A primary pulmonary artery sarcoma associated with multiple lesions
Jing Wang, Tian-lang Li, Shu Zheng
1 Department of Geriatric, the Second Aff iliated Hospital of Zhejiang University School of Medicine, Hangzhou, China 2 Institution of Tumor, the Second Aff iliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
Dear editor,
Primary pulmonary artery sarcoma (PPAS) is an uncommon neoplastic disease that can masquerade as pulmonary thromboembolism (PE). The nonspecific symptoms of this condition generally vary from mild dyspnea to severe respiratory distress and right heart failure. A male patient is discussed in this report who presented with fever, cough, and shortness of breath. An enhanced contrast computed tomography (EC-CT) scan revealed an intraluminal hypoattenuated area originating from the main pulmonary artery and extending further into the left pulmonary artery and the bilateral lobar pulmonary arteries, evidence for multiple lesions in both lungs. The spindle cell tumor, positive for smooth muscle actin, was pathologically diagnosed as PPAS using a needle biopsy of the lower lobe of the left lung.
CASE
A 65-year-old male patient was admitted to our department with fever, cough, and dyspnea ongoing over the last eight days. The cough and fever had worsened under a one-week course of empirical antibiotic therapy for pneumonia. The patient reported that he felt chest tightness and could not lie on his back the day before admission. At admission, the patient’s temperature was recorded as 38.4 °C, his respiratory rate was 22 breaths/minute, his pulse rate was 102 beats/minute, and he had a blood pressure of 109/72 mmHg. A clinical lung examination revealed diminished breath sounds in the left lower lung and occasional basilar crackles.A heart examination revealed expansion of the left heart border and a 3/6 systolic murmur at the upper sternal border. There was no cyanosis, digital clubbing,or evidence for deep venous thrombosis. Laboratory tests revealed white blood cell (WBC) count 12,900/mm³ with 80.7% neutrophils, C-reactive protein (CRP) 150 mg/L,and erythrocyte sedimentation rate 75 mm/hour. The serum f ibrinogen level was 9.29 g/L (normal range 3.00-5.50 g/L),lactate dehydrogenase (LDH) 399 U/L (normal range 50-240 U/L), and the D-dimer level 0.20 μg/mL (normal range 0-0.25 μg/mL). Serum electrolytes, liver enzymes, and glucose levels were found to be within normal limits.
At admission, the patient’s X-ray revealed left pleural effusion and an enlarged left main pulmonary artery (Figure 1). A EC-CT scan of the chest showed an intraluminal hypoattenuation mass which had invaded the pulmonary artery wall, extending from the main pulmonary artery into the left main pulmonary artery,the bilateral lobar pulmonary arteries, the lower lobe of the left lung, and the upper lobe of the right lung.Additionally, a moderate left pleural effusion was also present (Figure 2). A transthoracic echocardiogram(TECG) revealed a mild aortic regurgitation and a substantial mass within the pericardial cavity (Figure 3).
A thoracentesis was performed the day after admission. The pleural fluid appeared bloody-colored with an adenosine deaminase value of 21 U/L (normal range <25 U/L) and a carcinoembryonic antigen (CEA)level of 1.3 μg/L (normal range 0-5.0 μg/L). Specimen smears showed a nucleated cell account of 550 cells/μL(normal range 0-200 cells/μL), with 15% of neutrophil,33% of lymphocyte, and 52% of mesothelial cells, but no trace of malignant cells.
In order to make a def initive diagnosis, a CT-guided transthoracic needle biopsy was performed into the lower lobe of the left lung. Subsequent histological examination of this tissue revealed a proliferation of spindle cells with pleomorphic nuclei in a myxoid background. Smoothmuscle actin was positive in an immune-histochemical(IHC) test (Figure 4). A diagnosis of an intimal smoothactin positive spindle cell PPAS was made based on morphological and IHC data.

Figure 1. Chest X-ray showed a left pleural effusion and an enlarged left main pulmonary artery (arrow).

Figure 2. Chest EC-CT at admission (A) and coronary multi-planar reconstruction (B). A: areas of intraluminal hypoattenuation were located in the main pulmonary artery (arrow) alongside a tumor mass in the lower lobe of the left lung and a left pleural effusion; B:pulmonary artery sarcoma originating from pulmonary artery trunk and extending into left pulmonary artery (arrow). EC-CT: enhanced contrast computed tomography.
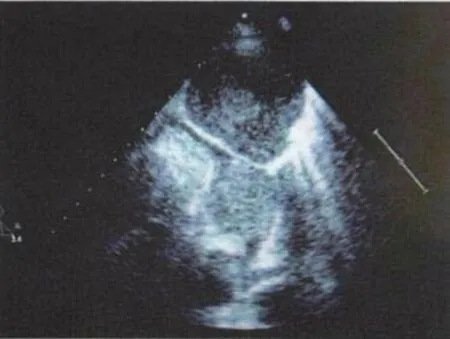
Figure 3. TECG at admission. Mild aortic regurgitation and a substantial tumor mass can be seen within the pericardial cavity(arrow). TECG: transthoracic echocardiogram.
As this patient refused surgical treatment, two courses of paclitaxel/cisplatin combination chemotherapy were applied (every three weeks). Following the first course of chemotherapy, hyperthermia was cured, but there was no clinical improvement in dyspnea. The pleural effusion decreased, and the WBC count reduced to 5,200/mm³ with 51.6% neutrophils. CRP decreased to 20 mg/L, LDH decreased to 156 U/L, and the D-dimer level increased to 1 μg/mL. After two months of treatment, chest EC-CT showed no shrinkage in the intraluminal hypoattenuation area; metastatic disease progressed, and multiple small lesions were seen in both lungs (Figure 5). Despite the progression of the disease,the patient received neither additional chemotherapy nor radiotherapy and died four months after PPAS diagnosis.
DISCUSS ION
PPAS arising from mesenchymal cells within the vascular intima of the pulmonary artery is unusual and has a poor prognosis if left untreated.[1,2]Indeed,estimating the incidence of this condition is diff icult due to the lack of reported data worldwide. Limited reported incidence-estimations mainly rely on mono-institutional retrospective observations, small numbers of scanned patient reports, and isolated case reports.[3-7]Incidence estimation is further hindered by PPAS simulating radiologically and clinically PE, which appears clinically quite often.[2,8]
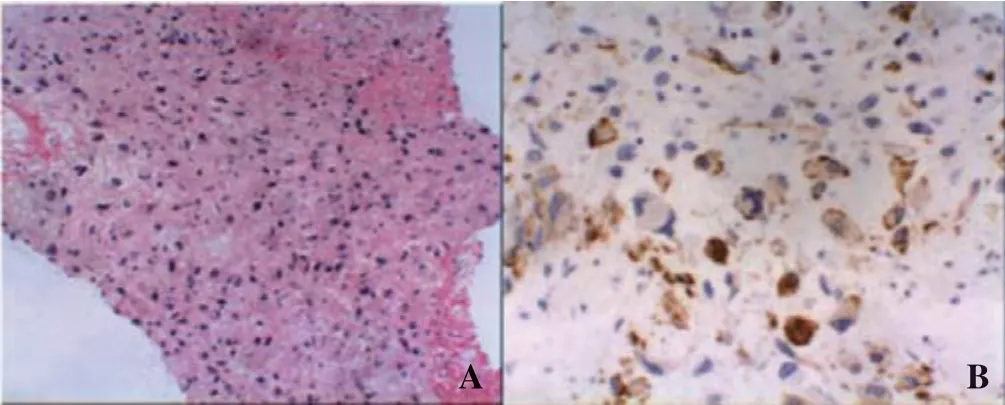
Figure 4. Tumor histologies (A) and IHC (B). A: proliferation of spindle cells with pleomorphic nuclei against myxoid and collagenic backgrounds (HE stain, original magnif ication ×100); B: positive for smooth muscle actin. IHC: immune-histochemical.
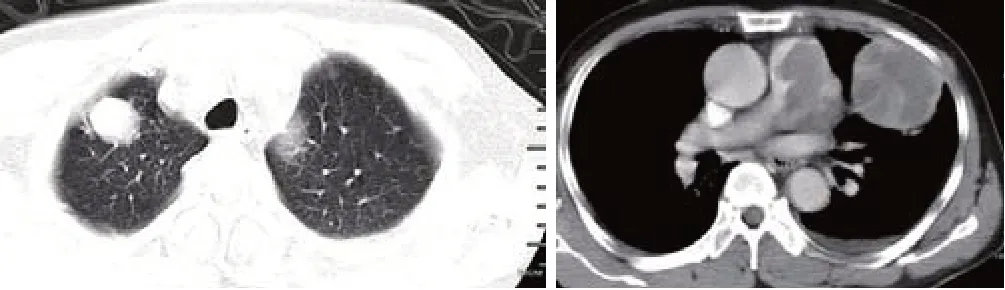
Figure 5. Chest EC-CT two months after diagnosis. Lesions in the lung were visible and the pulmonary artery had deteriorated. EC-CT:enhanced contrast computed tomography.
The PPAS reported here has a lifespan ranging from two months and 89 years old and mainly affects patients between 40 years old and 60 years old without any gender specification.[3]Symptoms vary from mild dyspnea to severe respiratory distress and right heart failure and are mostly nonspecific, including chest pain, cough, hemoptysis, and fever.[3]Invasion of the pulmonary valve and trunk can lead to decreased cardiac output, and can subsequently propagate to central vascular events leading to sudden death. Radiological findings, resembling those of PE, include intraluminal filling defects on CT and MRI angiography as well as TECG.[8]
Additional common radiologic findings include pulmonary infiltrations as well as nodules or masses,presumably as a result of embolic processes with, or without, lung infarction or metastasis. Hemorrhagic pleural effusions are also common, as observed in our patient in the absence of tumor cells. PPAS EC-CT might also demonstrate partial or complete obstruction of the central pulmonary arteries by large tumors.[9]To address the difficulty of discriminating PPAS from PE, we summarized several radiographic criteria in this analysis. In the first place, PPAS tends to exhibit contiguous lower-attenuation f illing defects and occupies the entire luminal diameter of the primary or proximal pulmonary artery. In contrast, PE rarely presents as a luminal obliteration of the proximal pulmonary arteries.Secondly, PPAS can appear in inhomogeneous areas characterized by high or low attenuation, representing hemorrhages and necrosis as well as soft-tissue density in pulmonary arteries, while PE is characterized based on uniform lesion density without enhancement. Thirdly,PPAS often destroys blood vessels with extra-luminal invasion, while PE is typically located inside the vascular lumen.[10]In some cases, PPAS has been observed to grow proximally, affecting the pulmonary valve and the right ventricle.[11]PPAS often inf iltrates the mediastinum and the heart.[12]Incidences of pulmonary metastasis from PPAS have been reported in 40% of published cases while extrapulmonary metastasis has been found in 20%.[4]
A D-di mer and LDH can be used as biomarkers to differentiate PPAS from PE. In one study, Guo et al[13]reported that D-dimer values were within the normal range in all nine PPAS cases. This means that, in general,PE can be excluded if the D-dimer is negative. Similarly,although LDH is a nonspecif ic biomarker for malignant tumors, elevated values are seldom seen in PE patients.In an earlier study, Pu et al[14]reported that 77.8% and 88.9% of PPAS patients exhibited elevated levels of serum LDH and CRP, respectively, while just 11.8% of patients with pulmonary embolisms had elevated levels of the former marker.[15]These results indicate that serum inf lammatory markers might be of importance to screen for PPAS or at lea st differentiate it from PE.
Many PPAS cases might be misdiagnosed as pneumonia due to symptoms of fever and the consolidation or infiltration on chest CT scans; this can lead to ineffective therapy. The presence of a hypoattenuating mass with no sign of an air bronchogram(as illustrated by an EC-CT) can also be used to help rule out pneumonia.
PPAS is often discovered late during treatment because of rare, nonspecif ic symptoms, insidious growth,and potential PE confusion. Many reported cases were identified by surgeries or bronchoscopy fine-needle aspiration biopsies.[2]In the cases of PPAS reported by Pollock et al,[16]a transvenous catheter suction biopsy was used to obtain specimens. This method is challenging to perform, however, because of a high risk of bleeding complications and is thus not widely recommended.The diagnos is of PPAS here was assured with a tumor specimen obtained through a CT scan guided suction biopsy of the pulmonary mass. This enhances our knowledge of the limitations of this method.
Significant variations are seen between different institutions in the use of chemotherapy and radiotherapy.Tumor types are an essential factor upon which dosing and schedules are determined. The efficacy of these treatments, however, remains unproven and reflects the clinical diversity of PPAS as well as its unique nature.The most effective therapeutic option for prolonging survival in patients with a PPAS diagnosis seems to be complete tumor resection.[17]In thi s case, unfortunately,PPAS determination was possible only at the most advanced stage, and so radical resection was almost impossible. Radiotherapy and chemotherapy can be applied for patients who have unresectable or recurrent PPAS to prevent distal tumor micro-embolization and prolong survival.[3]Chemotherapy and radiation therapy can be used separately and within a multi-modality approach, with or without surgery.[18]In one case, Maekura et al[19]reported radiotherapy combined with single-agent ifosfamide, enabling control of the tumor for one year,while Cantaloube et al[20]used carboplatin/vinorelbine as f irst-line therapy for metastatic PPAS. This treatment seems to be tolerable, as shown by a CT scan with an objective response over eight months. Similarly,Nakahira et al[21]introduced paclitaxel/carboplatin postoperatively alongside chemotherapy and achieved a remarkable survival extension (56 months). Our patient,however, failed to respond to paclitaxel/cisplatin chemotherapy most likely because of PPAS spindle cell primary resistance.
CONCLUSIONS
PPAS is a highly aggressive and rapidly evolving kind of tumor, often misdiagnosed as PE. We successfully differentiated PPAS from PE in this case with the help of EC-CT scans and lab tests (D-dimer and LDH). CT angiography revealed the presence of a f illing defect characterized by contrast enhancement in the pulmonary artery and raised the suspicion of malignancy.Extraluminal tumor extension and distant metastases are also reliable signs for distinguishing PPAS from PE. This case reconf irmed the fact that advanced disease prognosis remains poor as chemotherapy was only marginally useful for this patient.
Funding:None.
Ethical approval:The study protocol was reviewed and approved by the Institutional Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine.
Conflicts of interest: The authors declare that there are no conf licts of interest regarding the publication of this paper.
Contributors:JW propos ed and wrote the f irst draft. All authors contributed to the design and interpretation of the study and to further drafts.
 World journal of emergency medicine2020年4期
World journal of emergency medicine2020年4期
- World journal of emergency medicine的其它文章
- Improving antibiotic prescribing in the emergency department for uncomplicated community-acquired pneumonia
- Outcome prediction value of National Early Warning Score in septic patients with community-acquired pneumonia in emergency department: A single-center retrospective cohort study
- Effects of f luid balance on prognosis of acute respiratory distress syndrome patients secondary to sepsis
- Effects of sepsis on hippocampal volume and memory function
- Death and do-not-resuscitate order in the emergency department: A single-center three-year retrospective study in the Chinese mainland
- The general public’s ability to operate automated external def ibrillator: A controlled simulation study
