Fluidised bed granulation of two APIs:QbD approach and development of a NIR in-line monitoring method
,Tior Csin,Alin Porfire,Sestin Porv,Ion Voin,Alexndru Opre,Ion Tomut
aFaculty of Pharmacy,Iuliu Hatieganu University of Medicine and Pharmacy,Cluj-Napoca 400012,Romania
b National Institute for Research and Development of Isotopic and Molecular Technologies,Cluj-Napoca 400293,Romania
c S.C.Laropharm SRL,Bragadiru 077025,Romania
Keywords:Quality by design Design space Risk assessment Process analytical technology Fluid bed granulation MicroNIR
ABSTRACT The study focused on the fluid-bed granulation process of a product with two active pharmaceutical ingredients,intended for coated tablets preparation and further transfer to industrial scale.The work aimed to prove that an accurate control of the critical granulation parameters can level the input material variability and offer a user-friendly process control strategy.Moreover,an in-line Near-Infrared monitoring method was developed,which offered a real time overview of the moisture level along the granulation process,thus a reliable supervision and control process analytical technology(PAT)tool.The experimental design’s results showed that the use of apparently interchangeable active pharmaceutical ingredients (APIs) and filler sorts that comply with pharmacopoeial specifications,lead to different end-product critical attributes.By adapting critical granulation parameters (i.e.binder spray rate and atomising pressure) as a function of material characteristics,led to granules with average sizes comprised in a narrow range of 280–320 μm and low nongranulated fraction of under 5%.Therefore,the accurate control of process parameters according to the formulation particularities achieved the maintenance of product within the design space and removed material related variability.To complete the Quality by design (QbD) strategy,despite its limited spectral domain,the microNIR spectrometer was successfully used as a robust PAT monitoring tool that offered a real time overview of the moisture level and allowed the supervision and control of the granulation process.
1.Introduction
Fluid bed granulation is frequently an indispensable step in solid oral dosage form manufacturing,which consists in obtaining granules by spraying a binder solution over a fluidised powder bed.The main purposes of granulation are to improve flow characteristics,blend uniformity,compression properties and to reduce the dust in the manufacturing areas [1].Like in the case of all complex manufacturing processes,there are a series of critical key parameters that need to be monitored and controlled during the fluid bed granulation to ensure the delivery of constant quality products,such as inlet air flow rate,air temperature,air humidity,spray angle,flow rate,atomisation pressure[2].The effects assessment of all mentioned variables requires high amounts of experimental work and data analysis.Moreover,materials used in pharmaceutical product preparation are also exposed to some variability.It is not uncommon for a pharmaceutical company to be forced to change the suppliers of active pharmaceutical ingredients (API) or excipients because of changes in the quality of the raw material,shortages of raw material,regulatory aspects or even natural disasters [3].To overcome these issues,companies often initiate a dual-supply strategy for each API/excipient as backup for unanticipated interruptions.However,even if the substances seem to be equivalent and meet all the pharmacopoeial specifications,they are not necessarily interchangeable:processability and critical quality attributes of the end-product may be affected[4,5].
Quality by design (QbD) approach endorsed by the drug regulatory authorities,offers a manner to identify and evaluate the sources of variability involved in a manufacturing process.After having established the quality target product profile (QTPP) and critical quality attributes (CQAs) of the desired product,Ishikawa diagrams and failure mode effects analysis (FMEA) can be used as risk assessment tools to rate the critical process parameters (CPPs) and critical material attributes (CMAs) [6,7].Out of a plethora of factors that can influence the quality of the end-product,those associated to high risk scores can be further studied through design of experiments (DoE).This strategy was successfully applied for numerous dosage forms,including immediate release tablets,prolonged release drug delivery systems,oral lyophilisates and as a result it yielded design spaces that granted process flexibility and high product robustness [8,9].Kushner et al.identified the changes caused by excipient variability in a QbD approach,however a proper mitigation strategy was not proposed to overcome the variability effects[10].Working within the design space equalizes the effects of input material variability and leads to products that consistently meet the final requirements [4].To confirm that the manufacturing process was carried out within the limits of design space and the quality of intermediate and final products,a real-time measurement of CQAs is necessary,with the possibility to perform immediate adjustment to correct the errors and prevent batch loss.This is why modern analytical technologies were developed and encouraged by regulatory authorities within the process analytical technology (PAT) initiative.PAT framework envisages the real-time information collection/gathering regarding all critical aspects of a manufacturing process,through process analysers as Raman and NIR spectrometers.PAT environment allows real-time process measurements by including the process analysers into the process stream or in its close proximity for at-line,on-line or in-line assessment[11–14].
In-line process monitoring is a promising solution that could enhance the understanding of a process and increase its reliability.But in order to be suitable for real time monitoring,an analytical method should have some distinct characteristics,such as high detection speed,the samples or the product should remain intact after the analysis,it should be process adaptable and with no interference in the processes.Near-infrared (NIR) spectroscopy holds all the mentioned characteristics,which is why it is being implemented for such purposes for a few years now [15].Several authors have already developed real time NIR monitoring methods for the moisture content assessment during fluid-bed granulation and process endpoint determination [2,16–19].The moisture level reached along the binder spraying phase plays the most important role in the formation of granules,their growth,morphology and density.This parameter is also worth monitoring further,during the drying phase,to signal the end-point of the process;otherwise,unnecessary drying could lead to a decrease of granule size through erosion[1,15,20].
Lately,due to the widely known potential of NIR spectroscopy in pharmaceutical product quality and process monitoring,considerable attention has been given to the miniaturisation and portability of the spectroscopic devices[21].The microNIR spectrometer is an ultra-compact,light weight device,suitable for integration in many process points in a non-invasive manner,but with limited spectral domain,950–1650 nm.Its use was reported for the assessment of active principles in coffee beans and acerola fruits but to our knowledge its utility in in-line monitoring of fluid-bed granulation processes was not described yet[22].
This work focused on the fluid-bed granulation process of a product with two active pharmaceutical ingredients,intended for coated tablets preparation and further transfer to industrial scale.The experiment aimed to prove that an accurate control of the critical granulation parameters can level the input material variability and offer a user-friendly process control strategy.Risk assessment strategy was applied to rank the formulation and process variables as risk sources.Following an experimental design,design space was defined for granulation process.For CQAs’assessment,in-line monitoring of granules’moisture content was performed by NIR spectroscopy coupled with multivariate data analysis.
2.Materials and methods
2.1.Materials
Different sorts of the two APIs and intragranular filler were used in the formulation.The ingredient sorts were obtained from different suppliers or the same supplier,but presenting different characteristics.All samples were of commercial grade and complied with the European Pharmacopoeia specifications.For confidential reasons,samples were codded as:Ibu A,B,Cfor ibuprofen sorts; Par A,B,Cfor paracetamol sorts andMCC A,Bfor the two tested microcrystalline cellulose sorts.
The sodium starch glycolate used as disintegrant was Explosol from Blanver (Brazil) and the chosen binder was hydroxypropyl methylcellulose-Methocel E5LV kindly donated by Colorcon(UK).
During preformulation studies,in order to gain as much information as possible about the different sorts of APIs and excipients,X-ray powder diffraction and differential scanning calorimetry studies were performed (results not shown).Those studies did not reveal any polymorphism or behaviour differences between the different sorts.Besides this,all substances were stored in the same conditions,with the intention to minimize any differences in the materials’moisture content.
2.2.Scanning electron microscopy(SEM)
Active ingredients from different suppliers were evaluated for their morphology by SEM.Samples were sputter-coated with Pt/Pd in an Agar Automatic Sputter coater (Agar Scientific,USA),then images were captured at 15 kV with a Quanta 3D FEG electronic microscope(FEI,USA).
2.3.Granulation formulation and process parameters
The same formulation was used during all granulation runs,this consisted of a mixture of ibuprofen,paracetamol,microcrystalline cellulose,sodium starch glycolate and hydroxypropyl methylcellulose,resulting into a batch size of 200 g.The binder was sprayed over the fluidised powder bed as a 10% aqueous solution in order to granulate the dry components.The experimental runs were performed in a laboratory scale fluid bed granulator (Aeromatic Strea 1,GEA,Switzerland).During each run,the powder bed was fluidised with an air flow of 3–4.5 m3/min,heated at an inlet temperature of 30°C.At first,the powder mixture was preheated and homogenised for 10 min.After this first step,the binder solution was sprayed from the top of the granulation vessel,through a 0.8 mm nozzle,with variable pressures and the spraying rates according to the experimental design specifications (Section 3.2 and Table 3).After the binder spraying,the formed granules were dried in the same apparatus,over a period of 10 min.
2.4.Sampling and evaluation of granules
Along each run,samples of~3 g were withdrawn from process,through a sampling valve placed on the side of the expansion vessel wall,at the height of the fluidised bed.A total of 6 samples were collected,3 during the binder spraying and 3 during the drying,each time at one third,two thirds and at the end of each phase.The samples moisture content was analysed off-line for loss on drying (LOD),by keeping the samples in a Venticell 55 drying cabinet (MMM Medcenter Einrichtungen,Germany)for 48 h,at 50°C.
The granulometry was measured for each end product by using a set of 8 sieves (Retsch,Germany) with sizes ranging between 100 and 800 μm.A quantity of~130 g was analysed from each granulation batch.Mean size and distribution were calculated.An important purpose of the industrial granulation process is reducing the dust [1],for this reason,one more property of obtained granulates was analysed,namely the non-granulated fraction.The particles under 100 μm found in the collecting pan after the sieve analysis were considered non-granulated fraction and expressed as dust percentage from the total amount of analysed granules.
2.5.NIR process monitoring
All granulation runs were monitored using a MicroNIR PATU spectrometer (Viavi Solutions,USA),controlled with JDSU MicroNIR Pro software.The apparatus is equipped with Linear Variable Filter technology,which allows the reduction of the devices size and the direct attachment to the expansion vessel wall.The spectrometer was attached on the expansion vessel at the same height as the sampling valve,using a custom 3D printed support to ensure direct contact of the NIR detector with the fluidised powder bed and avoiding any interferences with process.Spectra were recorded continuously at 10 s intervals,in reflectance mode,over the whole range of the spectrometer,950–1650 nm,with a resolution of 6 nm.Each spectra was the average of 200 scans,recorded with an integration time of 7 ms per scan,which resulted in a total of 1400 ms necessary for a full spectral acquisition.Until the next measurement,in the spare time of the 10 s interval the device entered automatically in stand by mode.
2.6.Analysis of the spectral data
By continuously collecting spectra during all performed runs,a large amount of spectral data was gathered,therefore requiring proper multivariate data analysis in order to obtain the desired information.For this purpose the data was imported and analysed using the SIMCA 14.0 software(Sartorius Stedim,Sweden).Principal Component Analysis(PCA) was performed in order to identify which spectral domain is specific for changes in the moisture content of the powder bed.This dimensionality reduction technique is designed to efficiently extract and describe systematic variation present in the NIR spectra,represented by the physico-chemical properties of the powder bed and to facilitate data interpretation[23,24].By analysing the loading plots and comparing them with the pre-processed spectra,an appropriate spectral domain could be identified.
The multivariate prediction model was developed using orthogonal partial least squares(OPLS)method,which had the purpose to separate X-specific spectral systematic variation into predictive and orthogonal (uncorrelated) fractions.An optimal number of OPLS factors was chosen based on the highest fraction of X variation modelled in the component(R2X),fraction of Y variation predicted according to crossvalidation,using the X model (Q2) and low root mean square error of cross-validation (RMSEcv),avoiding in the same time the overfitting of the model[24].
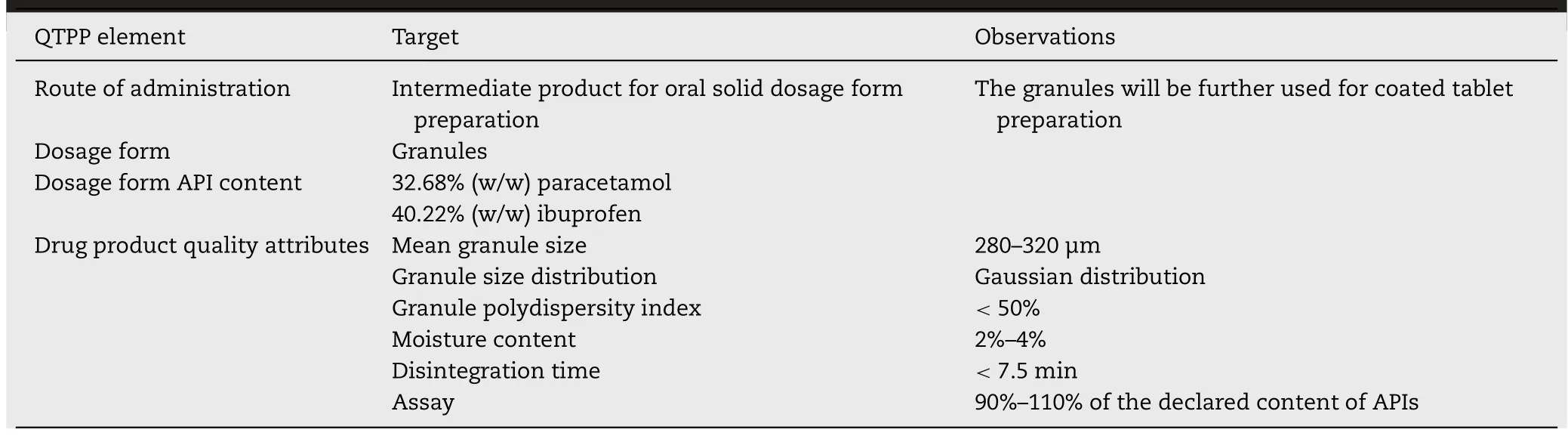
Table 1–Quality target product profile of granules containing paracetamol and ibuprofen.
2.7.Identification of granules’quality target product profile(QTPP),critical quality attributes(CQAs)and the risk analysis of CQAs
According to the International Council of Harmonisation(ICH)Q8,QTPP is the basis of product development design.Thus,defining QTPP,as shown in Table 1,was the starting point of the present research study.Further,out of QTPP,CQAs were revealed as physical characteristics that should be in appropriate limits to ensure the desired product quality.The CQAs were evaluated by means of Ishikawa diagrams,in order to identify the potential variables that could have an impact on the CQAs[6,8].
2.8.Risk assessment by failure mode effects analysis(FMEA)
Further,failure mode and effect analysis (FMEA) was applied in order to identify and prioritize the failure modes that are most probable to lead to process failure and thus to an improper product [8].The overall failure risk was assessed based on three criteria:occurrence frequency (O),effect severity (S) and detection difficulty (D),each of them ranked on a scale from 1,as the low level of the mentioned criteria to 5,as the high level of the criteria.The final score,the risk priority number(RPN)was obtained by the multiplication of the scores registered for each of the three criteria [25].CPPs and Critical Material Characteristics(CMCs)that met the highest risk scores were studied in detail in DoE.
2.9.Design of experiments
Before the development of an appropriate experimental design which would allow the in depth study of the process,some preliminary experiments were performed in order to establish a stable and reliable granulation process (data not shown).This part aimed to define the granules composition,batch size and the basic process parameters.The MODDE 11.0 software(Sartorius Stedim,Sweden)was further used in order to develop a D-optimal DoE,which allowed the introduction of anticipated variability that could normally occur in an industrial scale manufacturing process and also the study of the effects of such variability.A D-optimal approach is based on the selection of experimental runs so that they span the largest possible volume of the variability matrix and for this reason,it is recommended when combinations of qualitative and quantitative multilevel factors are studied in the same experimental design[26].
3.Results and discussions
3.1.QTPP,CQAs,CPPs and FMEA
A team of researchers with in-depth knowledge on the fluid bed granulation process was brought together.During several discussion sessions,QTPP of the granules was established according to the type of dosage form and its intended use,for the preparation of coated tablets,as shown in Table 1.The selected CQAs that emerged from QTPP were those susceptible to variations during a large scale manufacturing process:granule average size,polydispersity index and humidity.
Further,risks associated with every step of process,from the raw materials properties to the final granules characteristics were identified,analysed and evaluated in detail [25].The variables related to granule formulation,manufacturing process,analytical methods and equipment performance were summarised in an Ishikawa diagram(Fig.1).CPPs and CMCs were further analysed using FMEA method for their associated risk,revealed as RPN(Table 2).
Once the qualitative and quantitative composition of a pharmaceutical product is established,few changes can occur with respect to formulation; however,variations in particle size,shape,polymorph of APIs or excipients could appear when switching suppliers.Such variability could influence the quality of granules,especially when the major components are concerned.Therefore,in our particular case,FMEA revealed that variations in the sort of APIs or filler could exert a higher risk (RPN=60) than in the disintegrant agent or the binder(RPN=24 and 16 respectively).
Among the process parameters,the mixing phase was considered less critical to the process,compared to the spraying and the drying phases.The inlet air temperature during mixing/heating of the powder blend was considered less important due to the fact that the binder solution is added only when the powder reaches an established temperature.The binder spray rate and atomising pressure were evaluated as the most hazardous,as their values could change because of equipment malfunction or human error and they have a high impact on granule size and distribution.The drying parameters are also critical to the product quality:if the drying time is exceeded,granules could become friable and erode with impact on flow properties.These phenomena can be prevented by NIR monitoring,thus they were ranked lower than the granulation step,with RPN 48.An experimental design was developed based on the parameters that expose product quality to the highest risk,with RPN of 60:the APIs and filler sorts,binder spray rate and atomising pressure.
3.2.Design of experiments
When developing a D-optimal DoE,that includes quantitative as well as qualitative factors,the selection of experiments included in the design matrix is critical and needs to be decided based on scientific means.In this case,two evaluation criteria had to be considered:condition numberandG-efficiency.Thecondition numberassesses the sphericity and symmetry of the design,representing the ratio of the largest and smallest values of the variability matrix.Its ideal value is 1,representing an orthogonal design,the orthogonality of the design evolving inversely proportional with this parameter.In the case of an optimisation design with a mixture of quantitative and qualitative factors,thecondition numbervalue could increase substantially,hence a DoE with a condition number<8 is still considered very good.TheG-efficiencyis a criteria that expresses the design performance by comparing it to the performance of a fractional factorial design,being expressed in perfectness.For a high quality,reliable D-optimal DoE,aG-efficiencyabove 60%-70%is recommended[26].
The risk analysis indicated that supplier changes can occur during the life span of a product,so most of the companies rely on a dual-supply strategy that comes with a variation in the physical characteristics of the materials.Three CMCs,namely the different sorts of APIs and filler were selected to be studied as qualitative variables of the DoE.
Based on the Ishikawa diagram and the conducted risk assessment,two quantitative variables represented by the binder spraying parameters were chosen for further investigation.Their control was meant to level the effects of the qualitative variables.
The DoE was developed based on the data presented in Table 3,consisting in a total amount of 39 experimental runs,including 4 centre points(Table S1).
The generated DoE registered aG-efficiencyvalue of 70.21%and acondition numberof 7.08.The studied responses were the moisture content levels of the samples withdrawn during each experimental run,the average size and the nongranulated fraction of the obtained granules (see Section 2.4).For the experimental design matrix and results,consult Table S1.
The registered response values were centralised,introduced into the design matrix and further,the fitting of the experimental data was accomplished by applying multiple linear regression(MLR)and was evaluated using the standard,most reliable statistical parameters,namelyR2-goodness of fit,Q2 -goodness of predictionand the response reproducibility.R2reflects the fraction of the response variation explained by the model,whileQ2gives the model prediction capacities.The model reproducibility is calculated and represented strictly based on the replicates specified in the design matrix.A good fitting is represented by high values of the model performance indicators,as close to one as possible.Furthermore,for a valid model,the difference between R2and Q2should not exceed 0.2–0.3,and the reproducibility should be well over 0.5[26].


Table 3–Variables of the experimental design.
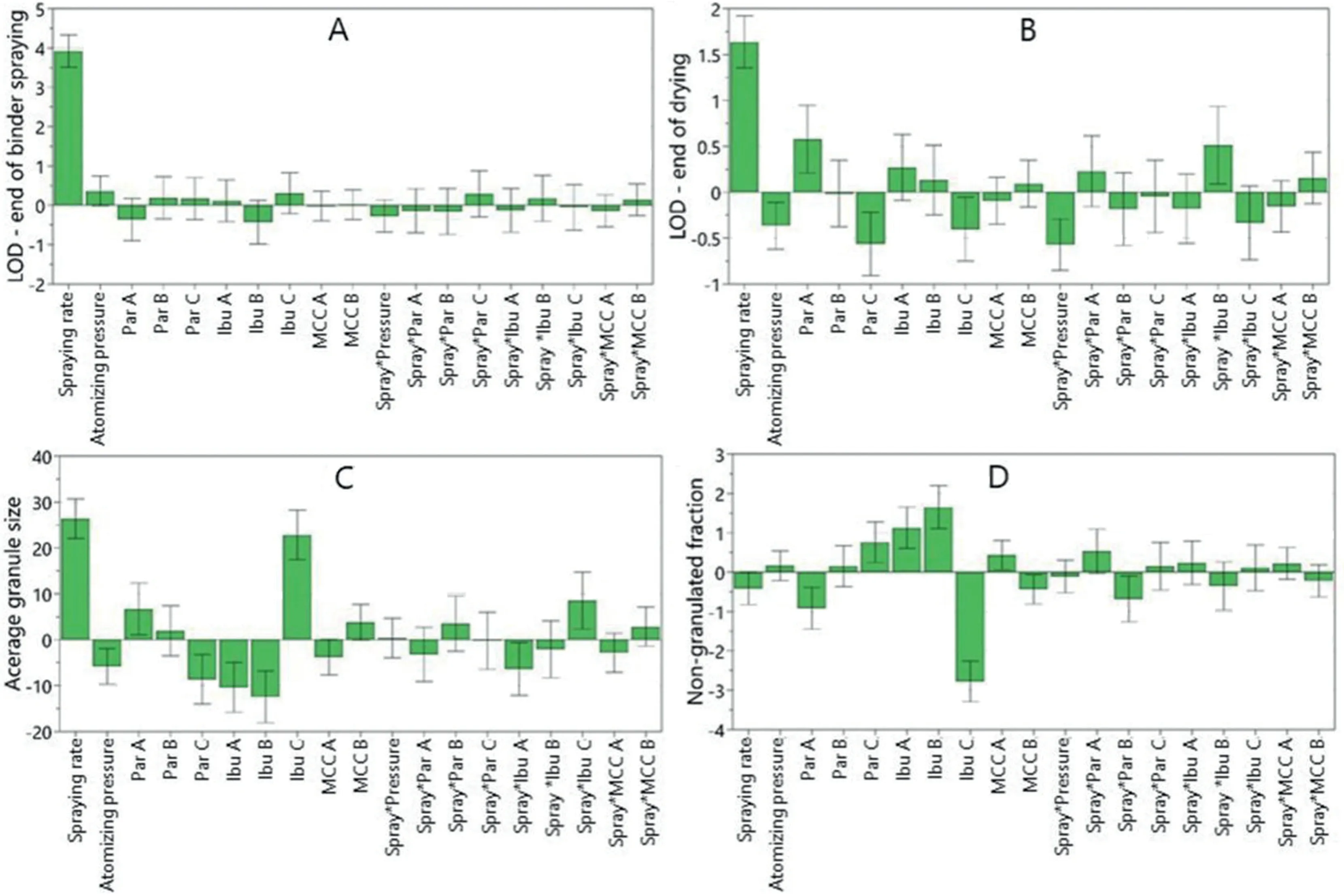
Fig.2–Scaled and centred coefficient plots–factor influence over the:(A)LOD%at the end of the binder spraying phase;(B)LOD%at the end of the drying process;(C)average size of the granules;(D)the non-granulated fraction.
The summary of fit and the statistical parameters were calculated based on the experimental design data.The chosen model presented excellent quality,with R2greater than 0.86 and Q2greater than 0.64 for all the eight studied responses.The model validity was also confirmed by a reliable reproducibility,with statistical values of over 0.84 for all studied responses/outputs(statistical values included in Table S2).
3.3.Independent variables effects on the process and granules properties
Based on the developed DoE model,regression coefficients were automatically calculated for the studied output variables.Fig.2 presents the scaled and centred coefficient plots which describe the influence of the studied factors over the moisture levels registered at the end of the binder spraying phase and drying phase,i.e.end of the process,and over the average granule size and non-granulated fraction of each batch.
The first important observation is that the binder spraying rate had the highest effect over the average granule size and as expected,an increase of the spraying rate would also lead to an increase of granule size [20].Naturally,a higher spraying rate will lead to higher moisture levels along the process,which promotes the granule forming and growth,especially when the formulation contains ingredients that are soluble in the spraying solvent,as it is the case.

Fig.3–Scanning electron microscopy micrographs for the 3 studied ibuprofen sorts.
The influence of the second studied process parameter,the atomising air pressure used for the spraying of the binder solution,seemed to be minor,but still significant,representing that an increase of atomising pressure would reduce the average granule size.By increasing the atomising pressure,the size of the formed droplets decreases,favouring their evaporation before they actually get in contact with the fluid powder bed,thus limiting the binder effect[27].
Neither of the two studied process parameters seemed to have statistical significant influence over the non-granulated fraction size of each batch,which meant that only the formulation ingredients needed to be considered in this case.
Regarding the sort of paracetamol used in the formulation,it resulted that usingPar Awould lead to obtaining slightly larger granules,with a lower non-granulated fraction,whilePar Cpresented opposite effects.The actual total granule size variation was about 10–20 μm.In the present model,this global effect is shared between all the threeParsorts,meaning that the sum of model coefficients equals zero,therefore the coefficients having opposite signs does not imply a substantial change in terms of particle size.However,this API sort presented a positive influence over the LOD% registered at the end of the process,which translates through a higher moisture content of the final product.On the other hand,Par Csignificantly decreased the final LOD%,due to its slightly lower bulk density (declared in the product’s bulletin of analysis),favouring the final drying process,thus representing a viable alternative to a more reliable process.
The most influential qualitative factor was the sort of ibuprofen,the strongest effect in the coefficient plot appearing forIbu C.This sort appeared to increase the granulometry,substantially reducing the dust fraction of the final product and,in the same time,favouring the drying process.The two other API sorts tested in the formulation had opposite effect compared toIbu C,reducing the average granule size and increasing the dust fraction.
In order to identify the occurring phenomena causing those substantial differences,scanning electron microscopy was performed,the obtained images are shown in Fig.3.It is well known that ibuprofen comes as acicular crystals,fact also observed in the figure for the first two sorts.TheIbu Chad homogenous,large particles,with sizes averaging 500 μm;Ibu Ahowever,presenting a much higher dispersity of particles size and shapes.In the case ofIbu B,which was a micronised sort,the particles were much finer and the acicular shapes can not be identified any more,most probable being broken through the micronisation process.
The larger particles size ofIbu Cis the property which aids to dust reduction,the particles being already larger than the considered non-granulated fraction of under 100 μm.The granule growth phenomenon caused byIbu Ccould be explained by the particle size and shape differences between it and the particles of all three studied paracetamol sorts(Fig.S1).It is probable that the larger,acicular ibuprofen crystals would act as cores,on whose surface adhere the much smaller,irregular paracetamol particles,thus forming granules.
The high influence of the ibuprofen sort over the studied responses suggests that this would be an important formulation factor that needs to be taken into consideration when adapting the CPPs.The influence of the MCC sort used in the granules composition did not seem to have statistically significant effects,nor had the factor interactions that can be observed in Fig.2.Still,those factors aided the fitting of the experimental data,assuring the obtaining of a statistically valid model.
Up to this stage of the research,results showed that the use of apparently interchangeable APIs and filler sorts that comply with pharmacopoeial specifications,lead to different end-product critical attributes.Thus,in order to maintain the final product’s desired characteristics,the critical process parameters and the Design Space should be adapted accordingly.
3.4.NIR monitoring method development
Parallel with the experimental design analysis,a noninvasive NIR method was developed for the in-line monitoring of the fluid bed granulation process by predicting the moisture content.
At first,all the acquired NIR spectral data was loaded into the SIMCA software and pre-processed by applying the first Savitzky–Golay derivative which removed the baseline shifts,reduced the additive effects and improved the overall spectral resolution[28].
The raw recorded reflectance spectra are illustrated in Fig.4A,while Fig.4B illustrates the spectral data pre-processed with the first Savitzky–Golay derivative.The highest spectral intensity variation can be noticed around the first–OH group overtone,around 1390–1450 nm.Especially in this specific region,the 1st derivative pre-processed spectral intensity increases with the increase of water content registered during the binder spraying and decreases when the water content reduces during the drying of the product[2].

Fig.4–Raw(A)and pre-processed first derivative(B)spectra registered during the performed granulation runs.

Fig.5–LOD%measured vs.NIR predicted values for 3 granulation runs performed with different binder spraying rate.Abbreviations:N11,N14,N32–experimental runs performed in different conditions,according to the Design of Experiments.
A PCA model was developed including only the 1300–1600 nm spectral domain,situation where only one principal component(PC)was sufficient to explain 97%variability.
Further,for the moisture prediction model development,the 39 performed and recorded experimental runs were divided into two groups,chosen so that each of the two groups covered the entire studied variability.The first group contained 25 runs and was used for the multivariate calibration of the model,the second group contained the rest of 14 experiments and was used for validation.The calculated OPLS model included two factors,one for each fraction of the model.For the predictive fraction,high statistical parameters could be registered,with values of 0.937 for R2X and 0.906 for Q2.On the other hand,the orthogonal(uncorrelated)fraction registered only a low R2X value of 0.057 but which improved the overall model quality.The RMSEcv calculated for the model with 2 factors was 1.55.The second group,formed from the remaining 14 experimental runs,was used for the validation of the OPLS prediction model.The method was validated with a good prediction recovery,close to 100% and low relative bias.
The measured LOD% values were plotted against the NIR predicted values; Fig.5 illustrates 3 of the monitored granulations,framed in the validation data set,preformed using different binder spraying rates.The correlation coefficient calculated between the measured LOD% and the NIR predicted values was greater than 0.98 for all 39 granulation runs,which shows good predictive capacities.
Even though the microNIR spectrometer has limited spectral domain compared to FT-NIR spectrometers,the development of accurate moisture content prediction method was possible,thus proving to be a powerful tool for pharmaceutical applications.
3.5.Design space and process optimisation
The DoE approach lead to an in depth understanding of the variables’influence over the process.Further,based on the initial investigation domain,the statistics software was used to generate a design space by introducing a set of constraints according to the QTTP [29,30].The pre-set ingredients and the CQAs targets and variation intervals are presented in the upper part of Table 4.The intermediate LOD% limits were set based on the values obtained along the performed experimental runs with set process parameters inside the design space.The final LOD% and average granule size were set based on the QTPP specifications,and the non-granulated fraction was minimised.Depending on APIs and filler sort,each of them varied on 3,3 and respectively 2 levels,18 Design Spaces could be generated.As an example,three of them as a function ofIbusort are shown in Fig.6.Ibuwas chosen for its high impact over all studied responses.ThePar Csort was kept constant for its positive influence over the granule drying phase,whileMCC B,due of its tendency to aid the granule growth,reducing the dust fraction(see Section 3.3).
Each of the three plots includes a different area where the specified constraints are fulfilled,highlighting that the same characteristics of granules can be obtained with different API sorts,by rigorously controlling the CPPs throughout the process.The different acceptable areas of the three Design Spaces plotted in Fig.6 can be easily explained based on the influence of the different ibuprofen sorts described in Section 3.3 and depicted in Fig.2.As it can be observed,the first two Design Spaces(Fig.6A and B)present similar acceptable areas– by using a formulation withIbu AorIbu B,the granulation process needs to be performed with high spraying rate and low atomising pressure in order to ensure the required granulation conditions,leading to a final product which would comply with the QTPPs specifications.The high spray rate is needed to compensate the tendency ofIbu AandIbu Bto provide smaller granules and a higher dust fraction.Moreover,the acceptable area of theIbu Bformulation is slightly smaller than the onecalculated for theIbu Aformulation,fact explained based on theIbu Btendency to reduce the granule size and to increase the non-granulated fraction even more than Ibu A,thus lowering the probability of obtaining appropriate granules.

Table 4–CQAs and results of the optimal granulation process.
Fig.6–Design Spaces for granulation processes that provide optimal granules,adapted according to the sort of ibuprofen used in the formulation.(A):Ibuprofen A(Ibu A);Paracetamol(Par C);Microcrystalline cellulose(MCC B);(B):Ibuprofen B(Ibu B);Paracetamol(Par C);Microcrystalline cellulose(MCC B);(C):Ibuprofen C(Ibu C);Paracetamol(Par C);Microcrystalline cellulose(MCC B).

Fig.7–Moisture content measured and predicted along the optimal granulation process.
By usingIbu Cin the granules’formulation,the process parameters acceptable variation range is completely different compared to the ones obtained for the previously twoIbusorts.This sort substantially increases the particle size and reduces the dust fraction,favouring the drying process of the final product.The use ofIbu Cin the formulation allows the performing of a successful granulation with average binder spraying rate,tolerating a variation of the atomising pressure over almost all the studied domain.
To confirm the robustness of the model and NIR monitoring method,thedesign space explorerfunction of the software generated the following process parameters:spraying rate of 11 g/min and atomising pressure of 0.6 atm for the qualitative formulation indicated in Table 4.The aforementioned granulation process was carried out and simultaneously monitored using the same NIR setup.The measured vs.predicted intermediate and end-product characteristics are listed in the lower part of Table 4.Fig.7 pictures the LOD%evolution throughout the granulation process:NIR predicted LOD% values overlapped with the DoE predicted ones and were confirmed by the experimental measurements.All the outputs fell within the set intervals,hence the DoE could be considered valid for further use for the optimisation of process parameters according to the desired formulation factors.Moreover,as a non-invasive in-line method,the developed NIR spectroscopic technique yields LOD% values each 10 s of the process,compared with the classical measurements which allowed the analysis of a total of 6 samples per process.
4.Conclusion
The study demonstrated that the fluid bed granulation can be adapted through an accurate control of CPPs to eliminate the variability brought by possible API or excipient changes.Therefore,assuring a consistent end product quality and maintaining its characteristics within the QTPP is possible with a constant monitoring of the manufacturing process.
Practically,a multiple supplier strategy for all used substances prevents unexpected shortages in the manufacturing process.A simple QbD research provides enough information to handle ingredient variability in order to ensure constant product quality which falls into the limits of the Design Space.The developed model allows the easy and timely adaptation of CPPs as a function of the selected excipient,improving the process control.Process adjustment can be performed immediately,without the need of a process variation approval from the authorities.
However,if a new API or excipient sort,which has not been studied from beginning,needs to be introduced in the formulation,some additional experimental runs need to be performed.This does not mean repeating the entire study,sometimes the influence of a new substance sort over the endproduct’s critical attributes,may be similar to one that was already studied.This observation highlights how important it is for the manufacturer to choose reliable suppliers.
Moreover,the developed in-line microNIR monitoring method offers a real time overview of the moisture level and allows its maintenance in the desired intervals,thus a reliable PAT tool for the supervision and control of the granulation process.
Such an approach grants that the quality of the medicine gets not only to be tested,but built into the product.
Declaration of interest
The authors declare that there is no conflicts of interest.
Acknowledgements
The authors are thankful to S.C.Laropharm SRL (Bucharest,Romania)for kindly providing the APIs and excipients used in the study.Funding:This work was supported by the Romanian National Authority for Scientific Research and Innovation,CNCS-UEFISCDI[project number PN-III-P2-2.1-BG-2016-0201].
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2019.03.003.
 Asian Journal of Pharmacentical Sciences2020年4期
Asian Journal of Pharmacentical Sciences2020年4期
- Asian Journal of Pharmacentical Sciences的其它文章
- Biointerface engineering nanoplatforms for cancer-targeted drug delivery
- Tumor microenvironment responsive drug delivery systems
- Advanced delivery strategies facilitating oral absorption of heparins
- Engineered targeting tLyp-1 exosomes as gene therapy vectors for efficient delivery of siRNA into lung cancer cells
- Let-7 miRNA and CDK4 siRNA co-encapsulated in Herceptin-conjugated liposome for breast cancer stem cells
- In situ apolipoprotein E-enriched corona guides dihydroartemisinin-decorating nanoparticles towards LDLr-mediated tumor-homing chemotherapy
