefore,ONOO—can be employed as an early sign in the progression of DILI.Exploring the changes of ONOO—is of great significance for understanding various physiological and pathological processes caused by abnormal liver function.
Peroxynitrite(ONOO—),which is originated from the diffusion control reaction of nitric oxide(NO)and superoxide anion radicalacts as a strong oxidant and nitrating agent in living system[15].It can cause lipid reactions,DNA breakage,protein modification,and irreversible damage to biological substances,so it could induce cardiovascular disease,inflammation,and nervous system diseases[16].However,the special nature of ONOO—under physiological conditions,extremely short half-life(<10 ms),and high chemical reactivity make it difficult to achieve direct trackingin situ[17,18].Recently,fluorescence imaging technology has attracted much attention due to its excellent performance in non-invasive imaging,high sensitivity and satisfactory temporal and spatial resolution[19—22].And quite a few of organic fluorescent probes have been developed for ONOO—detection in biological systems[23—35].However,these ONOO—probes generally have the disadvantages of small Stokes shift,short emission wavelength,and susceptibility to interference from biological tissues and excitation light.These drawbacks can easily cause crosstalk between the excitation spectrum and the emission spectrum,limit the sensitivity and accuracy of imaging and sensingin vivo.Therefore,the exploitation of far red to near-infrared(FR-NIR)large Stokes shifts small molecular fluorescent probes for ONOO—is imminent.
Herein,we proposed an activated FR-NIR fluorescent probe(LSDQ-ONOO—)with large Stokes shift for real-timein situmonitoring ONOO—variation during drug-induced liver injury.The probe displayed superior performancein vitro,including excellent sensitivity(detection limit=25.8 nmol/L),prominent extrinsic response(20-fold),excellent selectivity,and was not interfered by other reactive species.We adopted three drugs,acetaminophen(APAP)[10,11],valproic acid(VPA)[36,37],isoniazid(INH)[38],to induce liver injury,and attempted to elaborate the role of ONOO—in the process of DILI.Using this probe,we showed the upregulation of ONOO—levels during DILI in HepG2 cells and in mice model,which helps us to further understand the pathological process of liver injury.
2 Experimental
2.1 Synthesis of Probe LSDQ-ONOO‒
Compound DQF-565 was prepared according to the method previously reported by our group[39].4-Nitrophenylglyoxylic acid(19.5 mg,0.1 mmol),1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate(HATU)(57.0 mg,0.15 mmol)and triethylamine(2 μL)were added into 1.0 mL of anhydrous dichloromethane,the mixture was stirred at room temperature for 40 min under nitrogen atmosphere.Then compound DQF-565(48.2 mg,0.1 mmol)was poured into the mixture,which was further stirred at room temperature for 2 h.The solvent was removed under evaporated,and the mixture was purified by column chromatography on silica gel with dichloromethane and ethanol[V(CH2Cl2)/V(EtOH)=80∶1]to afford a light red solid(7.1 mg,10.7%yield).1H NMR(400 MHz,CDCl3),δ:8.61(s,1H),8.33(s,1H),8.18(d,J=7.8 Hz,2H),8.10(d,J=8.5 Hz,2H),7.56—7.48(m,2H),7.04—6.96(m,2H),6.79(d,J=6.3 Hz,1H),6.67(s,1H),6.24(d,J=7.6 Hz,1H),5.79(s,1H),3.46—3.41(m,1H),3.24(s,1H),2.85(s,4H),1.48—1.40(m,2H),1.19—1.16(m,2H),1.03(d,J=7.4 Hz,10H).13C NMR(100 MHz,CDCl3),δ:190.8,173.6,171.4,165.8,162.4,155.7,144.8,142.3,141.1,136.8,135.1,134.1,133.3,128.0,127.6,115.5,112.4,111.9,100.1,64.4,56.1,36.2,34.9,34.0,31.9,29.1,27.3,18.9,16.9.HRMS(ESI)C38H35N4O7+[M]+,calcd.:659.2500;found:659.2501.
2.2 Cell Culture and Imaging
All the experiments were conducted in live cells.HepG2 cells and L02 cells were cultured in high glucose Dulbecco’s Modified Eagle Medium(DMEM,Hyclone)supplemented with 10%fetal bovine serum(FBS,BI),and 1%antibiotics(100 U/mL penicillin and 100 μg/mL streptomycin,Hyclone)at 37 ℃ and 5%CO2.Cells were carefully harvested and split when they reached 80%confluence to maintain exponential growth.The microscopic imaging uses confocal laser scanning microscope(Nikon,Japan)with an excitation filter of 561 nm and the collection wavelength range is from 640—750 nm.
2.3 Fluorescence Imaging of Drug-induced Liver Injury Mice Model
All female BALB/c mice(18—20 g)were employed in strict accordance with institutional ethics committee regulations and guidelines on animal welfare and were divided into two groups.Mice from the first group were the control group,fasted overnight and received an intraperitoneal injection of saline.Mice from the second group were fasted overnight and received an intraperitoneal injection of 300 mg/kg APAP.All the mice were then injected intravenously with 50 μL PBS containing 100 μmol/L LSDQ-ONOO—forin vivoimaging.Thein vivo(living mice)imaging was carried out using anIn⁃Vivoimaging system(IVIS Lumina XR).
3 Results and Discussion
3.1 Design and Synthesis of Fluorescent Probe for ONOO‒
Previously,our lab fabricated two ONOO—probes by incorporating theα-keto amine into chromophores rhodamine and aminonaphthalimide,which had superior selectivity and sensitivity[33].In this design,we also employedα-keto amine(i.e.4-nitrophenylglyoxylic acid)[33,40]as the reaction site for the construction of probe,owing to its good selectivity and high sensitivity,which can resistant to interference with other highly active substances.It’s well known that Stokes shift is an important characteristic of fluorescence,and increasing the Stokes shift can effectively improve the performance of fluorophore-based biosensing[41].So,we introduced DQF-565 as a fluorophore into our probe,because the dye DQF-565,proposed from our group[39],has the advantage of large Stokes shift(120 nm)(Fig.S1,see the Electronic Supplementary Material of this paper),good photo-stability and cell compatibility.Thus,we judiciously constructed a new large Stokes shift FR-NIR fluorescent probe(LSDQ-ONOO—)for specifically ONOO—detection by usingα-keto amine as the recognized group and our FR-NIR dye as the fluorescent reporter.LSDQ-ONOO—was readily preparedviaone-step procedure outlined in Scheme S1(see the Electronic Supplementary Material of this paper)and characterized by HRMS,1H NMR,and13C NMR.
3.2 Fluorescence Response of Probe Towards ONOO‒
We first tested the stability of the large Stokes shift dye DQF-565 to ONOO—.As shown in Fig.S2(see the Electronic Supplementary Material of this paper),DQF-565 displayed minor fluorescence enhancement with increasing concentration of ONOO—,indicating the dye has good stability which can avoid false signals for biological imaging[8].Then the reactivity of LSDQ-ONOO—in the presence of ONOO—was examined.After addition with different concentrations of ONOO—(0—6 μmol/L),a dose-dependent fluorescence increment was observed(Fig.1),and the fluorescence intensity of LSDQ-ONOO—also increased more than 20-fold.Moreover,the fluorescence intensity also exhibited an excellent linear correlation,and the detection limit was as low as 25.8 nmol/L(Fig.2).
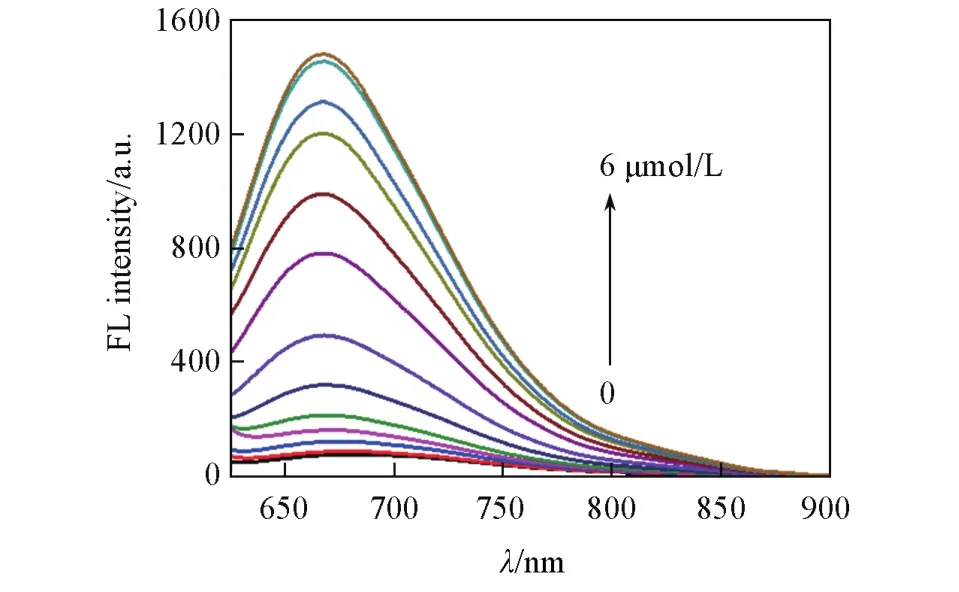
Fig.1 Fluorescence spectra of LSDQ⁃ONOO-(5µmol/L)upon addition of ONOO-(0—6 µmol/L)in PBS/EtOH(volume ratio 8∶2,pH=7.4)buffer solution
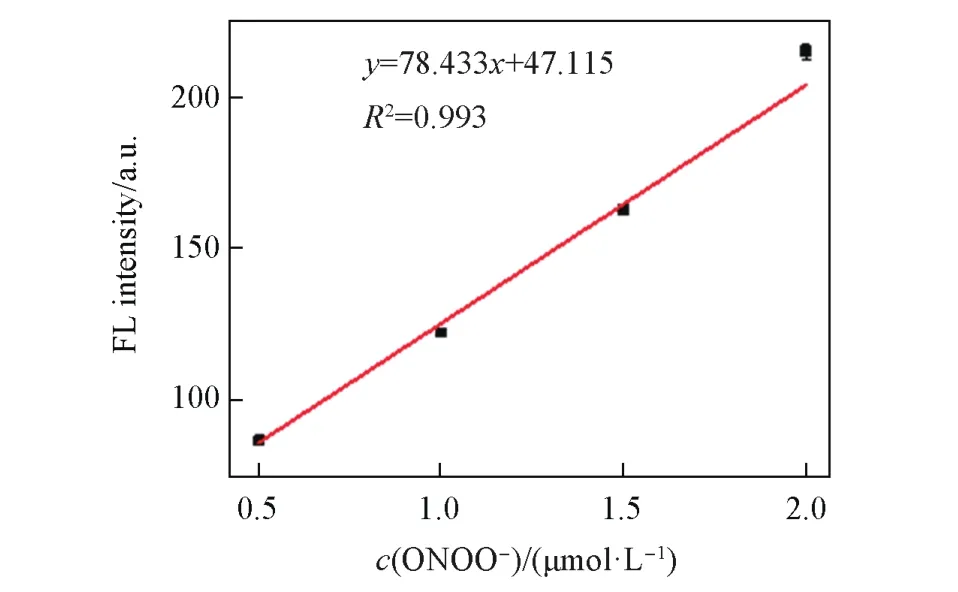
Fig.2 Linear correlation between the FL intensity of LSDQ ⁃ONOO-(5 µmol/L)and ONOO-concentration in PBS/EtOH(volume ratio 8∶2,pH=7.4)buffer solution
In addition,after reacting with ONOO—,the absorption peak of the probe shifted to 562 nm,and the solution color also gradually changed from light red to dark red(inset of Fig.3).Then,we evaluated the fluorescence recovery time of the probe for 20 min(Fig.S3,see the Electronic Supplementary Material of this paper).The proposed mechanism was also demonstrated by analyzing the reaction process of LSDQONOO—and ONOO—by MALDI-TOF-MS analysis(Fig.S4,see the Electronic Supplemen-tary Material of this paper).Simultaneously,we observed mass spectral peaks of probe and dye,which further validated our propose mechanism(Scheme 1),that was ONOO—-mediated selectively cleavage of theα-keto amide bridge and concomitant release of the fluorophore.Additionally,the probe offered weak fluorescence emission at pH of 4.0—10.0 and showed significant enhancement toward ONOO—within an extremely narrow pH range of 7.0—8.0(Fig.S5,see the Electronic Supplementary Material of this paper),which was consistent with the pH range under physiological conditions.
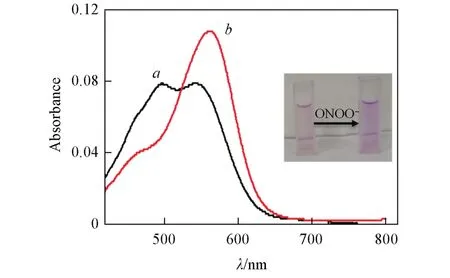
Fig.3 Absorption spectra of LSDQ⁃ONOO-(5µmol/L)in the absence(a)and in the presence(b)of ONOO-(5 µmol/L)in PBS/EtOH(volume ratio 8∶2,pH=7.4)buffer solution

Scheme 1 Proposed reaction mechanism of probe LSDQ⁃ONOO‒with ONOO‒
Subsequently,we evaluated the photo-stability of the probe.As shown in Fig.S6(see the Electronic Supplementary Material of this paper),our probe displayed good photo-stability than commercial probe.Then,we measured the selectivity of LSDQ-ONOO—by comparing the fluorescence response of probe with a series of biologically relevant species(Fig.4),including reactive oxygen species(,·OH,H2O2,HOCl,t-BuOOH),reactive sulfur species(GSH,Cys,H2S)and reactive nitrogen species(N),biorelevant anionsand cations(Cu2+,Fe2+,K+,Na+,Mg2+).As expected,LSDQ-ONOO—achieved remarkable fluorescence enhancement only in the presence of ONOO—,while other potential interfering substances had negligible fluorescence variations.H2O2possesses a peroxide moiety and activity similar to ONOO—,so it often cross-reacts with ONOO—probes.Therefore,we examined the potential disturbance of high amounts of H2O2,as shown in Fig.S7(see the Electronic Supplementary Material of this paper),there is no fluorescence response of the probe toward H2O2with a broad concentration from 0 to 1 mmol/L.All above results demonstrate that LSDQONOO—can be used for the specific detection of ONOO—levels under complicated physiological conditions.
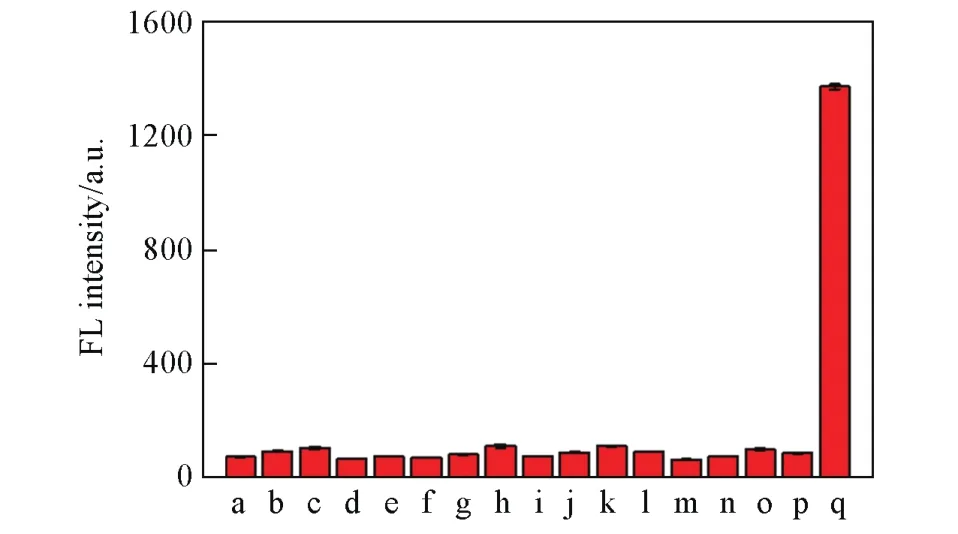
Fig.4 Fluorescence intensity of LSDQ⁃ONOO-(5µmol/L)in PBS/EtOH(volume ratio 8∶2,pH=7.4)buffer solu⁃tion toward various analytes(100µmol/L)
3.3 Organelle Distribution of LSDQ-ONOO‒
We first carried out the cytotoxicity experiments(Fig.S8,see the Electronic Supplementary Material of this paper),which showed the probe had tiny biological toxicity.Then the probe organelle distribution measurements were employed.HepG2 cells were pretreated with bacterial endotoxin lipopolysaccharide(LPS)and pro-inflammatory cytokine interferonγ(IFN-γ),which were known to trigger cellular apoptosis and produce ROS and RNS,including ONOO—,and then incubated with LSDQ-ONOO—and subsequently incubated with Mito-Tracker Green or Lyso-Tracker Green.As shown in Fig.5,the red fluorescence of LSDQ-ONOO—almost completely overlapped with the signal from Mito-Tracker Green,and the Pearson’s colocalization coefficient was determined to be as high as 0.82.It is well known that lipophilic cations are more likely to accumulate in the mitochondria,so the electrostatic interaction between the positively charged probe and the negative charge mitochondrial membrane may be the main factor for the probe to distribute to the mitochondria.Thus,we speculated LSDQ-ONOO—has the ability to image in the mitochondria where ONOO—primarily originates,and can further explore related biological processes.
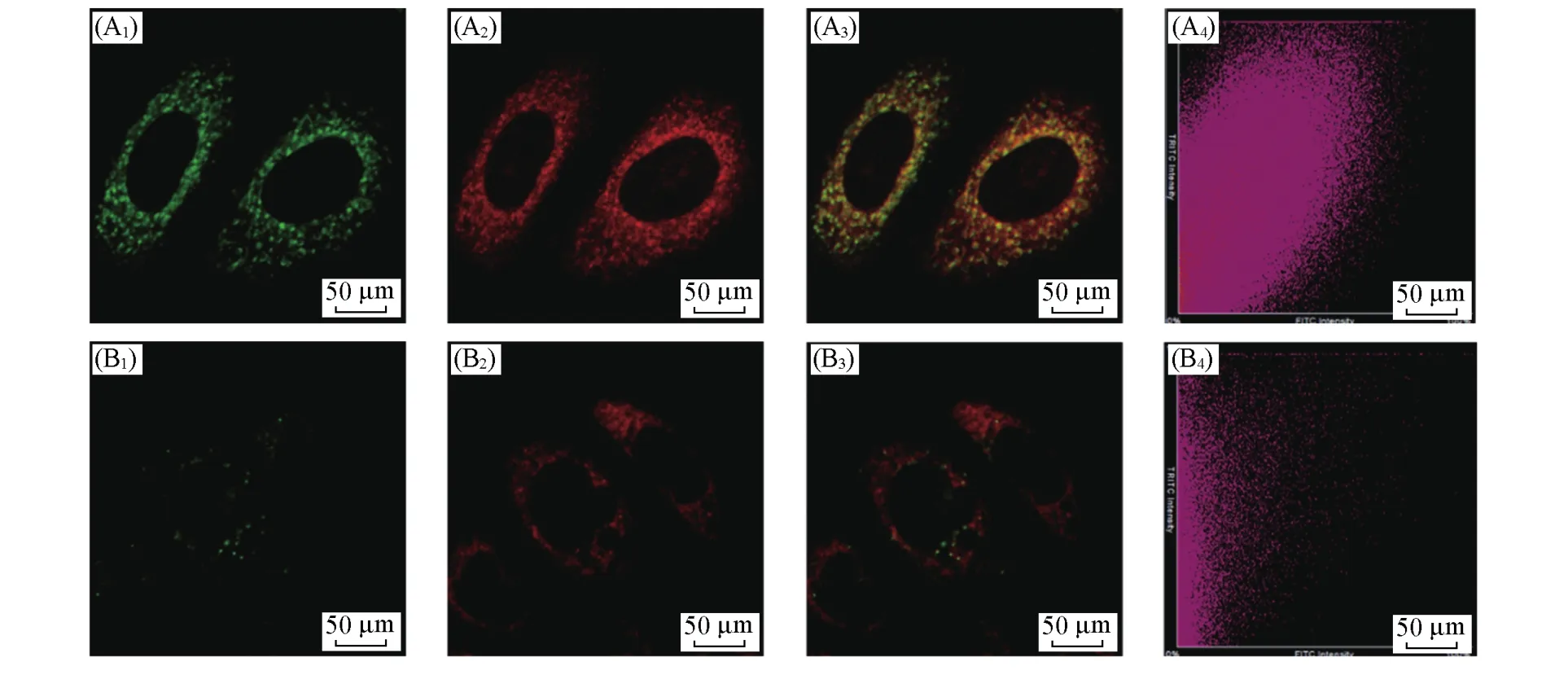
Fig.5 Distribution of LSDQ⁃ONOO-in HepG2 cells

Fig.6 Fluorescent images of LSDQ⁃ONOO-in HepG2 cells under different conditions
3.4 Fluorescence Imaging of Endogenous and Exogenous ONOO-Production in Living Cells
Considering the outstanding performance of probe LSDQ-ONOO—in vitro,we further explored the selectivity of LSDQ-ONOO—in living cells.Here,we selected LPS and IFN-γto stimulate the cells to produce ONOO—.HepG2 cells were first stained with probes and then stimulated with the corresponding reagents or control(PBS),respectively.As shown in Fig.6,the control group emitted almost no fluorescence,as did the H2O2(a strong ONOO—interferent)-treated cells.In contrast,the cells co-stimulated by LPS and IFN-γhad a significant fluorescence increment,suggesting that ONOO—was largely produced during the stimulation process.Additionally,when the cells were preincubated with superoxide scavenger(2,2,6,6-tetramethylpiperi-dine-N-oxyl,TEMPO)[42]or nitric oxide synthase inhibitor(amino-guanidine,AG)[43],the fluorescence intensity in HepG2 cells would decrease,as the reduced production of ONOO—.Furthermore,we adopted 3-morpholinosydnonimine hydrochloride(SIN-1),an ONOO—generator,to image exogenous ONOO—.As the concentration of SIN-1 increased,the cells illustrated a dose-dependent fluorescence enhancement(Fig.S9,see the Electronic Supplementary Material of this paper).These results elucidated that our probe can specifically and steadily detect ONOO—produced endogenously or exogenously without other interferences,indicating that the LSDQ-ONOO—can be applied for bioimagingin vivo.
3.5 Studying Drug-induced Hepatotoxicity in cell Models by Imaging of ONOO‒
To elaborate the role and the generation mechanism of peroxynitrite in the process of drug induced liver injury,we tried to monitor the change of ONOO—in living cells andin vivo.
We adopted three commercially available drugs that known to cause liver damage to illustrate the phenomenon of DILI.It is well known that excessive APAP can cause liver damage through enzyme biotransformation,resulting in overproduction of ONOO—[10,11].We first evaluated the generation of ONOO—in living cells with APAP.Cells were treated with APAP(0—800 μmol/L)for 12 h and then incubated with LSDQ-ONOO—(5 μmol/L)for 30 min.Obviously,the reaction-based fluorescence probe could easily capture the trace of ONOO—produced by APAP-induced liver toxicity,and the increased fluorescence signal was shown in Fig.7.The dose-dependent fluorescence intensity not only proved that ONOO—production in cells was related to APAP concentration,but also demonstrated the excellent sensitivity of LSDQ-ONOO—.These evidences indicated that ONOO—was mainly produced by mitochondrial oxidative stress when stimulated by APAP.APAP was also used to stimulate cells in the co-localization experiment,and the result was the same as before(Fig.S10,see the Electronic Supplementary Material of this paper).Seeking to whether our probe can also monitor ONOO—production in normal cells in real-time,we did the same APAP-stimulated experiment with normal liver cells L02(Fig.S11,see the Electronic Supplementary Material of this paper).The results implied that the LSDQ-ONOO—had good ability to monitor ONOO—in hepatotoxicity cells.In addition,we also evaluated the levels of ONOO—produced endogenously by other drugs,such as isoniazid(INH)[38]and sodium valproate(VPA)[36,37].The results revealed the ONOO—generation by INH and VPA-induced liver injury,demonstrating our probe possessed a potency to evaluate liver injury in different drug-caused liver injury(Fig.S12 and S13,see the Electronic Supplementary Material of this paper).

Fig.7 Fluorescent images of LSDQ⁃ONOO-in HepG2 cells
3.6 Studying Drug-induced Hepatotoxicity in Mouse Models by Imaging of ONOO‒
Motivated by these promising results in cells,we further carried out the experimentsin vivo.We established the mouse models to visualize ONOO—production in drug-induced liver injury.All Female BALB/c mice(18—20 g)were operated in accordance with institutional ethics committee regulations and guidelines on animal welfare.Firstly,the study was divided into two groups.Mice from the first group were the control group,which were fasted overnight and received an intraperitoneal injection of saline.Mice from the second group were fasted overnight and received an intraperitoneal injection of 300 mg/kg APAP.LSDQ-ONOO—was injected through tail vein after 45 min,and then the mice were imagedin situat different time points.The images illustrated significant fluorescence enhancement in the liver area from 60 to 90 min after administration of probe LSDQ-ONOO—(Fig.8).Additionally,ex vivoimaging of various organs showed that after reacting,both liver and kidney had obvious fluorescent signals(Fig.9).We speculated that the NH2would be exposed by the deacetylation process and led to increased water solubility of fluorophore and promoted the kidney metabolism.The above results confirmed the LSDQ-ONOO—can be suitable forin vivohepatotoxicity detection.
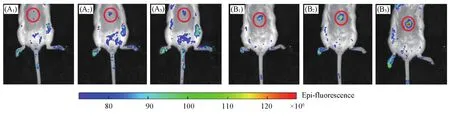
Fig.8 Representative images of BALB/c mice receiving saline(control,A1—A3),or APAP(300 mg/kg,intraperitoneally,B1—B3)followed by LSDQ⁃ONOO-(50 µL,100 µmol/L,intravenously)in 30(A1,B1),60(A2,B2)and 90 min(A3,B3)

Fig.9 Fluorescence images of representative organs of BALB/c mice receiving saline(control,A)or APAP(300 mg/kg,intraperitoneally,B)followed by LSDQ⁃ONOO‒(50 µL,100 µmol/L,intra⁃venously)after 90 min
4 Conclusions
In summary,we have presented a new activatable FR-NIR fluorescent probe,LSDQ-ONOO—,for monitoring ONOO—variation during drug-induced liver injury.LSDQ-ONOO—showed high sensitivity,prominent extrinsic response and excellent selectivity towards ONOO—.Using this probe,we found that ONOO—levels had an obvious upregulation in cells andin vivo,and it can be described an early biomarker of drug-induced liver injury.Therefore,we expected that our work could offer a new strategy for illustrating the process of drug-induced liver injury by constructing probes in the pathological environment through the large Stokes shifts fluorescent dyes.
Supporting Information:http://www/cjcu.jlu.edu.cn/CN/10.7503/cjcu20209236.
This paper is supported by the National Natural Science Foundation of China(Nos.21877029,21735001,21904035)and the China Postdoctoral Science Foundation(No.2018M642968).

