Study on chemical composition of Viscum coloratum and its HPLC fingerprint in different harvest periods
Ruizhen Zhang,Weiqing Wang,Rong Duan,Rong Rong,Zhiguo Yu,Yunli Zhao*
Department of Pharmaceutical Analysis,School of Pharmacy,Shenyang Pharmaceutical University,Shenyang,China
Abstract The purpose of this study was to evaluate the similarity of chemical profile of Viscum coloratum harvested in different months using high performance liquid chromatography (HPLC) fingerprint and multivariate statistical analysis,and to determine the optimal harvest time of Viscum coloratum.8 compounds were isolated from Viscum coloratum by a variety of isolation techniques,3 of which were isolated from the genus ViscumL.for the first time.The fingerprint of Viscum coloratum was established by HPLC,and 16 common peaks were obtained,4 of which were identified by comparison with the reference substances.Results showed that the Viscum coloratum harvested in 6 months could be roughly divided into three groups based on the chemical profiles,and the Viscum coloratum harvested in January had higher levels of the marked compounds.The HPLC fingerprint objectively reveals the characteristics of Viscum coloratum harvested in different months,which can be used to evaluate and control the quality of Viscum coloratum harvested in different months.
Key words:Viscum coloratum; quality control; harvest period; fingerprint; chemical composition
1 Introduction
Viscum coloratum(Komar.) Nakai (mistletoe),a semi-parasitic plant ofViscumL.,is an important medicinal plant.It has been reported thatViscum coloratumhas pharmacological effects,such as antitumor,anti-oxidation,regulating the cardiovascular system and the immune system [1-3].Therefore,it is generally used to treat cardiovascular disease,rheumatism and tumors.It also displays antihypertensive and anticoagulant bioactivities [4,5].The main chemical components of mistletoe include flavonoids,triterpenes,glycosides,and sterols [6-8].Since the composition and content of the active ingredients in the medicinal materials vary with the period of its growth and development,the difference of the harvest time directly affects the efficacy of the medicine [9].Although mistletoe is usually harvested in winter,no clear evidence shows that mistletoe harvested in winter has the best medicinal effect.
Therefore,in order to make better use of mistletoe and obtain better quality medicinal materials,we studied the chemical composition of mistletoe.Further,36 batches of mistletoe samples were evaluated based on the HPLC fingerprint established in the previous study [10],in order to provide reference for determining the optimal harvest time of mistletoe.
2 Experimental
2.1 materials and reagents
Mistletoe plants were harvested in Shenyang from March 2019 to January 2020,once every two months.Every time,six batches of samples were collected from the same six mistletoe.The sample details are shown in Table 1.The collected plant materials were dried in a well-ventilated unlighted room at room temperature and the voucher specimens were deposited in the state Key laboratory of traditional Chinese medicine (Shenyang Pharmaceutical University,China).Mistletoe decoction pieces for extraction and separation were purchased from Liaoning Pharmaceutical Industrial Co.,Ltd.(Liaoning,China).
Methanol (HPLC grade),tetrahydrofuran(HPLC grade) and glacial acetic acid (HPLC grade)were obtained from Tianjin concord Technology Co.,Ltd.(Tianjin,China).Ethanol (industrial),Petroleum ether (analytical reagent),Dichloromethane(analytical reagent),N-butanol (analytical reagent),and Methanol (analytical reagent) were obtained from Shandong Yuwang Industry Co.,Ltd.(Shandong,China).Pure water was purchased from Hangzhou Wahaha Group (Hangzhou,China).Silica gel (100-200 and 200-300 mesh) was obtained from Qingdao Marine Chemical Plant (Shandong,China).Sephadex LH-20 was obtained from Sweden Pharmacia company.Octadecyl silyl (ODS) was obtained from Japan YMC company.
Four reference substances,namely Syringin,Syringenin 4-O-β-D-apiofuranosyl (1→2)-β-Dglucopyranosides,Homoeriodictyo-7-O-β-Dapiosiyl-(1→2)-O-β-D-glucoside,and Isorhamnetin-3-O-β-D-glucoside,were all isolated from mistletoe by our research group,and the purity of all products was greater than 98%.
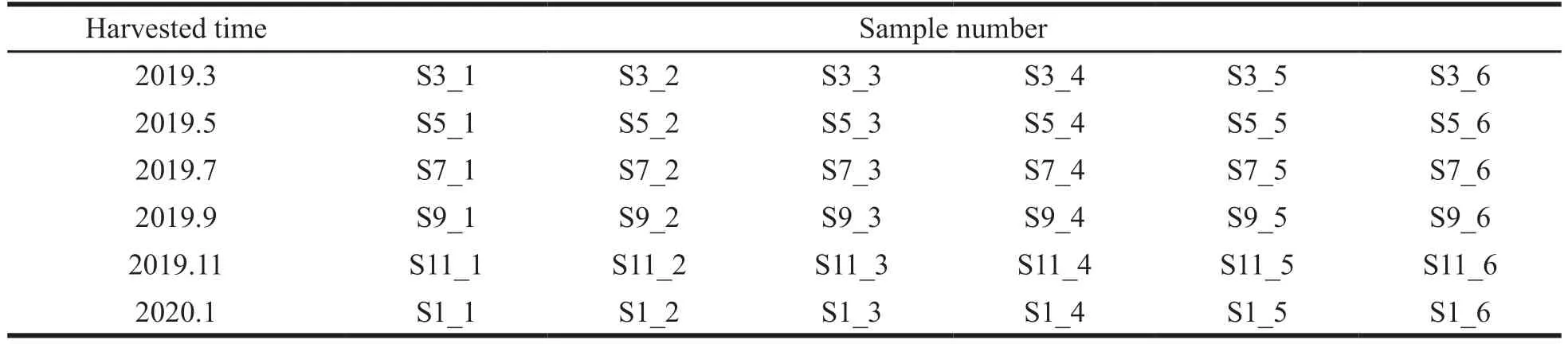
Table 1 Viscum coloratumharvested in different months
2.2 Instruments
The liquid chromatography system used for HPLC fingerprint research consisted of a LC-10ATvp pump (Shimadzu,Japan),a SIL-10AF auto sampler (Shimadzu,Japan) and an SPD-10Avp UVVIS detector (Shimadzu,Japan).Data acquisition and processing were carried out on LC solution workstation (Shimadzu,Japan).1H-NMR (400 MHz)and13C-NMR (100 MHz) spectra were recorded on Bruker ARX-400 spectrometer (Billerica,U.S.A),and TMS was used as internal standard.
2.3 Extraction and isolation
The dried leaves and twigs ofViscum coloratum(5.0 kg) were extracted with 95% ethanol under reflux three times,each time for 2 h,and the ratio of liquid to material was 8:1.This extract was suspended in H2O and successively partitioned with Petroleum ether,EtOAc and BuOH to obtain the Petroleum ether,EtOAc,and BuOH fractions.The BuOH fraction (230.0 g) was chromatographed on a silica gel column and eluted with a gradient of Dichloromethane−MeOH to obtain 18 subfractions(Fr.1-18).Fr.2 was chromatographed on a silica gel column and eluted with EtOAc-MeOH (3:1,v/v) to give five fractions (Fr.2_1-2_5).Fr.2-3 was chromatographed on an Octadecyl silyl(ODS) column and eluted with H2O-MeOH (9:1,v/v) to yield compound 1 (100.0 mg).Fr.15 was chromatographed on an ODS column and eluted with H2O-MeOH (9:1,v/v) to yield compound 2(25.0 mg) and compound 3 (8.0 mg).Fr.14 was chromatographed on a Polyamide column and eluted with H2O-MeOH (3:1-1:3,v/v) to give 13 fractions (Fr.14_1-14_13).Fr.14_2 fraction was chromatographed on Sephadex LH-20 and eluted with MeOH to yield compound 4 (50.0 mg) and compound 5 (18.0 mg).Fr.14_4 was chromatographed on HPLC and eluted with 53% MeOH to yield compound 6 (5.0 mg).Fr.17 was chromatographed on a silica gel column and eluted with EtOAc-MeOH (0:1-9:1,v/v) to give five fractions (Fr.17_1-17_9).Fr.17_2 was chromatographed on an ODS column and eluted with a gradient of H2O-MeOH to yield compound 7(3.0 mg) and compound 8 (4.0 mg).
2.4 Preparation of sample solutions
Mistletoe was powdered and accurately weighed 1.0 g.The powder was extracted with 25 mL of 50% MeOH under ultrasound for 30 min.The extracting solution was centrifuged at 13000 rpm for 10 min [10].The supernatant was filtered through a 0.22μm membrane for HPLC analysis.
2.5 Preparation of reference solution
An appropriate amount of reference substance was precisely weighed and dissolved by MeOH to obtain reference solution.The concentrations were 0.04 mg/mL Syringin,0.03 mg/mL Syringenin-4-O-β-D-apiofuranosyl(1→2)-β-D-glucopyranosides,0.31 mg/mL Homoeriodictyol-7-O-β-D-apiosiyl-(1→2)-O-β-D-glucoside and 0.03 mg/mL Isorhamnetin-3-O-β-D-glucoside,respectively.
2.6 Chromatographic conditions
Chromatographic separation was carried out on SynergiTMFusion-RP 80Å (250 mm × 4.6 mm,4μm) (Phenomenex,USA).Mobile phase A consisted of 0.1% glacial acetic acid and mobile phase B was composed of methanol/tetrahydrofuran(v:v=90:10).The gradient elution program was as follows:0.0-20.0 min,3-20% B; 20.0-60.0 min,20-70% B.The flow rate was 1 mL/min and the injection volume was 20μL.The UV detection wavelength was set at 270 nm.And the column temperature was kept at 30 °C [10].
2.7 Data analysis
Data of1H-NMR and13C-NMR were processed with the Bruker TOPSPIN 2.1 (Billerica,U.S.A).The similarity of fingerprints was evaluated in the Similarity evaluation system for chromatographic fingerprint of traditional Chinese medicine (TCM)(Version 2012.130723),and the multivariate statistical analysis was carried out in SIMCA 14.1(Umetrics,Umea,Sweden).
3 Results and discussion
3.1 Extraction and isolation
Compound 1 was isolated as colorless acicular crystal; ESI-MS ion at m/z 395.14[M+Na]+;1H-NMR (400 MHz,CD3OD) δ:6.75 (2H,s,H-3,5),6.54 (1H,d,J=15.79 Hz,H-7),6.33 (1H,dt,J=5.54;J=15.86,H-8),4.20 (2H,dd,J=1.45;J=5.7,H-9),3.80 (6H,s,OMe-2,6),4.87 (1H,d,J=7.3,H-1’),3.78 (1H,dd,J=2.36 Hz;J=11.76 Hz,H-6’),3.66 (1H,dd,J=5.32 Hz;J=11.75 Hz,H-6’).13C-NMR (150 MHz,CD3OD) δ:135.7 (C-1),154.1(C-2,6),105.2 (C-3,5),131.1 (C-4),135.1 (C-7),129.8 (C-8),63.4 (C-9),105.1 (C-1’),75.5 (C-2’),77.6 (C-3’),71.1 (C-4’),78.2 (C-5’),62.4 (C-6’),56.8 (OMe-2,6).Based on the examination of NMR data and comparison with references [8,11] and reference substance,compound 1 was identified as Syringin.
Compound 2 was isolated as colorless amorphous solid; ESI-MS ion at m/z 599.29[M+Na]+,579.39[M-H]-;1H-NMR (400 MHz,CD3OD) δ:1.81(1H,m,H-2),2.19 (1H,m,H-2),4.22 (1H,m,H-3),1.99 (2H,m,H-4),6.43 (1H,d,J=15.82 Hz,H-7),7.92 (1H,d,J=15.84 Hz,H-8),2.05 (1H,s,H-10),3.78 (2H,d,J=6.3 Hz,H-11),0.94 (1H,s,H-12),1.17 (1H,s,H-13),5.79 (1H,s,H-14),4.35 (1H,d,J=7.72 Hz,H-1’),3.14 (1H,dd,J=7.83 Hz;J=7.82 Hz,H-2’),3.35 (1H,m,H-3’),3.24 (1H,d,J=9.4 Hz,H-4’),3.42 (1H,m,H-5’),3.98 (1H,dd,J=9.7 Hz;J=2.1 Hz,H-6’),3.62 (1H,m,H-6’),5.04(1H,d,J=2.43 Hz,H-1’’),2.89 (1H,d,J=2.45 Hz,H-2’),3.97 (1H,d,J=9.75 Hz,H-4’’),3.77 (1H,d,J=9.75 Hz,H-4’’),3.58 (2H,m,H-5’’).13C-NMR(150 MHz,CD3OD) δ:49.9 (C-1),42.7 (C-2),74.0(C-3),42.9 (C-4),87.6 (C-5),83.2 (C-6),133.7(C-7),132.2 (C-8),21.1 (C-10),77.3 (C-11),16.4(C-12),19.7 (C-13),103.0 (C-1’),75.1 (C-2’),78.0(C-3’),71.8 (C-4’),77.1 (C-5’),68.6 (C-6’),110.9(C-1’’),78.1 (C-2’’),80.5 (C-3’’),75.0 (C-4’’),65.6(C-5’’).Based on the examination of NMR data and comparison with references [12,13],compound 2 was identified as Dihydrophaseic acid-4’-O-6’’-(βribofuranosyl)-β-glucopyranoside.
Compound 3 was isolated as white solid; ESIMS ion at m/z 527.19[M+Na]+,503.29[M-H]-;1H-NMR (400 MHz,CD3OD) δ:6.73 (2H,s,H-3,5),6.54 (1H,d,J=15.7 Hz,H-7),6.31 (1H,m,H-8),4.22 (2H,m,H-9),5.09 (1H,d,J=7.17 Hz,H-1’),3.69 (1H,m,H-2’),3.54 (1H,m,H-3’),3.17 (1H,m,H-4’),3.46 (1H,m,H-5’),3.74 (1H,m,H-6’),3.64 (1H,d,J=5.13 Hz,H-6’),5.48 (1H,brd,J=0.95 Hz,H-1’’),4.01 (1H,d,J=0.95 Hz,H-2’’),4.04 (1H,d,J=9.5 Hz,H-4’’),3.68 (1H,m,H-4’’),3.73 (1H,m,H-5’’),3.60 (1H,m,H-5’’).13C-NMR(150 MHz,CD3OD) δ:134.9 (C-1),154.5 (C-2,6),105.4 (C-3,5),135.3 (C-4),131.4 (C-7),129.8 (C-8),63.6 (C-9),102.7 (C-1’),78.6 (C-2’),78.7 (C-3’),71.3 (C-4’),78.0 (C-5’),62.6 (C-6’),110.4 (C-1’’),78.1 (C-2’’),80.9 (C-3’’),75.6 (C-4’’),66.4 (C-5’’).Based on the examination of NMR data and comparison with reference [14],compound 3 was identified as Syringenin 4-O-β-D-apiofuranosyl (1→2)-β-D-glucopyranosides.
Compound 4 was isolated as colorless crystal;ESI-MS ion at m/z 605.38[M+Na]+,581.39[M-H]-;1H-NMR (400 MHz,CD3OD) δ:2.6 (1H,dd,J=11.48 Hz;J=14.90 Hz,H-1),2.72 (1H,dd,J=4.8 Hz;J=15.2 Hz,H-1),1.7 (1H,m,H-2),2.0(1H,m,H-3),4.42 (1H,d,J=6.1 Hz,H-4),6.58(1H,s,H-8),3.54 (1H,dd,J=6.5 Hz;J=10.88 Hz,H-2α),3.63 (1H,m,H-2α),3.45 (1H,dd,J=4.0 Hz;J=10.0 Hz,H-3α),3.88 (1H,dd,J=5.57 Hz;J=9.94 Hz,H-3α),6.42 (1H,s,H-2’,6’),3.35 (3H,s,OMe-5),3.86 (3H,m,OMe-7),3.7 (6H,s,OMe-3’,5’),4.28 (1H,d,J=7.74 Hz,H-1’’),3.23 (1H,m,H-2’’),3.37 (1H,m,J=9.5 Hz,H-3’’),3.29 (1H,s,H-4’’),3.25 (1H,d,J=3.64 Hz,H-5’’),3.67 (1H,m,H-6’’),3.58 (1H,m,H-6’’);13C-NMR (150 MHz,CD3OD) δ:33.7 (C-1),40.5 (C-2),46.6 (C-3),42.7(C-4),147.5 (C-5),139.2 (C-6),148.5 (C-7),107.8(C-8),130.1 (C-9),126.3 (C-10),134.4 (C-1’),106.8 (C-2’,6’),148.8 (C-3’,5’),138.8 (C-4’),66.1(C-2α),71.4 (C-3α),60.1 (OMe-5),56.5 (OMe-7),56.8 (OMe-3’,5’),104.7 (C-1’’),75.1 (C-2’’),78.1(C-3’’),71.6 (C-4’’),77.8 (C-5’’),62.7 (C-6’’).Based on the examination of NMR data and comparison with references [11,15],compound 4 was identified as (+)-Lyoniresinol-3α-O-β-Dglucopyranoside.
Compound 5 was isolated as colorless crystal;ESI-MS ion at m/z 581.39[M-H]-;1H-NMR(400 MHz,CD3OD) δ:2.68 (1H,s,H-1),2.66 (1H,d,J=2.43 Hz,H-1),1.68 (1H,m,H-2),2.13 (1H,m,H-3),4.23 (1H,d,J=6.37 Hz,H-4),6.57 (1H,s,H-8),3.64 (3H,m,H-2α,3α),3.83 (1H,m,H-3α),6.41 (1H,s,H-2’,6’),3.34 (3H,s,OMe-5),3.85(3H,m,OMe-7),3.75 (6H,s,OMe-3’,5’),4.14 (1H,d,J=7.68 Hz,H-1’’),3.13-3.23 (m,H-2’’-5’’),3.69 (1H,dd,J=5.42 Hz;J=11.9 Hz,H-6’’),3.87(1H,m,H-6’’);13C-NMR (150 MHz,CD3OD) δ:33.7 (C-1),41.2 (C-2),46.5 (C-3),43.1 (C-4),147.5(C-5),139.4 (C-6),148.6 (C-7),107.7 (C-8),130.1(C-9),126.1 (C-10),134.5 (C-1’),107.0 (C-2’,6’),148.9 (C-3’,5’),138.8 (C-4’),66.1 (C-2α),71.9(C-3α),60.0 (OMe-5),56.5 (OMe-7),56.8 (OMe-3’,5’),104.2 (C-1’’),75.0 (C-2’’),78.1 (C-3’’),71.4(C-4’’),77.9 (C-5’’),62.6 (C-6’’).Based on the examination of NMR data and comparison with references [11,15],compound 5 was identified as(-)-Lyoniresinol-3α-O-β-D-glucopyranoside.
Compound 6 was isolated as white amorphous powder; ESI-MS ion at m/z 595.35[M-H]-;1H-NMR(400 MHz,CD3OD) δ:5.38 (1H,dt,J=12.78 Hz,H-2),2.76 (1H,dt,J=3.51 Hz;J=17.18 Hz,H-3),3.19 (1H,m,H-3),6.16 (1H,d,J=2.17 Hz,H-6),6.19 (1H,m,H-8),7.08 (1H,brs,H-2’),6.82 (1H,d,J=8.12 Hz,H-5’),6.92 (1H,d,J=8.11 Hz,H-6’),3.87 (3H,s,OMe-3’),Glu:5.05 (1H,t,J=6.39 Hz,H-1’’),3.6 (1H,m,H-2’’),3.43 (1H,m,H-3’’),3.39 (1H,brd,J=8.17 Hz,H-4’’),3.98 (1H,dd,J=3.7 Hz;J=9.7 Hz,H-5’’),3.67 (1H,m,H-6’’),3.85 (1H,brs,H-6’’),Api:5.41 (1H,brs,H-1’’’),3.94 (1H,d,J=1.02 Hz,H-2’’’),3.77 (1H,d,J=9.36 Hz,H-4’’’),3.52 (2H,s,H-5’’’).13C-NMR(150 MHz,CD3OD) δ:80.9/80.9 (C-2),44.1/44.3(C-3),198.5 (C-4),164.96/164.99 (C-5),97.9 (C-6),166.8/166.8 (C-7),96.8 (C-8),164.6 (C-9),104.9(C-10),131.4/131.5 (C-1’),111.3/111.4 (C-2’),149.1(C-3’),148.2/148.2 (C-4’),116.1 (C-5’),120.6/120.7(C-6’),56.5 (OMe-4’),99.8 (C-1’’),78.6 (C-2’’),78.4 (C-3’’),71.1 (C-4’’),78.1 (C-5’’),62.3 (C-6’’),110.9 (C-1’’’),78.1 (C-2’’’),80.7 (C-3’’’),75.4 (C-4’’’),65.9 (C-5’’’).Based on the examination of NMR data and comparison with references [8,16]and reference substance,compound 6 was identified as Homoeriodictyol-7-O-β-D-apiosiyl-(1→2)-O-β-D-glucoside.According to13C-NMR spectrum,it was identified as a mixture of isomers with a relative ratio of about 1:1.
Compound 7 was isolated as pale yellow solid;1H-NMR (400 MHz,DMSO-d6) δ:7.42 (2H,d,J=8.7 Hz,H-2,6),7.33 (1H,d,J=15.7 Hz,H-7),6.81(2H,d,J=8.6 Hz,H-3,5),6.41 (1H,d,J=15.8 Hz,H-8);13C-NMR (150 MHz,DMSO-d6) δ:115.8(C-3,5),118.9 (C-8),126.0 (C-1),129.3 (C-2,6),138.6 (C-7),158.9 (C-4),165.4 (C-9).Based on the examination of NMR data and comparison with reference [17],compound 7 was identified as (E)-p-Coumaramide.
Compound 8 was isolated as pale yellow solid;1H-NMR (400 MHz,DMSO-d6) δ:7.21 (1H,d,J=15.6 Hz,H-7),6.95 (1H,d,J=2.0 Hz,H-2),6.82(1H,dd,J=8.2 Hz,J=1.9 Hz,H-6),6.74 (1H,d,J=8.1 Hz,H-5),6.33 (1H,d,J=15.7 Hz,H-8);13C-NMR (150 MHz,DMSO-d6) δ:114.0 (C-2),115.9 (C-5),118.7 (C-8),120.4 (C-6),126.5 (C-1),139.0 (C-7),145.7 (C-3),147.4 (C-4),165.4(C-9).Based on the examination of NMR data and comparison with reference [17],compound 8 was identified as (E)-Caffeamide.
A total of eight compounds were isolated fromViscum coloratum,and summarized in Table 2.Compounds 2,7 and 8 were isolated from the genusViscumL.for the first time.
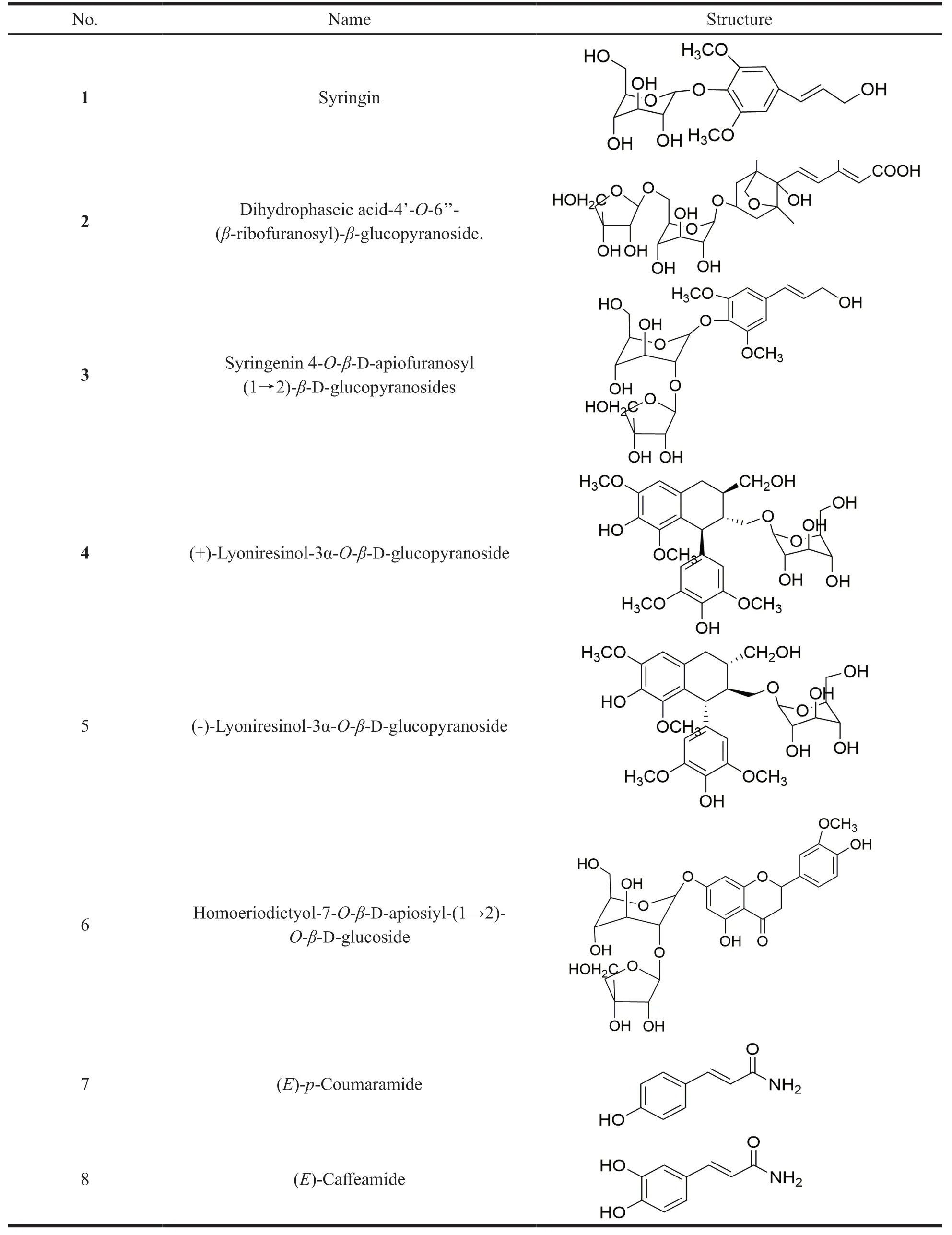
Table 2 Compounds isolated from Viscum coloratum
3.2 HPLC fingerprint analysis
3.2.1 Method validation
To validate the method,sample S1-1 was used.Precision of the procedure was determined by consecutively injecting the same sample solution six times.Sample stability was tested by injecting the same sample solution after standing for 0,2,6,8,12 and 24 h at room temperature.Repeatability was tested by six injections of independently prepared samples (S1-1).The Method Validation was determined by analyzing relative retention time(RRt.) and relative peak area (RA) of common peaks(No.0-15) of chromatogram profiles from mistletoe by taking the No.12 peak as the internal reference peak.All the results were summarized in the Table3.All theRSDvalues were within 5%,which suggested that these methods were effective and reliable.
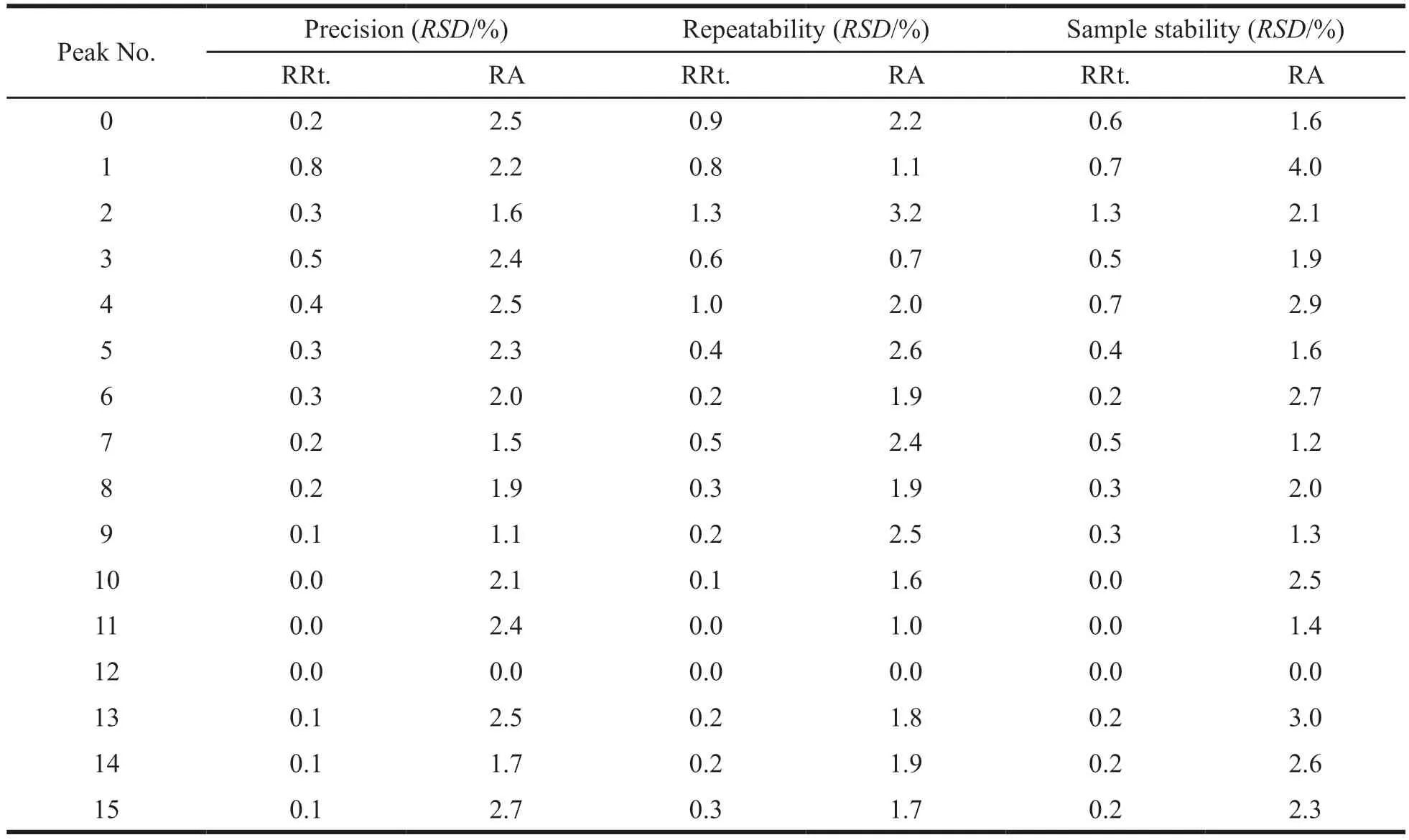
Table 3 Results of method validation
3.2.2 HPLC fingerprint
36 batches of mistletoe were prepared into sample solutions according to the description in Section 2.4.The sample solutions were analyzed chromatographically according to the description in Section 2.6 and their HPLC chromatograms were recorded.The fingerprints of 36 batches of samples were established in the Similarity evaluation system for chromatographic fingerprint of TCM (Version 2012.130723).Chromatogram of S1-1 was used as the reference spectrum.The time span was set to 0.1 min,and the control spectrum was generated using the median method.A total of 16 common peaks were obtained after multi-point correction.The typical chromatogram is shown in Fig.1,and the superposition chromatogram of 36 samples is shown in Fig.2.By comparison with the chromatogram of the reference solution,peak No.7 was identified as Syringin,peak No.8 as Syringenin 4-O-β-Dapiofuranosyl (1→2)-β-D-glucopyranosides,peak No.12 as Homoeriodictyol-7-O-β-D-apiosiyl-(1→2)-O-β-D-glucoside,and peak No.13 as Isorhamnetin-3-O-β-D-glucoside.The chromatogram of the reference solution is shown in Fig.3.Since flavonoids are the main chemical components of mistletoe,and peak No.12 is stable in the chromatogram,with a moderate peak area and good separation,peak No.12 was selected as the internal reference peak.
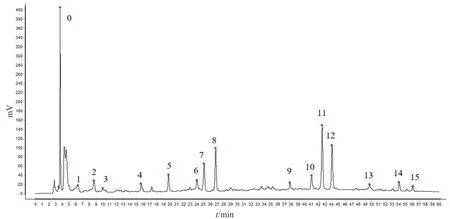
Fig.1 The typical chromatogram of Viscum coloratum

Fig.2 The superposition chromatogram of 36 samples
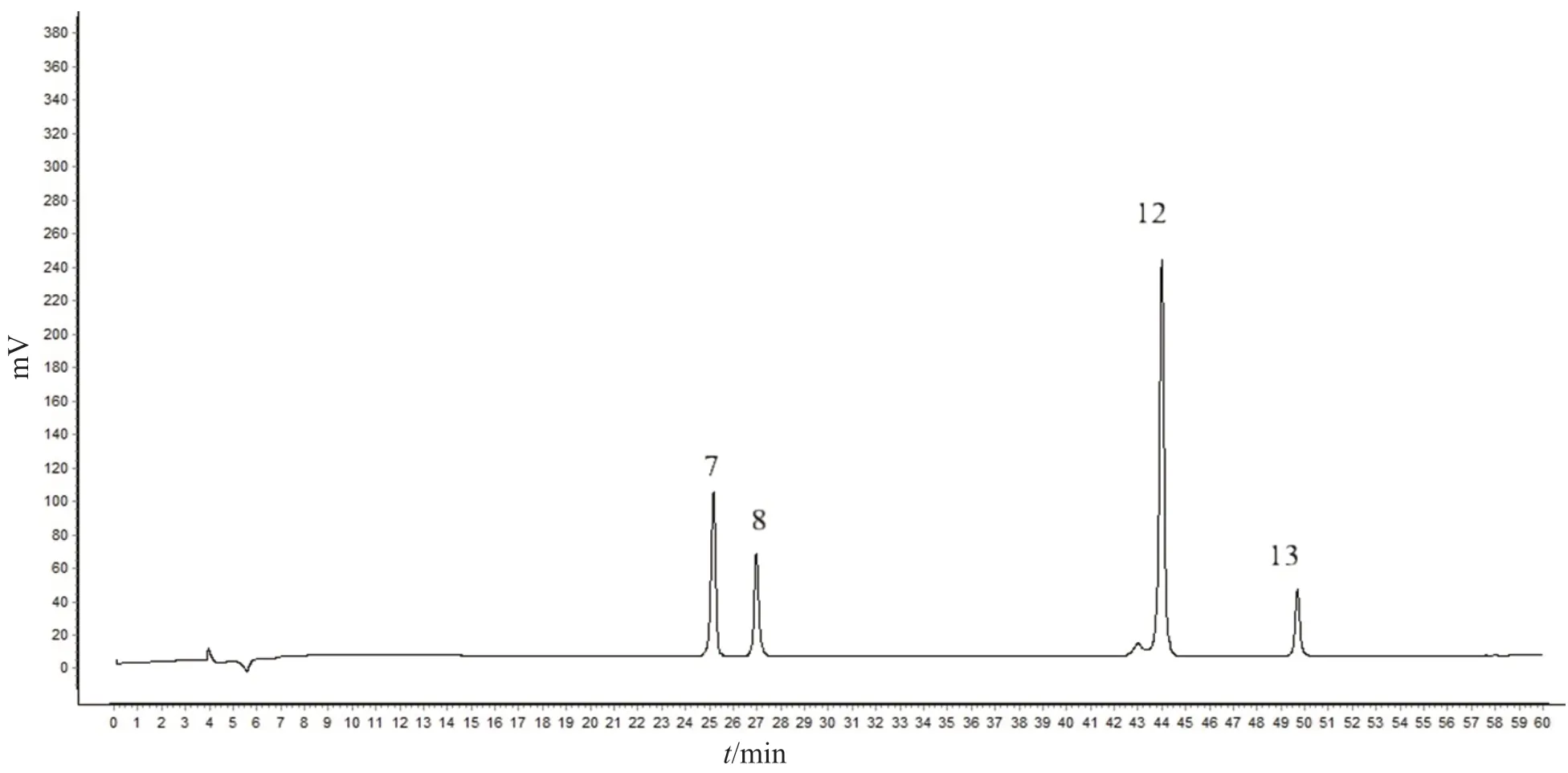
Fig.3 The chromatogram of the reference solution
3.2.3 Multivariate statistical analysis
The common peak area data of each sample was imported into the SCIMA 14.0 software for hierarchical cluster analysis (HCA) (Fig.4) and principal component analysis (PCA) (Fig.5).The result of HCA showed that 36 samples were divided into 3 groups:January; March and November; May,July and September.And the result of PCA showed that 36 samples were divided into two major parts along the vertical axis:January and other months.More specifically,the samples from March and November were mixed together,and the samples from the other 4 months were clustered together by month.The above analysis shows that mistletoe collected in January are different from those collected in other months,and the herbs collected in each month are slightly different except for March and November.
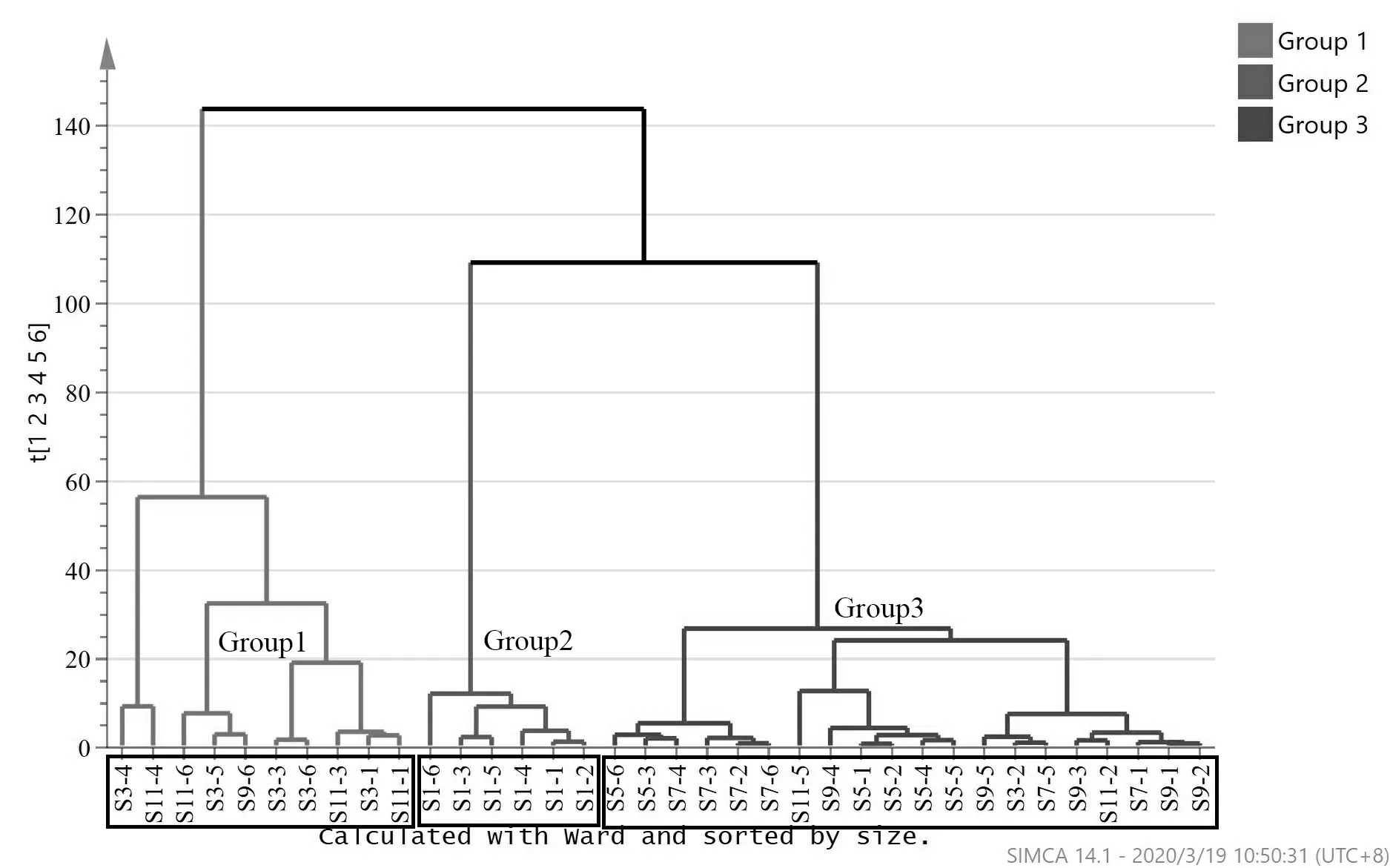
Fig.4 The HCA figure of 36 samples
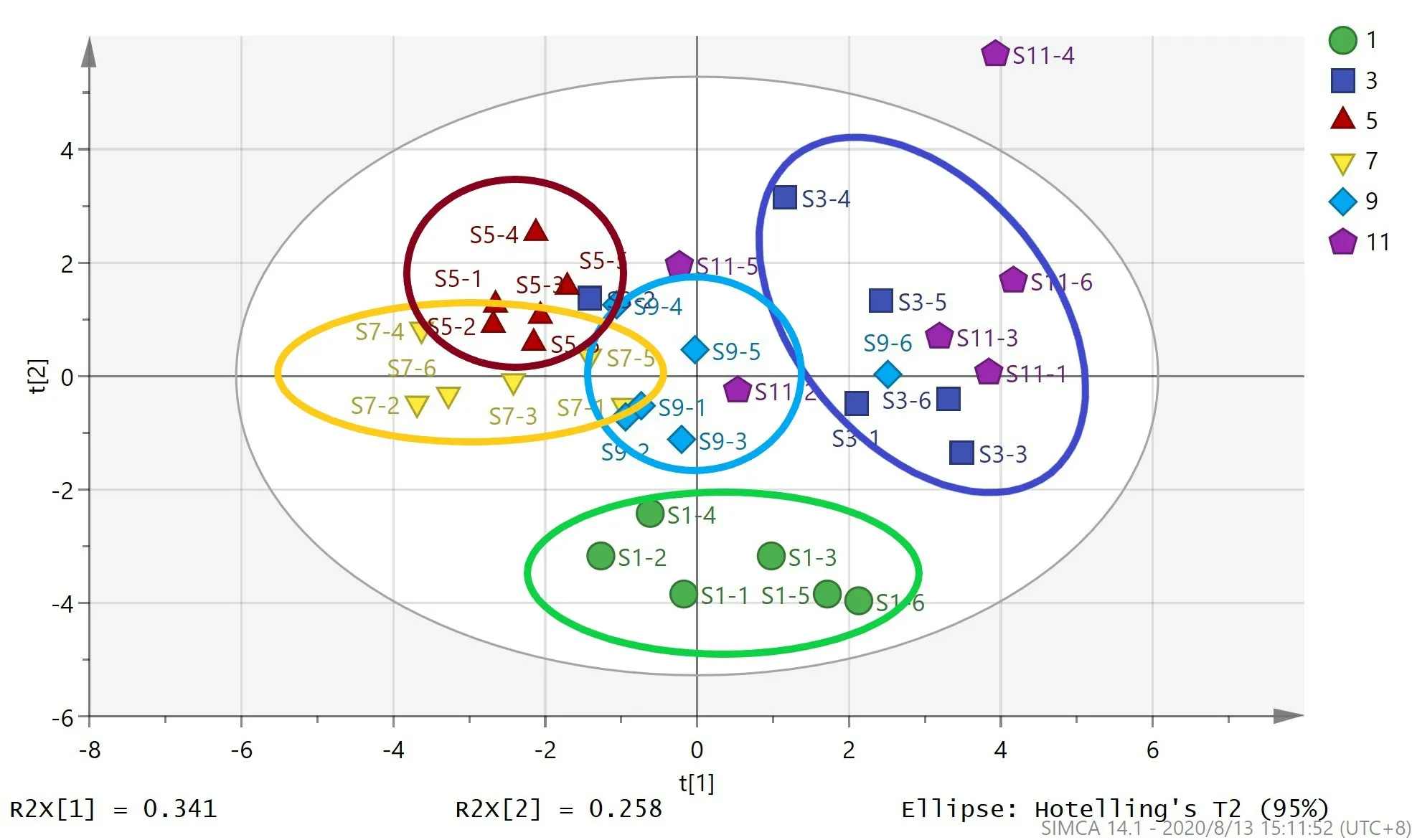
Fig.5 PCA score scatter plot of 36 samples
Orthogonal Partial Least-Squares Discriminant Analysis (OPLS-DA) was used to further analyze the differences in sample fingerprints between months:January-July; January-September; March-July; January-March.The OPLS-DA score scatter plot and VIP are shown in Fig.6.The parameters of the OPLS-DA models are listed in Table 4.All the parameters are > 0.5,indicating that the discriminating ability and prediction ability of models are reliable.
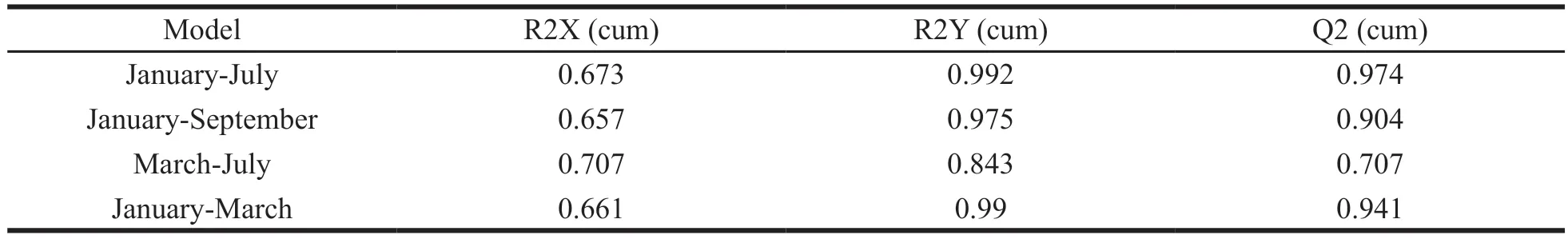
Table 4 Parameters of the OPLS-DA models
The VIP value represents the contribution of each peak to the difference between sample groups.With VIP > 1 as the criterion,the components that contributed significantly to differences between groups were screened out.The total area of marker peaks in each comparison group are summarized in Tables 5-8.According to the above data,it was found that the total marker peak area of the mistletoe medicinal materials in January was larger than that in July,March and September,and the total marker peak area of mistletoe medicinal materials in March was larger than that in July.The paired samplet-test was performed on the total area of the marked peaks in different months.The results are shown in Table 9.AllPvalues are less than 0.05,indicating that all peak areas differences are statistically significant.
Summarizing the above analysis,it was found that the compounds content of mistletoe was different in different months.The content of marked compounds in the medicinal materials harvested in January was higher than that in other months,suggesting that the medicinal materials in January might have better quality.
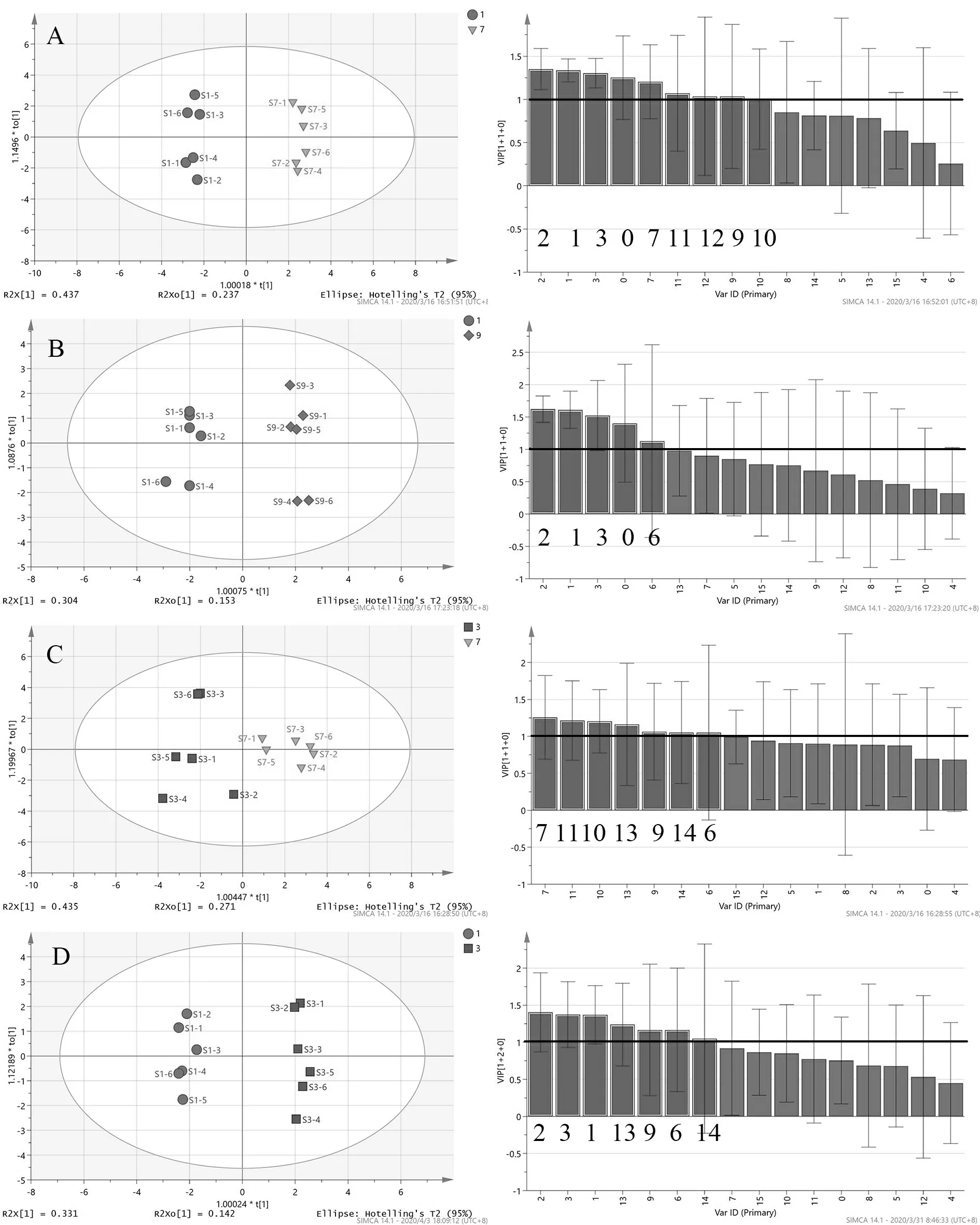
Fig.6 The OPLS-DA score scatter plot and VIP of three compare groups
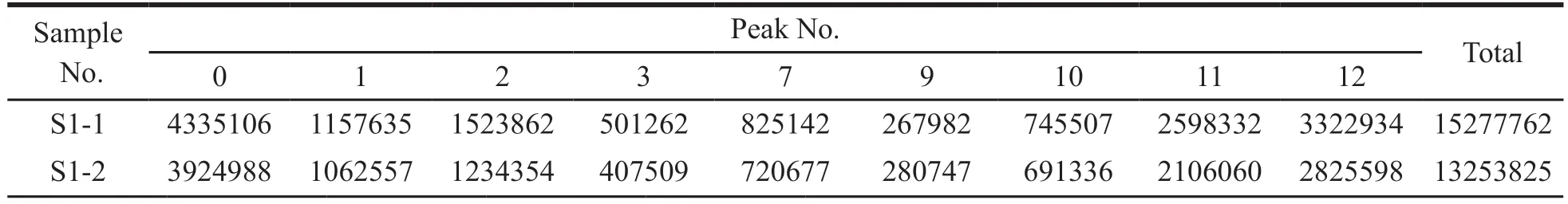
Table 5 The total peak area of marker in samples harvested in January and July

Continued table 5
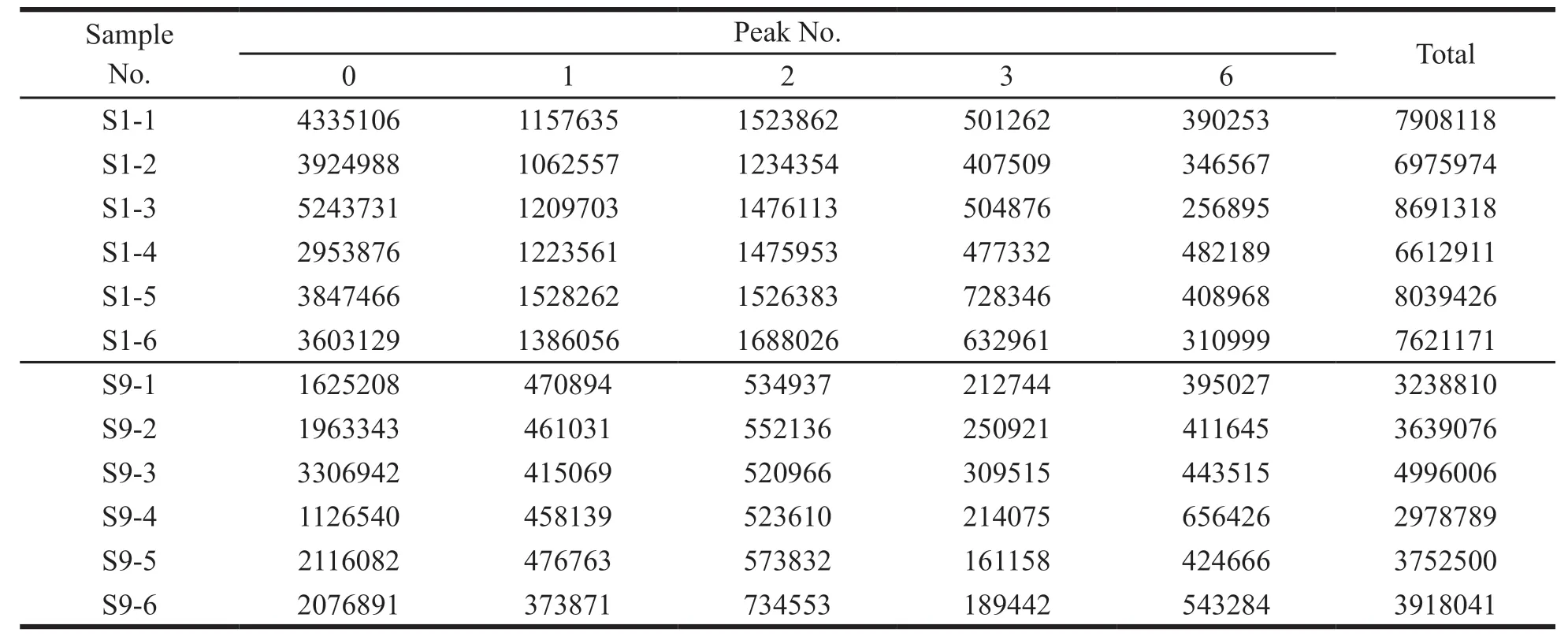
Table 6 The total peak area of marker in samples harvested in January and September
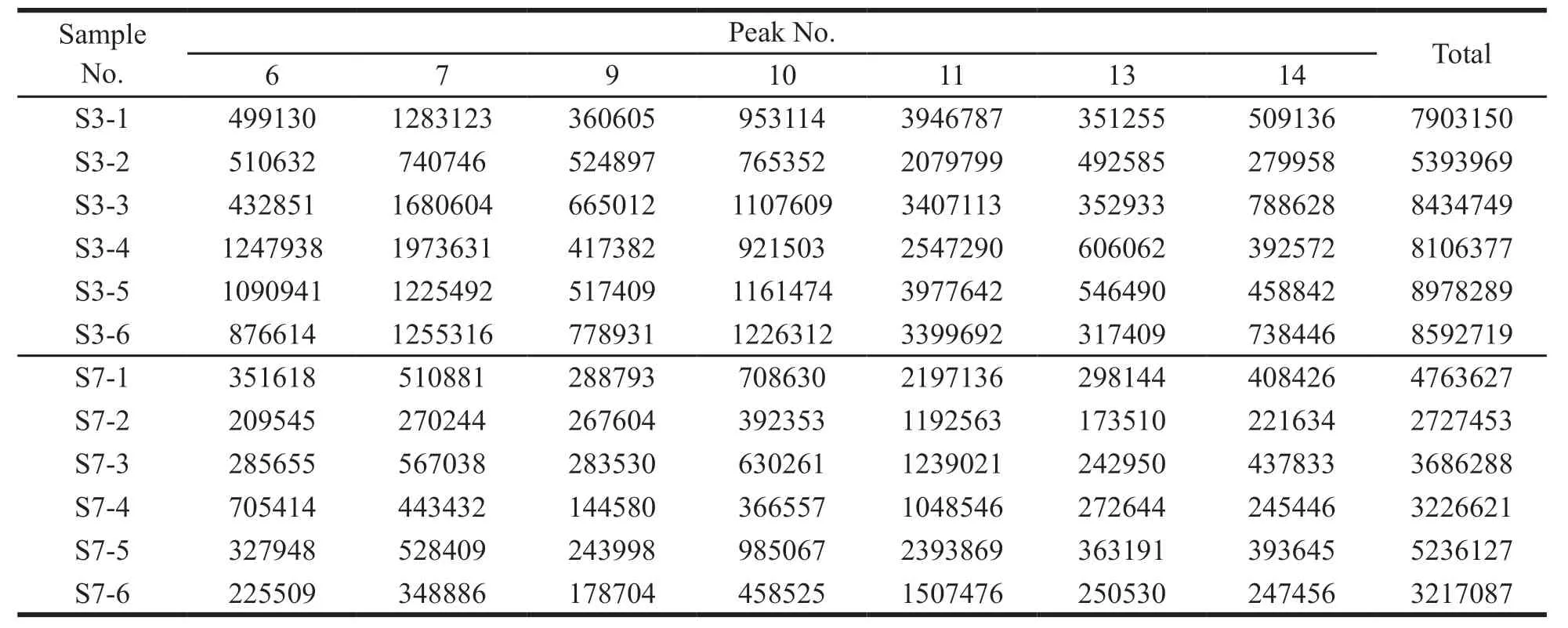
Table 7 The total peak area of marker in samples harvested in March and July
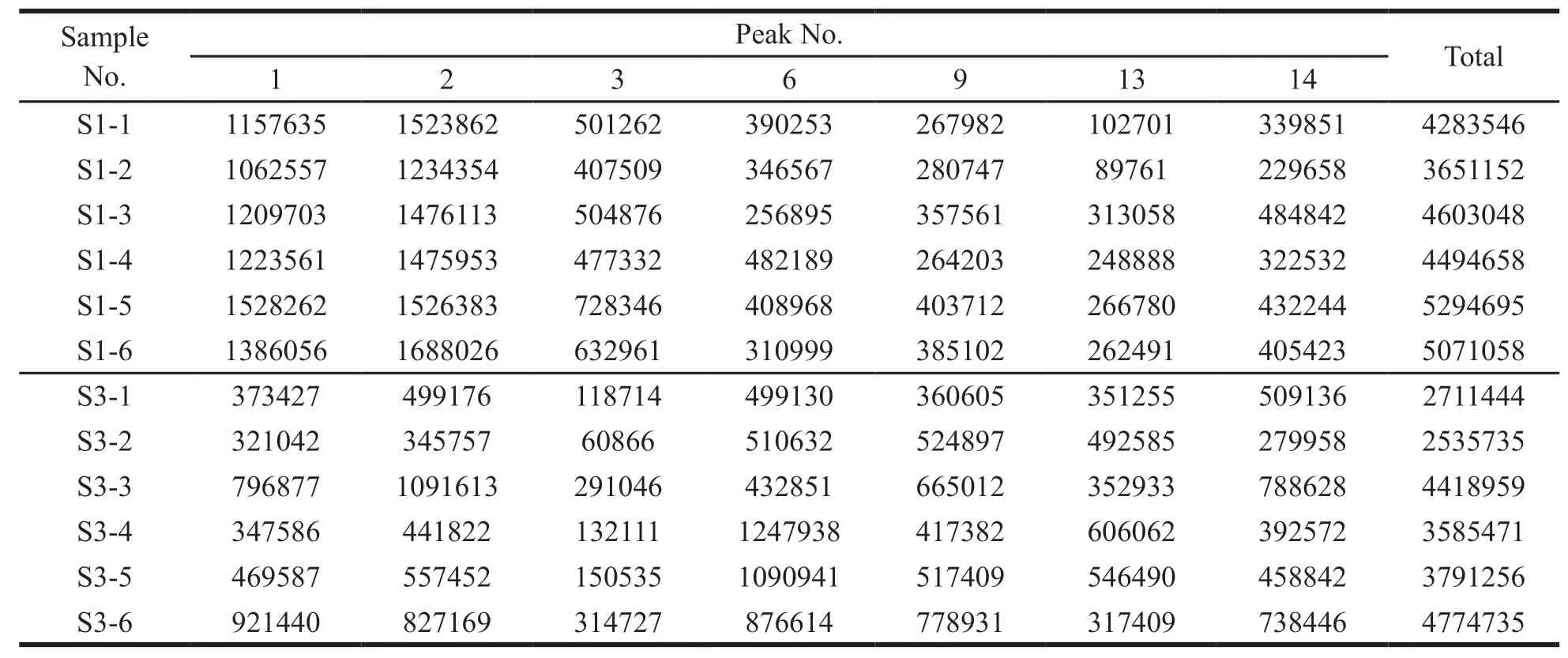
Table 8 The total peak area of marker in samples harvested in January and March

Table 9 Result of paired sample t test
4 Conclusion
The chemical constituents of mistletoe were studied.8 compounds were isolated,three of which were isolated from the genusViscumL.for the first time.In addition,based on the HPLC fingerprint method of mistletoe established in the previous study,16 common peaks were obtained,4 of which were identified.The established fingerprint was used to analyze the chemical profiles of mistletoe harvested in different seasons,and the results showed that the chemical profiles of mistletoe in different months were slightly different.Among them,the chemical profile of mistletoe harvested in January showed a marked difference compared with those harvested in other months,and the comparison of the total area of common peak indicated that the quality of mistletoe in January might be better.The HPLC fingerprint method reveals the chemical profile of mistletoe objectively and can be used to evaluate and control the quality of mistletoe medicinal materials harvested in different months.
Acknowledgement
This work was supported by the Natural Science Foundation of Liaoning Province (No.20180551065).
 Asian Journal of Traditional Medicines2020年4期
Asian Journal of Traditional Medicines2020年4期
- Asian Journal of Traditional Medicines的其它文章
- Contribution Regulations for Asian Journal of Traditional Medicines
- A review of phytochemistry and pharmacology perspectives of Gentiana rhodantha Franch.ex Hemsl.
- A review of research on coumarins from Radix Glehniae
- Phenolic compounds from Peanut testa
- Impact of polyherbal formulations MEF-4 and MEF-8 on high fat diet induced obesity in SD rats
- Flavonoids with cytotoxicities from the seeds of Pongamia pinnata (L.) Pierre
