Quantitative prediction of the bitterness of atomoxetine hydrochloride and taste-masked using hydroxypropyl-β-cyclodextrin:A biosensor evaluation and interaction study
aDepartment of Pharmaceutics,College of Pharmacy,Shandong University,Jinan,250012,China
b Department of Pharmaceutical Development,Shandong Dyne Marine Biopharmaceutical Limited Corporation,Rongcheng,264300,China
Keywords:Atomoxetine HCl E-tongue Quantitative prediction Host–guest interaction
ABSTRACT The bitterness of a drug is a major challenge for patient acceptability and compliance,especially for children.Due to the toxicity of medication,a human taste panel test has certain limitations.Atomoxetine hydrochloride (HCl),which is used for the treatment of attention deficit/hyperactivity disorder (ADHD),has an extremely bitter taste.The aim of this work is to quantitatively predict the bitterness of atomoxetine HCl by a biosensor system.Based on the mechanism of detection of the electronic tongue (Etongue),the bitterness of atomoxetine HCl was evaluated,and it was found that its bitterness was similar to that of quinine HCl.The bitterness threshold of atomoxetine HCl was 8.61 μg/ml based on the Change of membrane Potential caused by Adsorption (CPA)value of the BT0 sensor.In this study,the taste-masking efficiency of 2-hydroxypropyl-βcyclodextrin (HP-β-CyD) was assessed by Euclidean distances on a principle component analysis(PCA)map with the SA402B Taste Sensing System,and the host–guest interactions were investigated by differential scanning calorimetry (DSC),powder X-ray diffraction(XRD),nuclear magnetic resonance (NMR) spectroscopy and scanning electron microscopy(SEM).Biosensor evaluation and characterization of the inclusion complex indicated that atomoxetine HCl could actively react with 2-hydroxypropyl-β-cyclodextrin.
1.Introduction
Despite its unpleasant feeling,a bitter taste protects organisms from ingesting poisonous substances [1].The pharmaceutical industry is vulnerable to bitterness.Indeed,most active pharmaceutical ingredients have a terribly bitter taste that has a negative impact on patient acceptability and compliance,especially for pediatric formulations,as children are more bitter-sensitive than adults[2,3].
Due to the toxicity of bitter compounds,computational prediction andin vitrobiological evaluations have become considerably more important thanin vivoapproaches for bitterness assessment.For example,the Bahia group[4] investigated three structure-based computational approaches,including ligand-based methods,structurebased methods and machine learning techniques,which could predict the bitterness of most compounds [5,6].Although all of these methods worked well for prediction,there was a lack of access to the quantitative evaluation of bitterness [7].The electronic tongue (E-tongue) taste system has been widely used for the evaluation of the bitterness of medicines [8,9].Two types of E-tongues are commercially available,theα-Astree sensor (Alpha M.O.S.,Toulouse,France) and the SA402B/TS-5000Z sensor (Insent,AtsugiChi,Japan).Due to its reliability (in vitro-in vivocorrelation) and precision(reproducibility and repeatability)[10,11],the Insent System has been widely employed to quantify the bitterness intensity of pharmaceuticals with artificial lipid membrane sensor technology similar to a human tongue taste cell.
Atomoxetine HCl (Fig.1) is a selective norepinephrine reuptake inhibitor and has been approved by FDA asStrattera®for the treatment of attention deficit/hyperactivity disorder(ADHD) in children and adults [12].It is well known that atomoxetine HCl is a highly bitter drug[13,14],and therefore,several novel technologies have been developed to reduce its bitterness.A taste-masked multi-particulate system of atomoxetine HCl based on a drug-resin(Kyron T134)complex was prepared [15],and chewable dispersible taste-masked tablet formulations of atomoxetine HCl with Eudragit EPO were also formulated[16].Based on its“child friendly”clinical requirements,atomoxetine HCl oral solution bioequivalent to atomoxetine HCl capsules was developed by Eli Lilly Company [17].To mask the bitter taste,lots of sweeteners and raspberry flavor (14.7 mg/ml) were used in atomoxetine HCl oral solution (4 mg/ml) [13].However,the addition of sweeteners and flavors is not effective enough to mask the extremely disgusting taste of the atomoxetine HCl after swallowing.Cyclodextrins have also been widely used in the pharmaceutical industry due to their cavities,which can form inclusion complexes to mask the bitterness of drugs [18–20].Especially,HP-β-CyD (Fig.1),which has good solubility,has been applied to mask the bitter taste of lidocaine HCl [21],meloxicam[22]and diclofenac sodium[23].
In this study,the quantitative bitterness of atomoxetine HCl was measured by the E-tongue system using quinine HCl,a typical standard bitter substance,as a reference [24–27].In order to mask the bitter taste of atomoxetine HCl,the HP-β-CyD/atomoxetine HCl inclusion complex was prepared and characterized.The efficiency of bitterness suppression by HP-β-CyD was evaluated,and the host-guest interactions were studied by differential scanning calorimetry (DSC),powder X-ray diffraction (XRD),nuclear magnetic resonance(NMR)spectroscopy and scanning electron microscopy(SEM).
2.Materials and methods
2.1.Materials
Atomoxetine HCl was provided by Shandong Dyne Marine Biopharmaceutical Co.,Ltd.(China).HP-β-CyD (molecular mass=~1370 g/mol,degree of substitution (NMR)=4.07,purity (HPLC)=97.7%) and quinine HCl dihydrate were purchased from Aladdin.Potassium chloride (KCl),tartaric acid,potassium hydroxide,and hydrochloric acid (32%) were purchased from Sino Pharm Chemical Reagent Co.,Ltd.(China).
2.2.E-tongue evaluation
A Taste Sensing System (SA402B,Intelligent Sensor Technology Inc.,Japan) equipped with four liquid membrane sensors (three represent bitterness:BT0/AN0/C00,and one represent astringency:AE1) and two corresponding reference electrodes was used for quantitative evaluation.The bitterness of cationic (basic) substances such as famotidine was evaluated by an AN0 sensor,and a BT0 sensor was used for the bitterness evaluation of HCl salts,for example,alkaloids (quinine HCl).The bitterness of anionic (acidic)substances such as iso-alpha acid was evaluated by a C00 sensor[28].
Ten different concentrations of atomoxetine HCl solution(0.003–1.0 mg/ml),quinine HCl solution (0.004–1.36 mg/ml)and HP-β-CyD solutions (0.01–4.729 mg/ml) were prepared in deionized water.All the concentrations of atomoxetine HCl solution,quinine HCl solution and HP-β-CyD solution were at the same molarity(ranging from 0.010 to 1.028 mM).

Fig.1–Chemical structure of(A)atomoxetine HCl and(B)2-hydroxypropyl-β-cyclodextrin.
Table 1–Procedure for measuring dilute solutions.
The taste-masked formulations were prepared by mixing atomoxetine HCl and HP-β-CyD at 1:1,1:2,1:3,1:4 and 1:5 molar ratios.All samples were prepared based on 10 mM potassium chloride solution,and each sample was measured four times in a row.The data of the first cycle were deleted because measurement data of the first cycle tends to be higher than that of other cycles.The measurement procedure for each cycle was the same as that displayed in Table 1.The cleaning 2,3,4,5 solutions and conditioning solution,CPA (Change of membrane Potential caused by Adsorption) solution in Table 1,had the same composition,containing 0.3 mM tartaric acid and 30 mM potassium chloride.The cleaning 1 solution (in Table 1) was defined as negatively charged membrane washing solution(containing 30% ethanol and 100 mM hydrochloric acid) and positively charged membrane washing solution (containing 100 mM potassium chloride and 10 mM potassium added to 30% ethanol) for negatively charged membrane sensors BT0/AN0 and positively charged membrane sensors C00/AE1,respectively.The sensor output,denoted as relative value(R) for the initial taste and after taste as a CPA value,was determined in relation to the preliminary measured sensor response to the reference solution(Vr)[10].

The PCA map,a powerful data analysis method that can easily extract information from large datasets,was used to evaluate taste-masked formulation with HP-β-CyD.The PCA map containing principal component 1 (PC1) on thex-axis and principal component 2 (PC2) on they-axis is presented in a two-dimensional graph.The Euclidean distances (Eq.(3)) on the PCA map were measured to evaluate the tastemasking efficiency.Data processing,graphical representation and statistical analysis were carried out using Origin Pro 2018(Origin Lab Corporation Northampton,MA,USA).

2.3.In vivo evaluation:human taste panel assessment
In order to evaluate the results of the E-tongue,a human taste panel assessment was conducted through a singleblind study.Anin vivostudy was performed to evaluate the bitterness level of each formulation by six well-trained and healthy volunteers (age:26–38 years,gender:3 males and 3 females).Informed consent from all volunteers was obtained,and all the subjects were trained systematically according to the section“2.8.15”of European Pharmacopoeia 9.0 before initiating the study.Pure atomoxetine HCl solution and atomoxetine HCl/HP-β-CyD solution containing equal amounts of atomoxetine HCl (equivalent to approximately 1.028 mM) were prepared.All solutions were randomly taken and held in the mouth for 30 s by the subjects,and the bitterness level was scored from 0 to 4 by subjects,0 standing for no taste,1 for threshold,2 for slightly bitter,3 for bitter,and 4 being remarkably bitter.Between each test interval,participants were required to rinse their mouths well with mineral water.All scores for each sample from the six subjects were compared by at-test,with a significance level ofP <0.05.
2.4.Study on the interaction between atomoxetine HCl and HP-β-CyD
2.4.1.Preparation of inclusion complex
Different molar ratios(3:7,5:5,7:3)of atomoxetine HCl and HPβ-CyD were dissolved in deionized water at room temperature(25±1 °C),and then a solid form of the atomoxetine HCl/HPβ-CyD inclusion complex was obtained by freeze-drying over 48 h(Alpha 1–2 LD plus,Martin Christ,Germany)at 223 K and 0.03 mbar[29,30].
2.4.2.Differential scanning calorimetry
DSC measurements of pure drug,HP-β-CyD,physical mixture and inclusion complexes were performed using a DSC 1 Mettler Toledo instrument (Mettler-Toledo AG,Analytical,Schwerzenbach,Switzerland).STAReSoftware was used to compare the variation in the scans of pure drug and complexes.The samples (3–5 mg) were placed in aluminum pans and then heated from 25 to 350°C at a rate of 10°C/min,using an empty pan as a reference.
2.4.3.Powder X-ray diffraction
Powder XRD diffractograms of pure drug,HP-β-CyD,physical mixture and inclusion complexes were recorded using an X-ray powder diffractometer (Miniflex diffractometer 600,Rigaku,Japan).The diffraction patterns of atomoxetine HCl,HP-β-CD,the physical mixtures and atomoxetine/HP-β-CD inclusion complexes were obtained at angles of 5°–60°(2θ).
2.4.4.Scanning electron microscopy
The surface morphologies of pure drug,HP-β-CyD,physical mixture and inclusion complexes were observed by SEM(S-4800,Hitachi,Japan)with an excitation voltage of 5 KV and magnification factor of 500.
2.4.5.Nuclear magnetic resonance
To further study the interaction between the guest and cyclodextrin molecules,1H spectra were recorded by a Bruker Avance Ⅲ 600 MHz NMR spectrometer.Pure drug,HP-β-CyD and inclusion complexes were dissolved in DMSO–d6.

Fig.2–Radar map of the electronic sensor responses of(A)atomoxetine HCl and(B)quinine HCl at different concentrations.
3.Results and discussion
3.1.Quantitative evaluation of the bitterness of atomoxetine HCl
The quantitative evaluation of the bitterness of atomoxetine HCl was performed by the biosensor system for the first time.The responses of AN0,BT0,C00 and AE1 sensors corresponding to the different concentrations of atomoxetine HCl (0.003–1.0 mg/ml) are shown in Fig.2A.As the concentration of atomoxetine HCl increased,there was an obvious enhancement of the bitterness sensor responses(AN0,BT0) for initial taste (relative value),resembling the sensor output for after taste (CPA value) of the AN0 and BT0 sensors.The sensor output of the astringency sensor AE1 was weak,as was that of the C00 sensor.In addition,the BT0 and AN0 sensor responses were significantly increased at low concentrations from 0.003 to 0.3 mg/ml relative to other concentrations.
In order to understand the bitterness of atomoxetine HCl intuitively,the sensor outputs of quinine HCl dihydrate,as described in Fig.2B,were also measured as a reference drug.The trends of the responses of the BT0 and AN0 sensors between relative values and CPA values were similar to that of atomoxetine HCl.There was no change in the sensor output of the astringency sensor AE1 as well as the C00 sensor for both relative value and CPA value with increasing concentrations.The responses of the BT0 and AN0 bitterness sensors increased significantly at low concentrations from 0.004 to 0.41 mg/ml.
Compared to the responses of quinine HCl,the sensor outputs of the AN0 sensor for both the relative value and CPA value of atomoxetine HCl were greater than those of quinine HCl,as with the relative value of the C00 sensor (Fig.3B and 3C).Regarding the outputs of the BT0 sensor for relative value,the response trend was divided into two parts as shown in Fig.3A,and the sensor outputs of quinine HCl were higher at the concentrations from 0.01 to 1.028 mM and lower at the concentrations from 1.028 to 3.427 mM than atomoxetine HCl.This indicates that the bitterness of atomoxetine HCl is greater at high concentrations for relative value under the BT0 sensor and less at low concentrations than that of quinine HCl.Based on the detection mechanism of Etongue,the sensor response to bitter compounds requires that bitter materials are adsorbed on the hydrophobic part of the membrane,causing a change in membrane potential by changing the charge density [11,31–33].It was clearly shown that quinine was adsorbed more quickly at low concentrations than atomoxetine because of its stronger hydrophobicity.With increasing concentrations,the space steric hindrance mainly influenced the adsorption capacity and rate.The CPA value of the BT0 sensor for atomoxetine was lower than that of quinine.It was also suggested that atomoxetine should be removed more quickly from the lipid membrane due to its smaller molecular size and hydrophobicity.There was no difference between atomoxetine HCl and quinine HCl under the C00 sensor for CPA value,nor for the AE1 sensor for both relative value and CPA value,and especially,no difference for the C00 sensor for CPA value and the AE1 sensor for relative value(Fig.3C and 3D).
The concentration dependence of bitterness was also assessed by changing the concentration of quinine HCl and atomoxetine HCl.Based on classical Gouy–Chapman theory and the Poisson–Boltmann equation [11],the sensor outputs should have a linear relationship with the concentration according to the Nernst equation (4) [10].Calibration curves and the regression formula of quinine HCl are shown in Fig.4 and Table 2.

whereU:electrode potential,U0:standard electrode potential,R:gas constant,T:temperature (K),z:ionic valence of the substance,F:Faraday constant,fi:activity coefficient of the substance,ci:concentration of the substance.
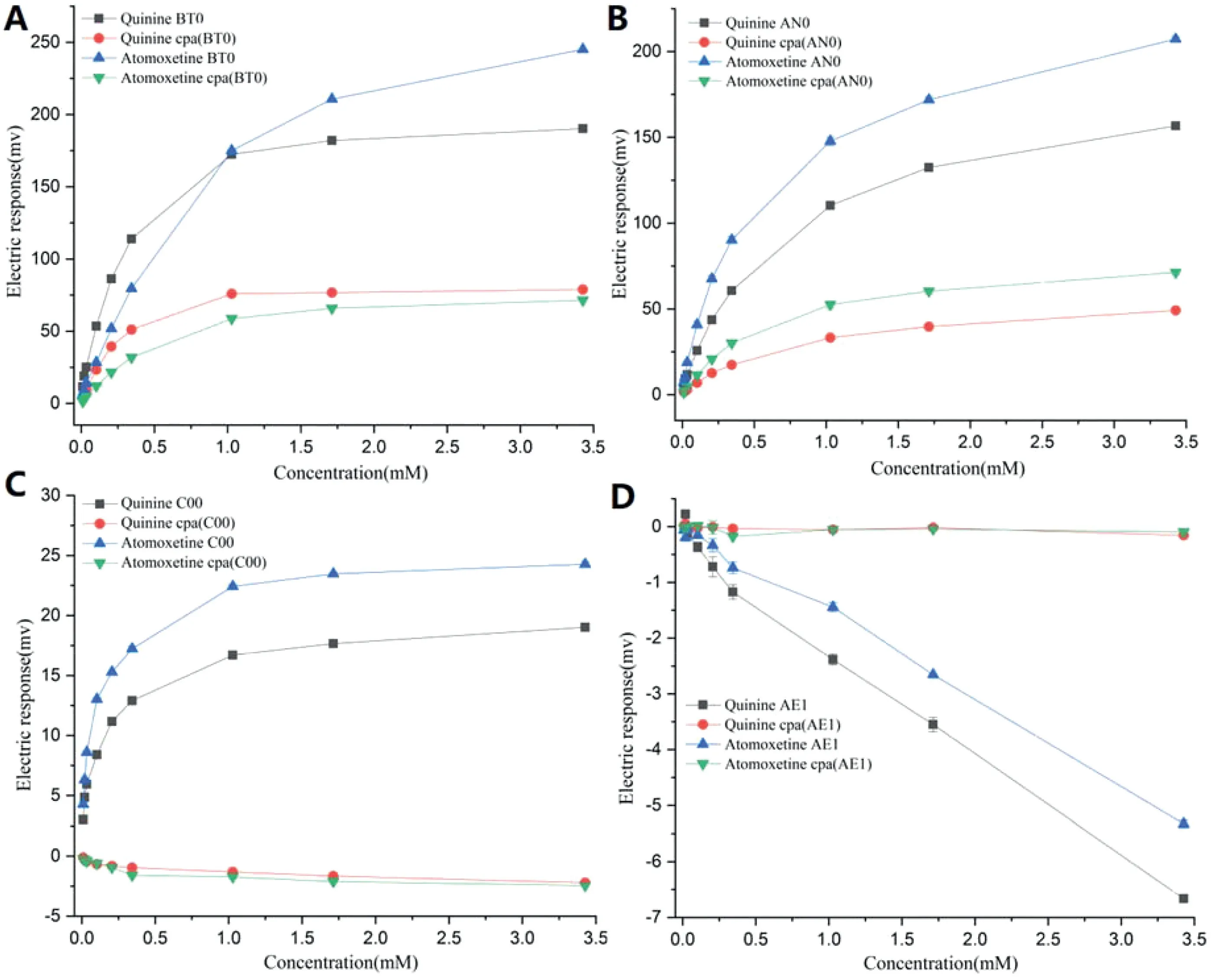
Fig.3–Electronic sensor response of quinine HCl at the same concentration compared to atomoxetine HCl.(A)BT0 sensor relative response and CPA value,(B)AN0 sensor relative response and CPA value,(C)C00 sensor relative response and CPA value,(D)AE1 sensor relative response and CPA value.
For the relative value of the BT0 sensor,when 28.44 mV at 0.03 mg/ml (0.103 mM) atomoxetine HCl was substituted into the equation “y=170.15+119.72logx” in Table 2,the corresponding concentration of quinine HCl was calculated as 0.065 mM.That is to say,the bitterness of atomoxetine HCl at 0.103 mM was equivalent to that of quinine HCl(0.065 mM).It was observed that the bitterness of quinine HCl was greater than that of atomoxetine HCl at concentrations from 0.1 mM to 1.0 mM under the BT0 sensor (Table 2).However,the trends of the responses under the AN0 and C00 sensors were different from the BT0 sensor; the BT0 sensor had the same outputs for atomoxetine HCl at lower concentrations as the corresponding quinine HCl (Table 2).It was suggested that atomoxetine HCl had more bitterness at the concentrations from 0.103 mM to 3.427 mM under the AN0 and C00 sensors.
As described above,both the BT0 and AN0 sensors are effective at detecting the bitterness of cationic substances,especially the BT0 sensor,which is used for detecting the bitterness of HCl salts such as quinine HCl.As reported in the literature,the AN0 sensor is used for basic materials such as famotidine[34],and the C00 sensor is used for acidic bitter materials such as iso-alpha acid [35].This may explain why the difference in the concentrations of atomoxetine HCl and quinine HCl at the same sensor output in the highconcentration region was larger,as shown in red in Table 2.
3.2.Definition of the bitterness threshold of atomoxetine HCl
As discussed above,the BT0 sensor is effective for the bitterness of HCl salts,including quinine HCl and azelastine HCl[36].In this study,the bitterness threshold of atomoxetine HCl under the BT0 sensor was analyzed.As shown in Table 3 and Fig.5,the concentration dependence of the bitterness of quinine HCl and atomoxetine HCl was assessed.According to the Nernst equation (Eq.4),the regression linear equation was found to give a reasonable description of the atomoxetine HCl concentrations from 0.003 to 0.01 mg/ml.The bitterness threshold of quinine HCl was assumed to be 0.01 mM,and then the relative value of 11.48 mV and the CPA value of 4.22 mV at 0.01 mM were introduced into the linear regression equation of atomoxetine HCl as shown in Table 3,the threshold concentrations of atomoxetine HCl were calculated as 6.48 μg/ml and 8.61 μg/ml,respectively.All of these results suggest that atomoxetine HCl is an extremely bitter-tasting drug.
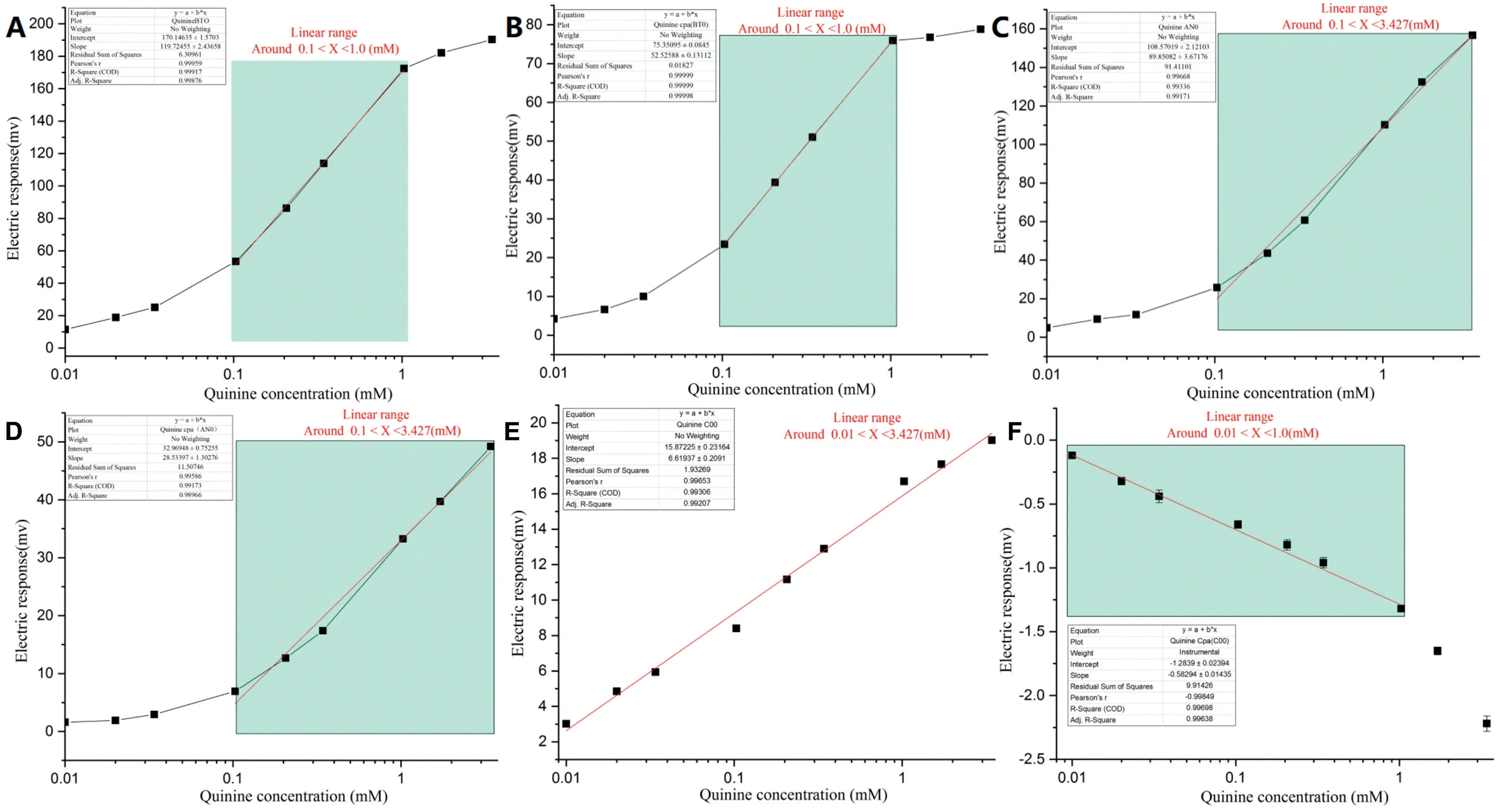
Fig.4–Experimental results and calibration curve of quinine HCl.(A)BT0 sensor for relative value,(B)BT0 sensor for CPA value,(C)AN0 sensor for relative value,(D)AN0 sensor for CPA value,(E)C00 sensor for relative value,(F)C00 sensor for CPA value.(mean±SD,n=3).
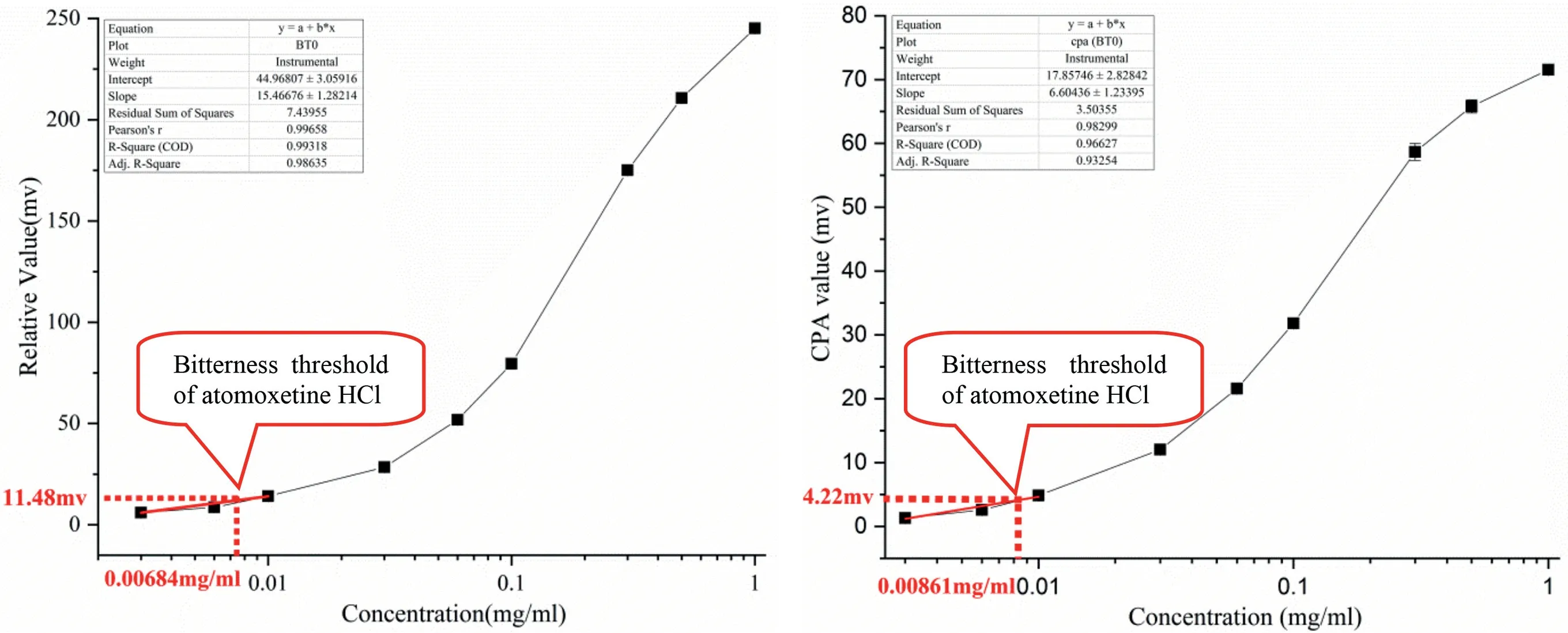
Fig.5–Bitterness threshold of atomoxetine HCl under the BT0 sensor.
3.3.Taste-masked formulations
The electronic sensor response of HP-β-CyD is shown in Fig.6,only the sensor outputs of the AN0 and C00 sensors slightly increased relative to those of atomoxetine HCl,and the other sensors barely responded.One possible explanation for these findings is that the selected sensors of the SA402B taste sensing system are not effective for neutral compounds,such as HP-β-CyD.As mentioned above,all three bitterness sensors(AN0,BT0,C00)are effective at detecting the bitterness of ionic substances.On the other hand,given the lack of response to HP-β-CyD,the taste sensing system could be used to evaluate the taste-masked effectiveness of formulation with HP-β-CyD.
To examine the taste-masking efficacy of formulations,PCA was used to identify the sensor outputs of four sensors.Clearly,as shown in Fig.7,there was a significant difference among atomoxetine HCl,HP-β-CyD,and quinine HCl at the concentration of 1.028 mM.As already illustrated with the extracted eigenvectors (Table 4),the BT0 sensor was closely linked with thex-axis (coefficient of PC1 is 0.86),while the AN0 sensor was aligned to they-axis(coefficient of PC1 is 0.84).The cumulative percentage had the highest eigenvalues and proportionally accounted for 100%of variance as shown in the first and second principle components (PC1:98.19% and PC2:1.81%)in Fig.7.The biplots showed that atomoxetine HCl and quinine HCl were close to thex-axis loaded for the BT0 sensor,thereby suggesting similar bitterness for both of them.The Euclidean distance between atomoxetine HCl and HP-β-CyD was calculated as 2.30,which was the same as the distance between quinine HCl and HP-β-CyD,a further explanation for the similar bitterness of atomoxetine HCl and quinine HCl.
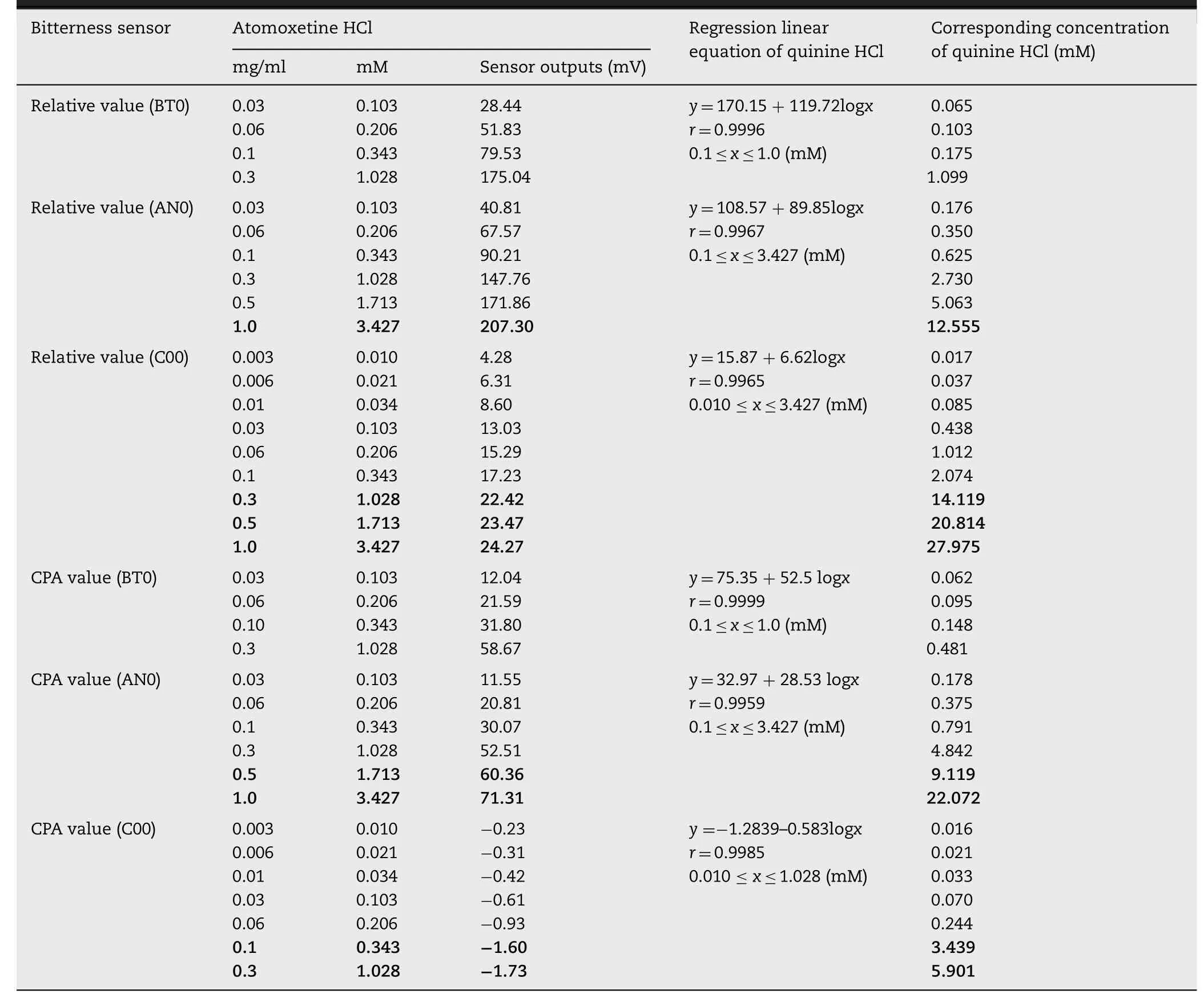
Table 2–Concentration dependency of quinine HCl and atomoxetine HCl for bitterness.

Table 3–Bitterness threshold of atomoxetine HCl.

Fig.6–Electronic sensor response of HP-β-CyD at different concentrations(mean±SD,n=3).

Fig.7–Principal component analysis of the electronic sensor response of atomoxetine HCl with those of quinine HCl dihydrate and HP-β-CyD under different sensors:BT0,AN0,C00,AE1.
The sensor outputs of five different taste-masked formulations with different molar ratios (1:1,1:2,1:3,1:4 and 1:5) (Table 5) under different sensors (BT0,AN0,C00,and AE1) were also evaluated by PCA.The concentration of 0.3 mg/ml (1.028 mM) of atomoxetine HCl was chosen for illustration,as shown in Fig.8A.According to Table 6A,only the two principle components were used,the first and second principle components (PC1 and PC2) accounted for approximately 98.90%,based on the significance level (lowPvalues,i.e.<0.05).The contribution of each sensor to each principle component is given in Table 7A.PC1 was primarily related to the BT0 sensor (for the bitterness of HCl salts,coefficient of PC1 is 0.66)and C00 sensor(for acidic substance bitterness,coefficient of PC1 is 0.75),while PC2 was related to the BT0 sensor(coefficient of PC2 is 0.72).
As presented in Table 8,the Euclidean distances were calculated to assess the taste-masked efficiency of the different formulations described above and pure drug.The distance between atomoxetine HCl and formulationsratios(1:1,1:2,1:3,1:4 and 1:5).increased from 1:1 to 1:5 molar ratios,which suggested that the suppressing efficiency increased as the molar ration of HP-β-CyD increased.The formulations of atomoxetine HCl at 0.3 mg/ml (1.028 mM) with a 1:1 molar ratio to 1:5 molar ratio had distances of 0.90,1.31,1.73,2.38,and 3.19,respectively(Fig.8A).
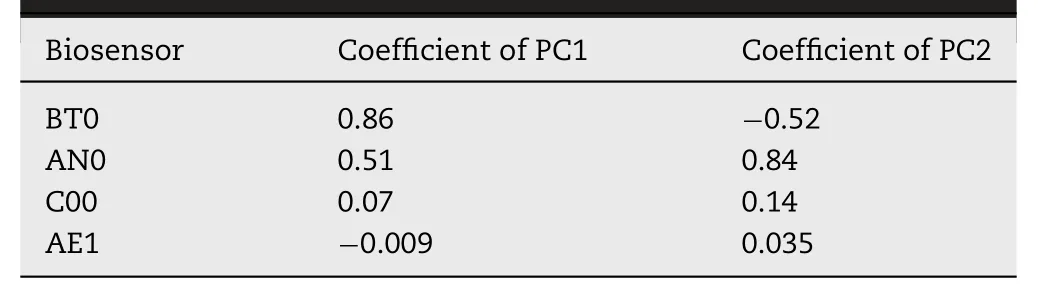
Table 4– Extracted eigenvectors for PC1 and PC2 and corresponding sensor allocation.

Table 5– Five different taste-masked formulations of atomoxetine HCl and HP-β-CyD with different molar
The human taste panel tests were applied to comprehensively evaluate the taste-masking efficiency of atomoxetine HCl/HP-β-CyD inclusion complexes.Atomoxetine HCl at a concentration of 0.3 mg/ml with 1:1–1:5 molar ratios was chosen.As shown in Table 9,according to the sensory test evaluation,the bitterness score decreased with increasing HP-β-CyD ratios,which had a significant relationship with the results of the E-tongue test.The subjects reported that pure drug and the formulation with the 1:1 molar ratio tasted almost the same,indicating that there was no masking effect generated by HP-β-CyD with a ratio of 1:1.The bitterness score of the pure drug and each formulation was defined byt-test.The statistical analysis indicated a significance difference for the formulations with 1:4 and 1:5 molar ratios relative to atomoxetine HCl(P <0.05).Thus,it was evident that the bitterness of atomoxetine HCl was efficiently masked by HP-β-CyD.
As shown in Fig.8A,there was a significant difference between the taste-masked formulations and pure drug on they-axis,indicating the remarkably suppressed bitterness of atomoxetine HCl with HP-β-CyD.Compared to formulations with 1:4 and 1:5 molar ratios,no significant difference was observed for the taste-masked formulations with 1:1–1:3 molar ratios on thex-axis.The biplot showed that both tastemasked formulations with 1:4 and 1:5 molar ratios were close to thex-axis loaded with the C00 sensor,suggesting an acidic bitter taste for both formulations.These findings may suggest that atomoxetine is included in the cavity of cyclodextrin,while hydrogen chloride remained free in the solution and prevented lipid molecule dissociation from changing the membrane potential and facilitated a response produced by the C00 sensor with a negatively charged membrane[11].
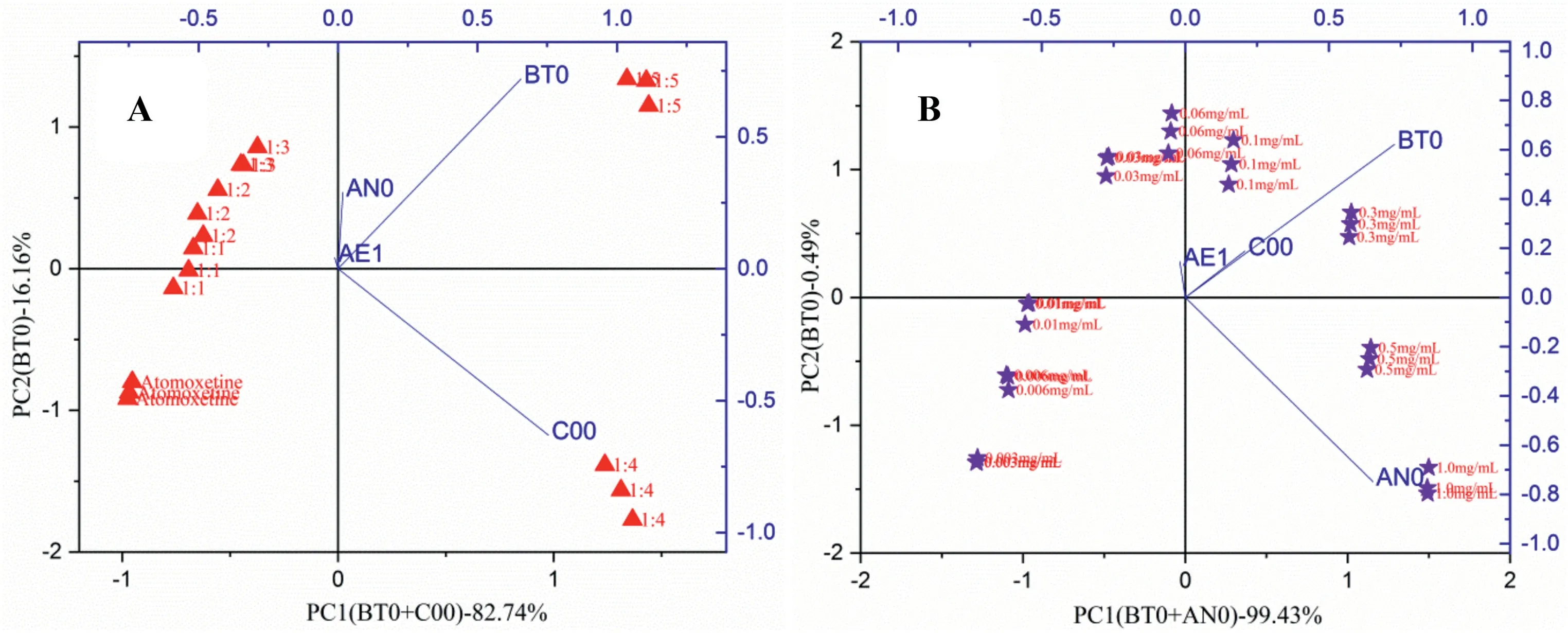
Fig.8–Principal component analysis of(A)five different taste-masked formulations of atomoxetine HCl(1.028 mM)and HP-β-CyD with different molar ratios(1:1,1:2,1:3,1:4 and 1:5),(B)nine different taste-masked formulations of atomoxetine HCl(0.01–3.427 mM)and HP-β-CyD with the molar ratio 1:5 under different sensors:BT0,AN0,C00,AE1.
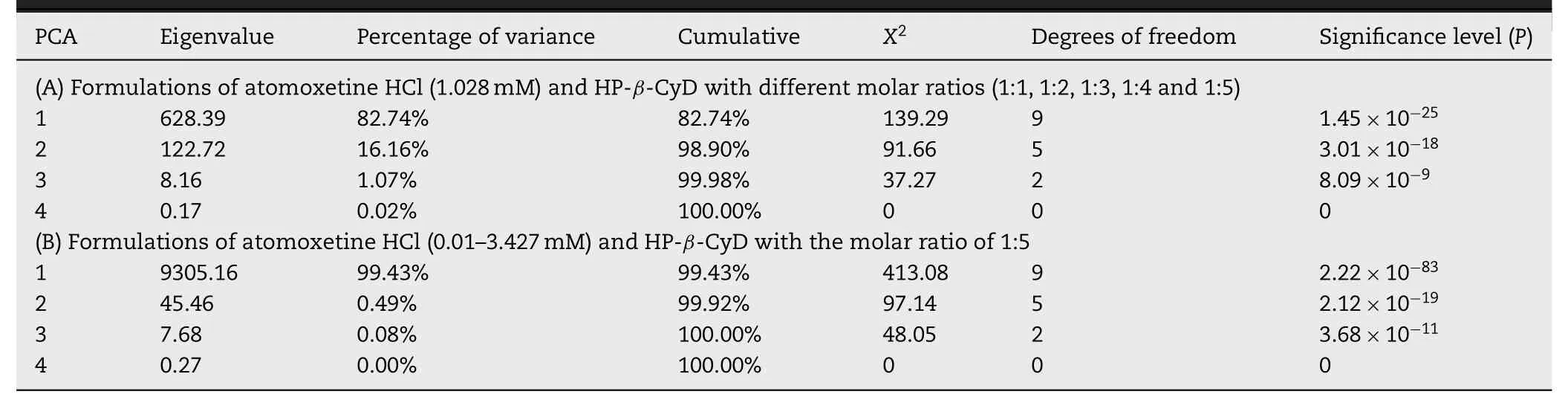
Table 6–Eigenvalues of the covariance matrix showing the contribution and significance of each principle component.
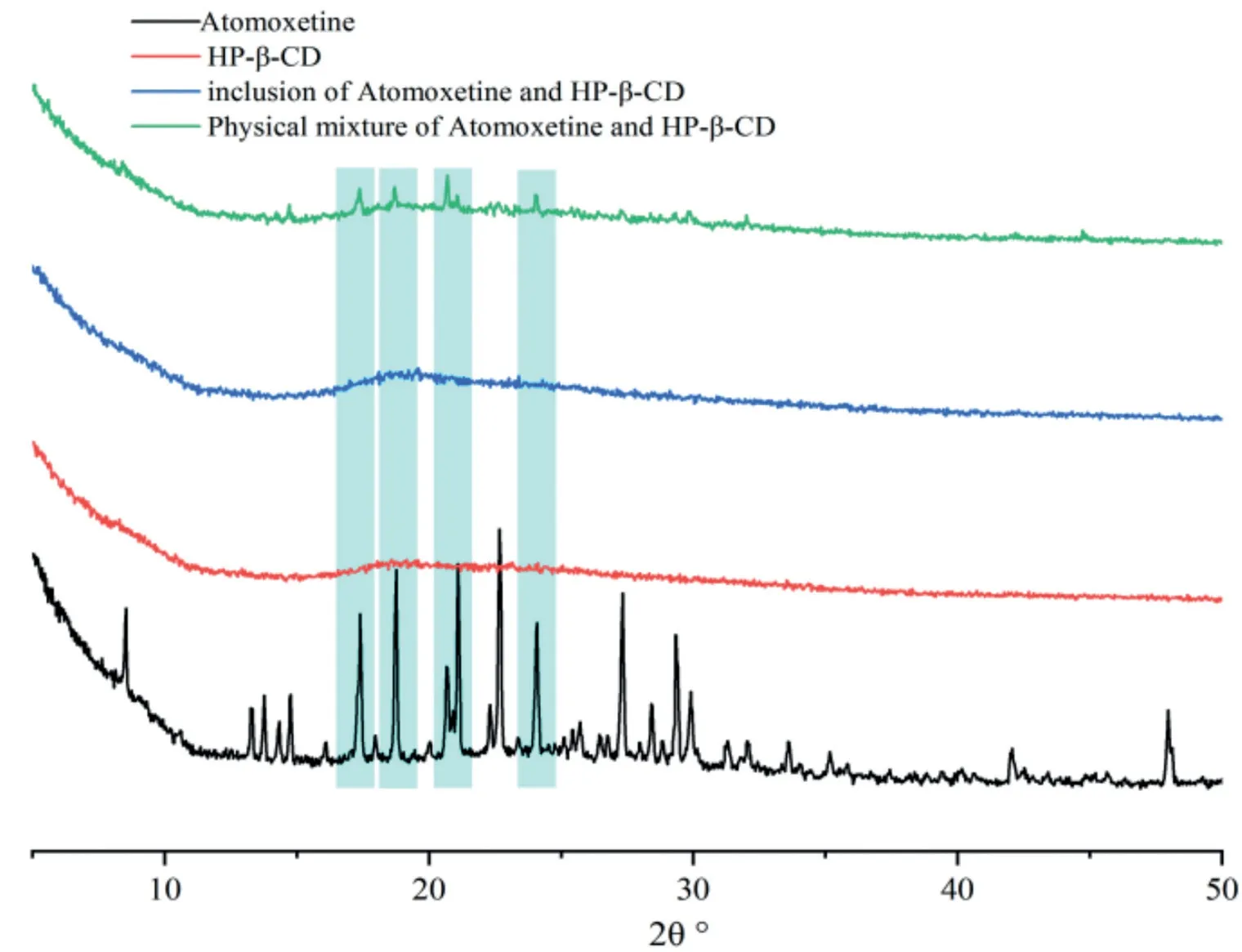
Fig.9–The XRD patterns of atomoxetine HCl,HP-β-CD,the physical mixtures and inclusion complexes from top to bottom.The characteristic peaks of original crystal of atomoxetine at 17.30°,18.70°,20.70°and 24.00°(2θ)are shaded in diffractograms.

Table 7– Extracted eigenvectors for PC1 and PC2 and corresponding sensor allocation.

Table 8– Euclidean distances of the taste-masked formulations with atomoxetine HCl/HP-β-CyD molar ratios(1:1,1:2,1:3,1:4 and 1:5).
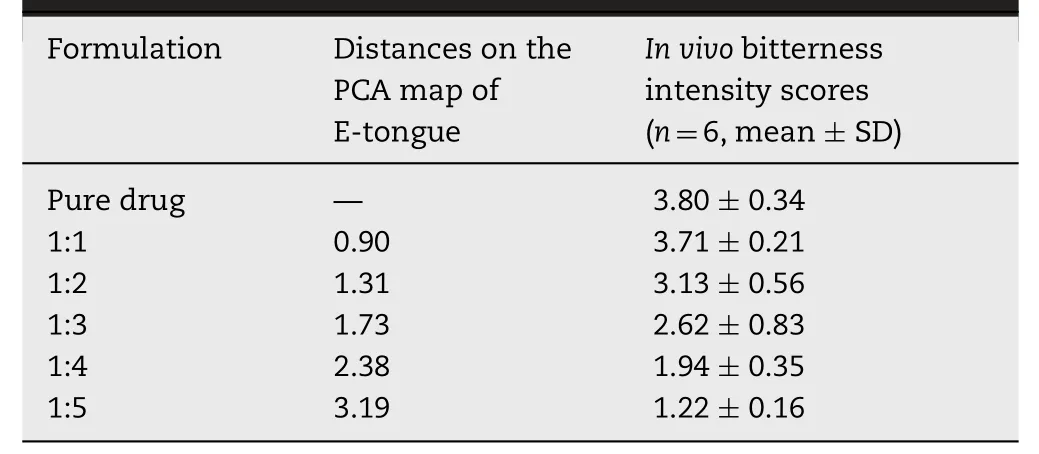
Table 9– Euclidian distances on the PCA map and bitterness intensity scores by the human taste panel tests between taste-masked formulations and pure atomoxetine HCl at the concentration of 0.3 mg/ml.
Meanwhile,the results for different concentrations of atomoxetine HCl with the same molar ratio(1:5)of HP-β-CyD were chosen for further investigation as illustrated in Fig.8B.The cumulative values displayed that PC1 and PC2 accounted for approximately 99.92% in Table 6B.For the extracted eigenvectors,it was clear that the highest contribution to PC1 was primarily related to the BT0 sensor and AN0 sensor,while PC2 was related to the BT0 sensor as shown in Table 7B.On the PCA map,it was observed that with increasing concentrations of atomoxetine HCl,the formulation distances further increased from each other on thex-axis loaded with the BT0 and AN0 sensors.
3.4.Exploration of the interaction between atomoxetine HCl and HP-β-CyD
3.4.1.Powder x-ray diffraction
PXRD diffractograms of atomoxetine HCl,HP-β-CyD,atomoxetine HCl/HP-β-CyD inclusion complexes and physical mixture are shown in Fig.9.The x-ray diffractogram of atomoxetine HCl displayed characteristic spectra of crystalline materials as evident from the number of sharp and intense peaks.The characteristic peaks of the original crystal of atomoxetine HCl at 17.30°,18.70°,20.70°and 24.00°(2θ) were shaded in the diffractograms as reported in the literature [16,37].However,the diffractograms of HP-β-CyD and inclusion of atomoxetine HCl showed an amorphous pattern,indicating the absence of crystallinity.Compared to the inclusion of atomoxetine HCl,the diffractogram of the physical mixture retained a few weak characteristic peaks as displayed by the crystal of atomoxetine HCl.These results indicate the formation of an atomoxetine HCl and HP-β-CyD inclusion complex.
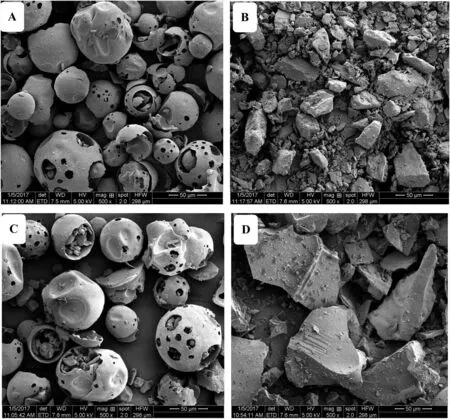
Fig.10–SEM micrographs of(A)HP-β-CyD,(B)atomoxetine HCl,(C)the physical mixtures and(D)atomoxetine HCl/HP-β-CD inclusion complexes.
3.4.2.Scanning electron microscopy
The surface morphologies of pure drugs,HP-β-CyD,physical mixture and inclusion complexes were observed by SEM (Fig.10) for further verification of the formation of atomoxetine HCl/HP-β-CD inclusion complexes.Both HP-β-CyD and its physical mixture were loose and spherical in appearance(Fig.10A and 10C) as reported in the literature [38,39],and some small scattered crystalline particles of atomoxetine HCl within and around the HP-β-CyD spherical particles could be observed (Fig.10C).All atomoxetine HCl was in crystal form with a layered texture according to its PXRD(Fig.10B).Atomoxetine HCl/HP-β-CD inclusion complex clearly showed non-spherical particles (Fig.10D),and although the morphology was similar to that of atomoxetine HCl,it was not crystalline according to its XRD.
3.4.3.Differential scanning calorimetry
The thermal properties of atomoxetine HCl,HP-β-CD,the physical mixtures and inclusion complexes were characterized using DSC as reported in Fig.11.The experiments were performed at the rate of 10 °C/min from 30 to 350 °C using open pans.The DSC thermogram of atomoxetine HCl showed a sharply endothermic onset peak at 170 °C,as well as the physical mixtures with a broad endothermic onset peak at the same temperature.It was clearly observed that the DSC thermographs of physical mixtures and inclusion complexes showed a significantly different form than HP-β-CD with a sharply endothermic onset peak at 230 °C,suggesting the successful interaction between atomoxetine HCl and HP-β-CD.

Fig.11–The DSC patterns of samples as follows:atomoxetine/HP-β-CyD inclusion complexes(M ratio 5:5),the physical mixtures,HP-β-CyD and atomoxetine HCl from top to bottom.
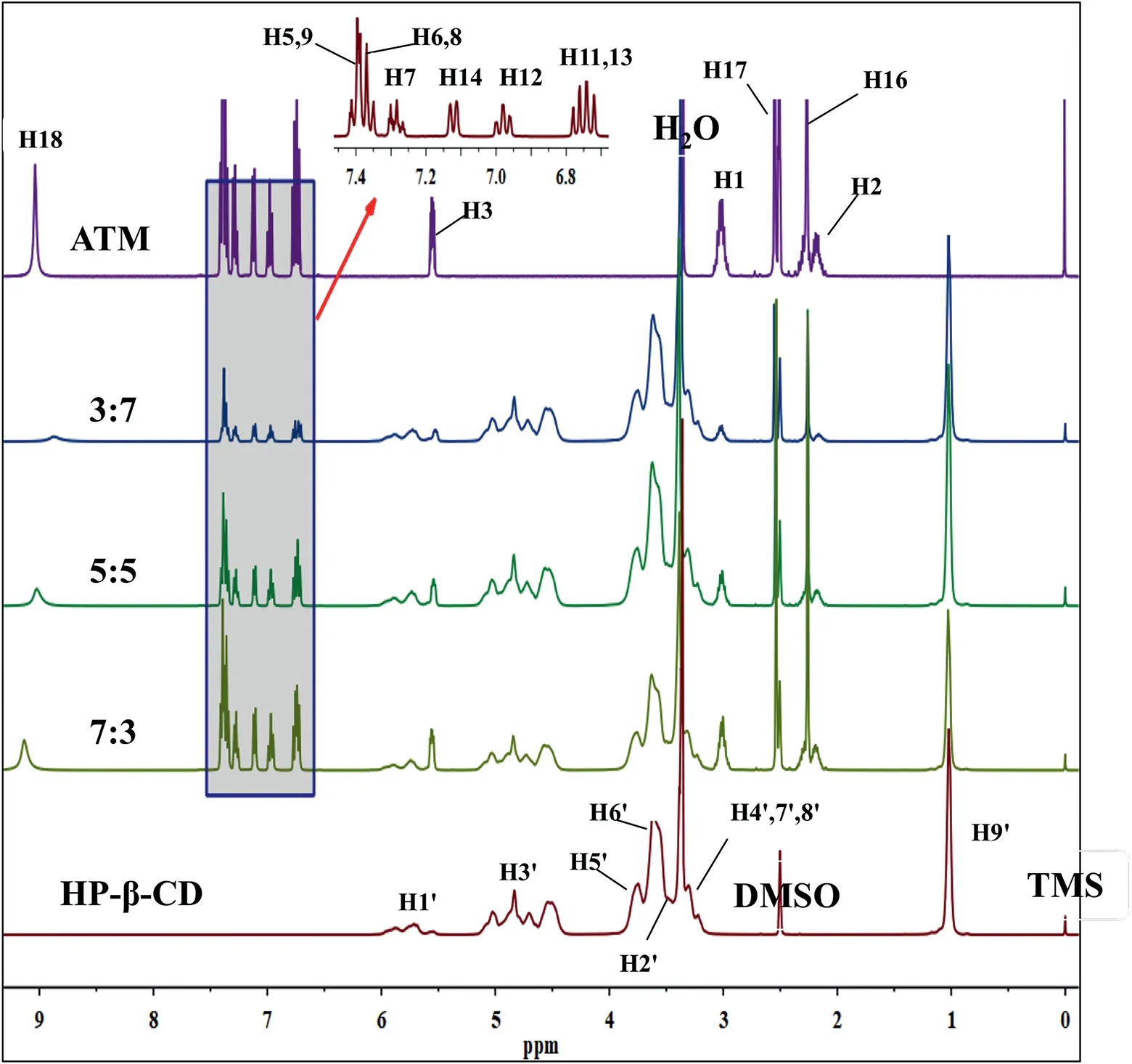
Fig.12–1H-NMR spectra of atomoxetine HCl(ATM),HP-β-CD and inclusion complexes(ATM:HP-β-CD)with different molar ratios(3:7,5:5,7:3)in DMSO–d6 at room temperature.
3.4.4.Nuclear magnetic resonance spectroscopy
In order to determine the interaction between atomoxetine HCl and HP-β-CD,NMR analysis was conducted to supply more information on the inclusion complexes reported in the literature[40–42].In order to clarify further the molecular mechanism of the inclusion complex,atomoxetine HCl/HPβ-CyD inclusion complexes with different molar ratios (3:7,5:5,7:3) were studied basing on the previous report [43].The1H NMR spectra of atomoxetine HCl,HP-β-CD and the inclusion complexes are presented in Fig.12.The proton chemical shift (δ) and the change in chemical shift (Δδ) of atomoxetine HCl and HP-β-CD are shown in Tables 10 and 11,respectively.
In this work,the variation in chemical shift (Δδ) was defined as (δcomplex–δfree).As shown in Table 10,the chemical shift of the H3′and H6′protons of HP-β-CD was significantly changed,indicating the formation of an inclusion complex.There was a great change in the chemical shift of the H2,H3 and H18 protons of atomoxetine HCl (Table 11) relative to other protons.The chemical shift of the H18 proton of atomoxetine HCl changed more drastically,and a possible explanation is that a hydrogen bond formed between the N atom of the drug and the H atom of HPβ-CD.Compared with free atomoxetine HCl and HP-β-CD,the changes in the chemical shift of the inclusion complex indicated that atomoxetine HCl reacted with HP-β-CD successfully.
4.Conclusion
In order to investigate the bitterness of atomoxetine HCl for the treatment of ADHD,a quantitative evaluation of the bitterness of atomoxetine HCl was performed for the first time by the E-tongue with four sensors (AN0,BT0,C00 and AE1).In a comparative study with quinine HCl,the trend of BT0 sensor outputs for relative values indicated two parts with 1.028 mM as a dividing point,and a possible explanation was investigated based on the detection mechanism of the electronic tongue.The bitterness threshold of atomoxetine HCl was similar to that of quinine HCl.
The bitterness of atomoxetine HCl was suppressed effectively by HP-β-CyD.The taste-masked formulations with different molar ratios of HP-β-CyD were effectively identified using the E-tongue system and human taste panel tests,and it was found that the optimal ratio was not simply 1:1 but rather 1:4 or 1:5.The taste-masked mechanism with HP-β-CyD was investigated.Compared to the pure drug,the inclusion complexes showed amorphous patterns in PXRD diffractograms and SEM,and the characteristic endothermic onset peak of the drug at 170°C in DSC thermographs disappeared.The chemical shift of H18 protons of atomoxetine HCl indicated the formation of a hydrogen bond between the N atom of the drug and the H atom of HP-β-CyD.These changes in the inclusion complex indicated that atomoxetine HCl reacted with HP-β-CyD successfully.The possible inclusion structure is displayed in Fig.13 with a ratio of HP-β-CyD to atomoxetine HCl of 4:1[44].Additionally,almost all chemical groups of atomoxetine HCl could be included in the complex structure,and the bitterproduced groups could not contact the taste receptors of the tongue within a short period with the inclusion complex staying in the oral cavity,so the bitter taste of the drug was masked.
Overall,this research involved the detailed quantitative investigation of the bitterness of atomoxetine HCl,highlighted the utility of the E-tongue system in the future development of taste-masked formulations and provided some information about the interaction between guest molecule and HP-β-CyD.

Table 10– Chemical shifts (δ) and chemical shift differences (Δδ) of HP-β-CD and inclusion complexes (atomoxetine HCl:HP-β-CD)with different molar ratios(3:7,5:5,7:3).

Table 11–Chemical shifts(δ)and chemical shift differences(Δδ)of atomoxetine HCl and inclusion complexes(atomoxetine HCl:HP-β-CD)with different molar ratios(3:7,5:5,7:3).

Fig.13–The possible inclusion and taste-masked mechanism when the ratio of HP-β-CD/atomoxetine HCl was 4:1.
Conflicts of interest
The authors report no conflicts of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgments
The authors acknowledge the financial support received from the National Major Scientific and Technological Special Project for “Significant New Drugs Development”during the Thirteenth Five-year Plan Period,P.R.China(2018ZX09721003-002-004),the Major Research Project of Shandong Province,P.R.China (2018GSF118004) and the Key Research and Development Program of Shandong Province,P.R.China(2018CXGC1411) for their support and encouragement in carrying out this work.
 Asian Journal of Pharmacentical Sciences2020年4期
Asian Journal of Pharmacentical Sciences2020年4期
- Asian Journal of Pharmacentical Sciences的其它文章
- Biointerface engineering nanoplatforms for cancer-targeted drug delivery
- Tumor microenvironment responsive drug delivery systems
- Advanced delivery strategies facilitating oral absorption of heparins
- Engineered targeting tLyp-1 exosomes as gene therapy vectors for efficient delivery of siRNA into lung cancer cells
- Let-7 miRNA and CDK4 siRNA co-encapsulated in Herceptin-conjugated liposome for breast cancer stem cells
- In situ apolipoprotein E-enriched corona guides dihydroartemisinin-decorating nanoparticles towards LDLr-mediated tumor-homing chemotherapy
