Flavonoids with cytotoxicities from the seeds of Pongamia pinnata (L.) Pierre
Wanru Jiang,Yuan Gao,Jiaxin Qi,Gang Chen,Di Zhou*,Ning Li*
Key Laboratory of Computational Chemistry-Based Natural Antitumor Drug,School of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University,Shenyang 110016,China
Abstract Twenty-eight compounds,including flavones (2,4-5,7-11),flavonols (1,3,6,21,22),flavanones (12-18),isoflavones (19,20),chalcones (23,24),phenylpropanoids (25,26),and others (27,28) were isolated from the ethanol extract of the seeds of Pongamia pinnata (L.) Pierre and identified on the basis of physic-chemical constants and spectral analysis (NMR,ECD,[α]20D).Among them,compounds 16,18,21,23,25 and 26 were obtained from thegenusfor the first time.The cytotoxicities of the purified flavonoids against H292 cells were evaluated using MTT assays.As a result,compounds 14 and 15 displayed moderate cytotoxicities.
Key words:Pongamia pinnata (L.) Pierre; constituent; flavonoids; cytotoxicities
1 Introduction
Pongamia pinnata(L.) Pierre (P.pinnata)is a semi-mangrove plant in Leguminosae family,growing near fresh water and coastal areas [1].P.pinnataoriginated from India and Southeast Asia,and has been successfully introduced into the hot and humid areas in China,the United States,Australia and other countries in recent years [2].
P.pinnatahas rich medicinal value.It is commonly used as folk treatment of leucoderma,leprosy,rheumatism and other diseases.At present,175 compounds have been isolated and identified from the seeds,leaves,flowers,bark,stem bark and heartwood ofP.pinnata,which are mainly divided into flavonoids,triterpenes and others [3-7].Modern pharmacological studies have shown that it has antiinflammatory,analgesic,anti-dysentery,anti-ulcer,anti-tumor,and other effects [3,4].
Researchers have selected the stems,seeds,and seedpods ofP.pinnataand compared their compositions and activities.Results showed that they all contain flavonoids and alkaloids,and flavonoids are the most important components.The components that exhibit anti-neuroinflammatory activity from the stems ofP.pinnataare pongaglabol methyl ether,lonchocarpin,and glabrachromene II [8],the component that exhibits cytotoxic activity from the seeds is lonchocarpin [9],and the component that exhibits anti-neuroinflammatory activity from the seedpods is lanceolatin B [7] (Fig.1).
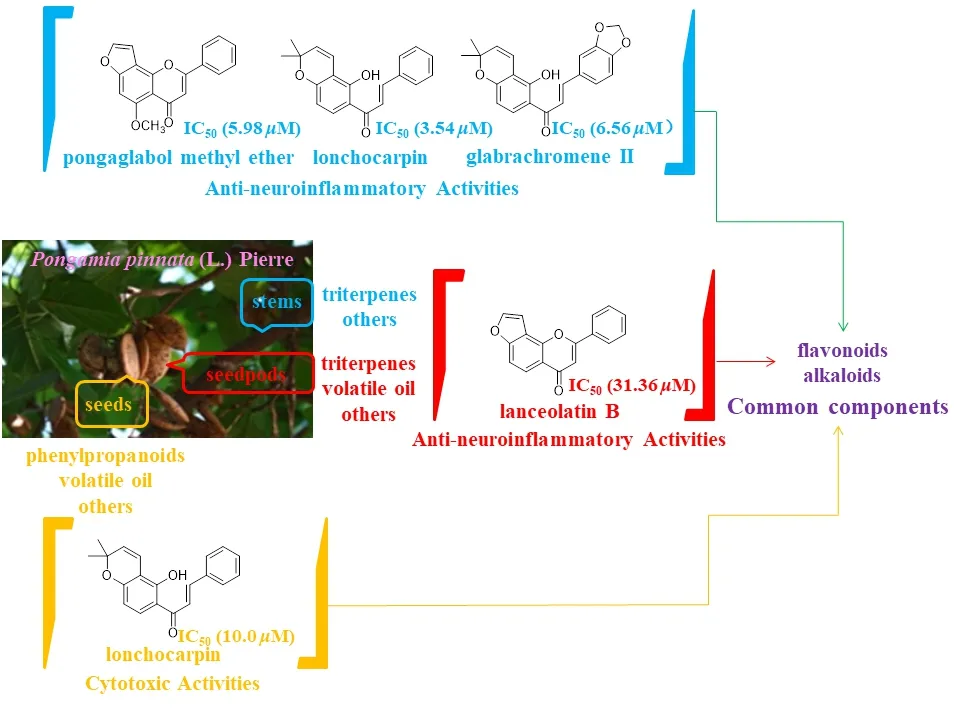
Fig.1 Comparison of compositions and activities of stems,seeds,and seedpods of P.pinnata
Our research group has investigated the antitumor activities of different medicinal parts (stems,seeds,seedpods) ofP.pinnata.In this paper,the chemical constituents and cytotoxicities of the seeds ofP.pinnatawere further studied in order to clarify the material basis of its pharmacodynamics.
2 Materials and methods
2.1 General experimental procedures
The chemical reagents used in the column chromatography were produced by Chemical Branch of Shandong Yuwang Industry Co.,Ltd..Methanol and acetonitrile used in HPLC were produced by Tianjin kangkede Technology Co.,Ltd..Dimethyl sulfoxide (DMSO),3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and cisplatin (DDP) were purchased from Sigma-Aldrich Company (St.Louis,MO,USA).RPMI-1640 medium and fetal bovine serum were obtained from Gibco company (NY,USA),and the 96-well cell culture plates from Thermo (USA).NCI-h292 cell line (ZQ0388) was provided by Shanghai Zhongqiaoxinzhou Biotech.
The NMR spectra were recorded on Bruker ARX-400 and Bruker-AV-600 spectrometers(Bruker Corporation,Bremen,Germany),using trimethylchlorosilane (TMS) as the internal standard.Analytical HPLC Unitary C18column (5μm,150 mm × 4.6 mm) was used and preparative HPLC was isolated with a Shimadzu LC-6AD instrument,using YMC-Pack ODS-A column (5μm,250 mm ×20 mm) and HPLC YMC-Pack ODS-A column(5μm,250 mm × 10 mm).Polarimeter was a Perkin-Elmer 341MC produced by Perkin Elmer Instruments Shanghai Co.,Ltd..CD spectrum was collected by Bio-Logic Science MOS-450 (French).Silica gel (200-300 mesh) was produced by Qingdao Ocean Chemical Factory.ODS (50μm) was produced by YMC Co.,Ltd..
2.2 Plant material
The seeds ofP.pinnatawere collected and identified by Professor Chunyan Yan,College of Pharmacy,Guangdong Pharmaceutical University.A voucher specimen (No.201307) is deposited in School of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University.
2.3 Extraction and isolation
The seeds ofP.pinnata(8.1 kg) were powdered and extracted with 95% EtOH.Then,the crude extract (1557.6 g) was suspended in hot water and partitioned with petroleum ether (PE),ethyl acetate(EtOAc) and n-butyl alcohol (n-BuOH) successively.
The PE extract (170.0 g) was subjected to silica gel column chromatography and eluted with petroleum ether-acetone (100:0-0:100) to give fractions A (100:2),B (100:5),C (100:20),and D(1:1).Fractions A and B were purified by an ODS column using a gradient solvent system MeOHH2O to give fractions A1,A2,B1,and B2.As a result,Compounds 7 (4.5 mg) and 28 (17.6 mg)were obtained from fraction A1,1 (5.3 mg) and 14(4.8 mg) from fraction B1,2 (4.5 mg) from fraction C,and 10 (6.2 mg) from fraction D by recrystallization,respectively.Fractions A2 and B2 were further purified by preparative HPLC.Compounds 12 (2.8 mg),13 (3.7 mg),17 (4.4 mg),20 (4.2 mg),and 21 (8.3 mg) were obtained from fraction A2.Compounds 3 (3.7 mg),4 (4.1 mg),9 (3.2 mg),11 (3.0 mg),and 22 (3.5 mg) were obtained from fraction B2.
The EtOAc extract (45.0 g) was subjected to silica gel adsorption column chromatography and eluted with CH2Cl2-MeOH (100:0-0:100) to give fractions E (100:0),F (100:0-100:2),and G (100:2-100:4).Fractions E and F were isolated by an ODS column using a gradient solvent system MeOHH2O as eluent and further purified by preparative HPLC.As a result,fraction E afforded compounds 14 (2.5 mg),15 (3.6 mg),and 16 (3.8 mg);fraction F gave 5 (2.8 mg),16 (4.9 mg),18(2.9 mg),19 (3.1 mg),20 (4.1 mg),21 (3.4 mg),23(3.5 mg),24 (8.2 mg),25 (3.2 mg),and 26 (3.6 mg).Fraction G was purified by an ODS column using a gradient solvent system MeOH-H2O to give fractions G1,and G2.Compound 1 (774.3 mg) was obtained from fraction G1 by recrystallization.Fraction G2 was further purified by preparative HPLC to get compounds 2 (6.2 mg),6 (5.3 mg),7 (3.2 mg),8 (5.2 mg),and 10 (2.6 mg).
Then-BuOH extract (42.5 g) was repeatedly subjected to silica gel column chromatography and gradient elution with CH2Cl2/MeOH solvent system,from which compound 27 (567.8 mg) was isolated.
2.4 Cytotoxic activities
The cytotoxicities of the identified flavonoids against H292 human lung cancer cells were determined by MTT assays.
DDP was selected as positive control.Samples were dissolved in DMSO to obtain stock solutions with concentrations of 1000.0 mM,400.0 mM,200.0 mM,100.0 mM,75.0 mM,50.0 mM,37.5 mM,25.0 mM,10.0 mM,and 1.0 mM.H292 cells were seeded into 96-well culture plates (5×104cells/well) for 24 h.The cells were cultured with different concentrations of sample solutions (1.0μM,10.0μM,25.0μM,37.5μM,50.0μM,75.0μM,100.0μM,200.0μM,400.0μM and 1000.0μM) for 48 h,and then cell survival rates were measured by MTT assays.The activity data of specific monomer compounds have been published in reference [9].
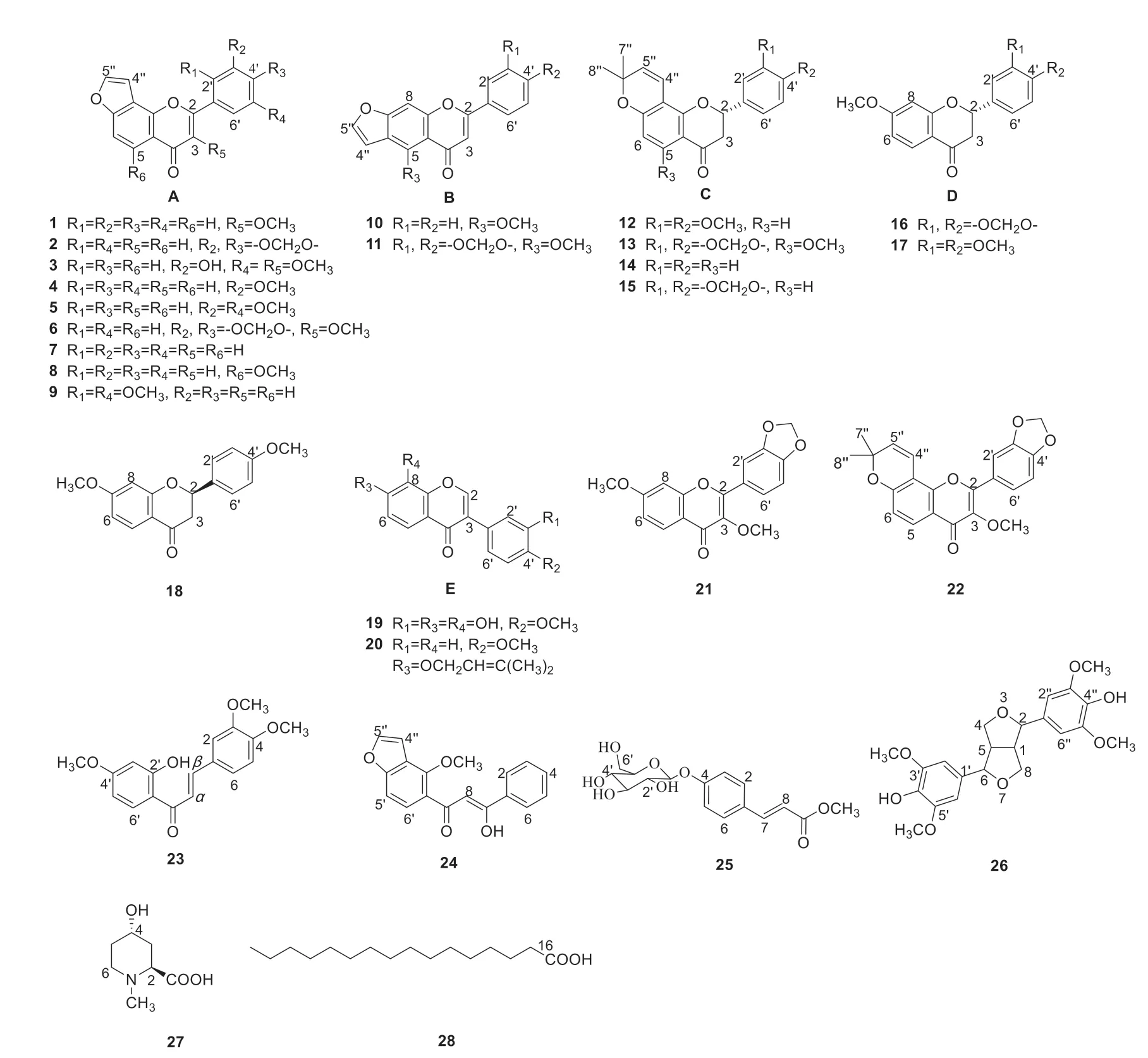
Fig.2 The structures identified from the seeds of P.pinnata
3 Results
Twenty-eight compounds in Fig.2,including flavones (2,4-5,7-11),flavonols (1,3,6,21-22),flavanones (12-18),isoflavones (19,20),chalcones(23,24),phenylpropanoids (25,26),and others (27,28) were isolated from 95% ethanol extract of the seeds ofP.pinnata.Among them,compounds 16,18,21,23,25 and 26 were obtained from the genus for the first time.
Compound 1 was isolated as a white crystal(PE-EtOAc).1H-NMR (400 MHz,DMSO-d6):δH8.16 (2H,m,H-2',6'),7.62 (3H,m,H-3',4',5'),8.03(1H,d,J=8.8 Hz,H-5),7.78 (1H,d,J=8.8 Hz,H-6),8.26 (1H,d,J=2.2 Hz,H-5''),7.46 (1H,d,J=2.2 Hz,H-4''),3.86 (3H,s,3-OCH3).Based on the above NMR data and compared with the known compound in reference [10],compound 1 was identified as karanjin.
Compound 2 was isolated as a colorless needle crystal (CH2Cl2-MeOH).1H-NMR (400 MHz,DMSO-d6):δH8.17 (1H,d,J=8.8 Hz,H-5),6.98(1H,d,J=8.8 Hz,H-6),7.21 (1H,d,J=2.0 Hz,H-2'),7.56 (2H,m,H-5',6'),7.42 (1H,d,J=2.2 Hz,H-4''),7.78 (1H,d,J=2.2 Hz,H-5''),6.82 (1H,s,H-3),6.11 (2H,s,3',4'-OCH2O-).Based on the above NMR data and compared with the known compound in reference [11],compound 2 was identified as pongaglabrone.
Compound 3 was isolated as a white needle crystal (CH2Cl2-MeOH).1H-NMR (600 MHz,CDCl3):δH8.19 (1H,d,J=8.8 Hz,H-5),8.17(1H,d,J=8.8 Hz,H-6),7.78 (1H,d,J=2.2 Hz,H-5''),7.74 (1H,d,J=2.2 Hz,H-4''),6.76 (1H,d,J=2.8 Hz,H-2'),6.69 (1H,d,J=2.8 Hz,H-6'),6.67 (1H,brs,H-4'),3.96 (3H,s,3-OCH3),3.87 (3H,s,5'-OCH3).Based on the above NMR data and compared with the known compound in reference [12],compound 3 was identified as pongapinnols A.
Compound 4 was isolated as a colorless needle crystal (CH2Cl2-MeOH).1H-NMR (600 MHz,CDCl3):δH6.87 (1H,s,H-3),7.78 (1H,d,J=2.2 Hz,H-5''),7.22 (1H,d,J=2.2 Hz,H-4''),8.17 (1H,d,J=8.8 Hz,H-5),7.57 (1H,m,H-6),7.48 (1H,brs,H-2'),7.46 (1H,m,H-5'),7.10 (1H,dd,J=8.2,2.6 Hz,H-4'),7.57 (1H,m,H-6'),3.92 (3H,s,3'-OCH3).Based on the above NMR data and compared with the known compound in reference [13],compound 4 was identified as 2-(3-methoxyphenyl)-4H-furo[2,3-h]-1-benzoyran-4-one.
Compound 5 was isolated as a white needle crystal (PE-Acetone).1H-NMR (600 MHz,DMSO-d6):δH7.21 (1H,s,H-3),8.27 (1H,d,J=2.2 Hz,H-5"),7.62 (1H,d,J=2.2 Hz,H-4"),7.99(1H,d,J=8.7 Hz,H-5),7.78 (1H,d,J=8.7 Hz,H-6),7.30 (2H,d,J=2.1 Hz,H-2',6'),6.76 (1H,t,J=2.1 Hz,H-4'),3.88 (s,6H,3',5'-OCH3).Based on the above NMR data and compared with the known compound in reference [14],compound 5 was identified as 3',5'-dimethoxy-[2",3",7,8]-furanoflavone.
Compound 6 was isolated as a light yellow needle crystal (CH2Cl2-MeOH).1H-NMR (400 MHz,DMSO-d6):δH8.23 (1H,d,J=2.0 Hz,H-5''),7.68 (1H,d,J=2.0 Hz,H-4''),7.99 (1H,d,J=8.8 Hz,H-5),7.75 (1H,m,H-6),7.75 (1H,m,H-6'),7.54 (1H,d,J=2.2 Hz,H-2'),7.14 (1H,d,J=8.3 Hz,H-5'),6.16 (2H,s,3',4'-OCH2O-),3.83 (3H,s,3-OCH3).Based on the above NMR data and compared with the known compound in reference [10],compound 6 was identified as pongapin.
Compound 7 was isolated as a light yellow needle crystal (CH2Cl2-MeOH).1H-NMR (600 MHz,DMSO-d6):δH7.98 (1H,d,J=8.7 Hz,H-5),7.76(1H,d,J=8.7 Hz,H-6),8.27 (1H,d,J=2.2 Hz,H-5''),7.62 (1H,m,H-4''),8.22 (2H,m,H-2',6'),7.62 (3H,m,H-3',4',5'),7.13 (1H,s,H-3).Based on the above NMR data and compared with the known compound in reference [15],compound 7 was identified as lanceolatin B.
Compound 8 was isolated as a colorless needle crystal (CH2Cl2-MeOH).1H-NMR (600 MHz,CDCl3):δH7.65 (1H,d,J=2.2 Hz,H-5''),7.11 (1H,d,J=2.2 Hz,H-4''),7.94 (2H,m,H-2',6'),7.55(3H,m,H-3',4',5'),6.81 (1H,s,H-3),4.04 (3H,s,5-OCH3),7.02 (1H,s,H-6).Based on the above NMR data and compared with the known compound in reference [16],compound 8 was identified as pongaglabol methyl ether.
Compound 9 was isolated as a white needle crystal (CH2Cl2-MeOH).1H-NMR (600 MHz,DMSO-d6):δH7.00 (1H,s,H-3),8.24 (1H,d,J=2.2 Hz,H-5''),7.46 (1H,d,J=2.2 Hz,H-4''),7.98(1H,d,J=8.7 Hz,H-5),7.76 (1H,d,J=8.7 Hz,H-6),7.52 (1H,d,J=3.1 Hz,H-6'),7.23 (1H,d,J=9.1 Hz,H-3'),7.18 (1H,dd,J=9.1,3.1 Hz,H-4'),3.90 (3H,s,2'-OCH3),3.84 (3H,s,5'-OCH3).Based on the above NMR data and compared with the known compound in reference [17],compound 9 was identified as millettocalyxins C.
Compound 10 was isolated as a yellow amorphous powder (CH2Cl2-MeOH).1H-NMR(400 MHz,CDCl3):δH6.76 (1H,s,H-3),7.62 (1H,d,J=2.4 Hz,H-5''),7.07 (1H,d,J=2.4 Hz,H-4''),7.41 (1H,s,H-8),7.93 (2H,m,H-2',6'),7.53 (3H,m,H-3',4',5'),4.22 (3H,s,5-OCH3).Based on the above NMR data and compared with the known compound in reference [16],compound 10 was identified as pinnatin.
Compound 11 was isolated as a white amorphous powder (CH2Cl2-MeOH).1H-NMR(600 MHz,DMSO-d6):δH6.77 (1H,s,H-3),8.07(1H,d,J=2.3 Hz,H-5''),7.30 (1H,brs,H-4''),7.68(2H,m,H-2',6'),7.11 (1H,d,J=8.7 Hz,H-5'),6.15 (2H,s,3',4'-OCH2O-),4.08 (3H,s,5-OCH3),7.75 (1H,s,H-8).Based on the above NMR data and compared with the known compound in reference [18],compound 11 was identified as gamatin.
Compound 12 was isolated as a light yellow needle crystal (CH2Cl2-MeOH),[α]20D-60.5 (c1.0,CH3CN).The1H-NMR (600 MHz,DMSO-d6):δH5.57 (1H,dd,J=12.6,3.0 Hz,H-2),3.22 (1H,dd,J=16.7,12.6 Hz,H-3α),2.75 (1H,dd,J=16.7,3.0 Hz,H-3β),6.55 (1H,d,J=10.1 Hz,H-4''),5.76(1H,d,J=10.1 Hz,H-5''),1.42 (3H,s,H-7''),1.39(3H,s,H-8''),7.59 (1H,d,J=8.6 Hz,H-5),6.51(1H,d,J=8.6 Hz,H-6),7.15 (1H,d,J=2.0 Hz,H-2'),7.06 (1H,dd,J=8.2,2.0 Hz,H-6'),6.99(1H,d,J=8.2 Hz,H-5'),3.78 (3H,s,3'-OCH3),3.77 (3H,s,4'-OCH3).Based on the above NMR data and compared with the known compound in reference [15],compound 12 was identified as ponganone III.
Compound 13 was isolated as a light yellow crystal (CH2Cl2-MeOH),[α]20D-46.0 (c1.0,CH3CN).The1H-NMR (600 MHz,DMSO-d6):δH5.51 (1H,dd,J=12.9,3.0 Hz,H-2),3.15 (1H,dd,J=16.8,12.9 Hz,H-3α),2.72 (1H,dd,J=16.8,3.0 Hz,H-3β),6.53 (1H,d,J=10.1 Hz,H-4''),5.76 (1H,d,J=10.1 Hz,H-5''),1.42 (3H,s,H-7''),1.40 (3H,s,H-8''),6.04 (2H,s,3',4'-OCH2O-),7.15 (1H,d,J=1.6 Hz,H-2'),7.01 (1H,dd,J=8.0,1.6 Hz,H-6'),6.94 (1H,d,J=8.0 Hz,H-5'),7.14 (1H,s,H-6),3.76 (3H,s,5-OCH3).Based on the above NMR data and compared with the known compound in reference [19],compound 13 was identified as isoglabrachromene.
Compound 14 was isolated as a white needle crystal (PE-Acetone),[α]20D-61.5 (c1.0,CH3CN).1H-NMR (400 MHz,DMSO-d6):δH5.68 (1H,dd,J=12.7,3.0 Hz,H-2),3.16 (1H,dd,J=16.8,12.7 Hz,H-3α),2.81 (1H,dd,J=16.8,3.0 Hz,H-3β),7.57 (1H,m,H-5),6.53 (1H,d,J=8.7 Hz,H-6),7.57 (2H,m,H-2',6'),7.41 (3H,m,H-3',4',5'),6.56 (1H,d,J=10.0 Hz,H-4''),5.76 (1H,d,J=10.0 Hz,H-5''),1.42 (3H,s,H-7''),1.40 (3H,s,H-8'').Based on the above NMR data and compared with the known compound in reference [11,20],compound 14 was identified as isolonchocarpin.
Compound 15 was isolated as a yellow crystal(CH2Cl2-MeOH),[α]20D-48.5 (c1.0,CH3CN).1H-NMR (400 MHz,DMSO-d6):δH5.56 (1H,dd,J=12.8,2.7 Hz,H-2),3.18 (1H,dd,J=16.8,12.8 Hz,H-3α),2.72 (1H,dd,J=16.8,2.7 Hz,H-3β),7.59 (1H,d,J=8.6 Hz,H-5),6.53 (1H,m,H-6),7.16 (1H,d,J=1.4 Hz,H-2'),7.01 (1H,dd,J=1.4,8.2 Hz,H-6'),6.95 (1H,d,J=8.2 Hz,H-5'),6.53 (1H,m,H-4''),5.76 (1H,d,J=10.1 Hz,H-5''),1.42 (3H,s,H-7''),1.39 (3H,s,H-8''),6.05(2H,s,3',4'-OCH2O-).Based on the above NMR data and compared with the known compound in reference [21],compound 15 was identified as ovalichromene B.
Compound 16 was isolated as a yellow crystal(CH2Cl2-MeOH),[α]20D-52.5 (c1.0,CH3CN).1H-NMR (400 MHz,DMSO-d6):δH5.53 (1H,dd,J=13.0,2.8 Hz,H-2),3.19 (1H,dd,J=16.8,13.0 Hz,H-3α),2.68 (1H,dd,J=16.8,2.8 Hz,H-3β),6.04 (2H,s,3',4'-OCH2O-),7.15 (1H,d,J=1.4 Hz,H-2'),7.00 (1H,dd,J=8.0,1.4 Hz,H-6'),6.94 (1H,d,J=8.0 Hz,H-5'),7.71 (1H,d,J=8.8 Hz,H-5),6.66 (1H,dd,J=8.8,2.4 Hz,H-6),6.62 (1H,d,J=2.4 Hz,H-8),3.81 (3H,s,7-OCH3).13C-NMR (100 MHz,DMSO-d6) can be seen in Table 1.Based on the above NMR data and compared with the known compound in reference [22],compound 16 was identified as 2-(benzo[d][1,3]dioxol-5-yl)-7-methoxychroman-4-one.
Compound 17 was isolated as a light yellow needle crystal (CH2Cl2-MeOH),[α]20D-42.5 (c1.0,CH3CN).1H-NMR (600 MHz,DMSO-d6):δH5.54(1H,dd,J=13.1,2.8 Hz,H-2),3.24 (1H,dd,J=16.8,13.1 Hz,H-3α),2.69 (1H,dd,J=16.8,2.8 Hz,H-3β),7.72 (1H,d,J=8.8 Hz,H-5),6.66 (1H,dd,J=2.4,8.8 Hz,H-6),6.63 (1H,d,J=2.4 Hz,H-8),7.16 (1H,d,J=2.0 Hz,H-2'),7.05 (1H,dd,J=8.3,2.0 Hz,H-6'),6.98 (1H,d,J=8.3 Hz,H-5'),3.81 (3H,s,7-OCH3),3.78 (3H,s,3'-OCH3),3.77 (3H,s,4'-OCH3).Based on the above NMR data and compared with the known compound in reference [23,24],compound 17 was identified as 3',4',7-trimethoxyflavanone.
Compound 18 was isolated as a white crystal(CH2Cl2-MeOH),[α]20D+3.0 (c1.0,CH3CN).1H-NMR (400 MHz,DMSO-d6):δH4.71 (1H,dd,J=8.7,4.6 Hz,H-2),3.43 (1H,dd,J=16.3,8.7 Hz,H-3α),3.20 (1H,dd,J=16.3,4.6 Hz,H-3β),7.49(1H,d,J=8.6 Hz,H-5),7.35 (2H,m,H-6,8),8.05(1H,d,J=2.2 Hz,H-2'),7.35 (3H,m,H-3',5',6'),4.12 (3H,s,7-OCH3),3.04 (3H,s,4'-OCH3).Based on the above NMR data and compared with the known compound in reference [25],compound 18 was identified as 7-methoxy-2-(4-methoxyphenyl)chroman-4-one.
Compound 19 was isolated as a yellow needle crystal (CH2Cl2-MeOH).1H-NMR (600 MHz,DMSO-d6):δH8.33 (1H,s,H-2),7.46 (1H,d,J=8.7 Hz,H-5),6.95 (1H,m H-6),7.05 (1H,brs,H-2'),6.95 (2H,m,H-5',6'),3.79 (3H,s,4'-OCH3).Based on the above NMR data and compared with the known compound in reference [26],compound 19 was identified as 3',7,8-trihydroxy-4'-methoxyisoflavone.
Compound 20 was isolated as a white needle crystal (EA-PE).1H-NMR (600 MHz,DMSO-d6):δH8.42 (1H,s,H-2),8.02 (1H,d,J=8.8 Hz,H-5),7.16 (1H,d,J=2.3 Hz,H-8),7.06 (1H,dd,J=8.8,2.3 Hz,H-6),7.53 (2H,m,H-2',6'),6.99 (2H,d,J=8.6 Hz,H-3',5'),5.47 (1H,t,J=6.7 Hz,H-3''),4.68(2H,d,J=6.7 Hz,H-2''),1.76 (3H,s,H-4''),1.74(3H,s,H-5''),3.79 (3H,s,4'-OCH3).Based on the above NMR data and compared with the known compound in reference [27],compound 20 was identified as maxima isoflavone J.
Compound 21 was isolated as a colorless needle crystal (CH2Cl2-MeOH).1H-NMR (600 MHz,DMSO-d6):δH6.16 (2H,s,3',4'-OCH2O-),7.96(1H,d,J=8.9 Hz,H-5),7.31 (1H,d,J=2.3 Hz,H-8),7.06 (1H,dd,J=8.9,2.3 Hz,H-6),7.69 (1H,dd,J=8.3,1.7 Hz,H-6'),7.62 (1H,d,J=1.7 Hz,H-2'),7.14 (1H,d,J=8.3 Hz,H-5'),3.90 (3H,s,3-OCH3),3.80 (3H,s,7-OCH3).Based on the above NMR data and compared with the known compound in reference [28],compound 21 was identified as maxima desmethoxykanugin.
Compound 22 was isolated as a white needle crystal (CH2Cl2-MeOH).1H-NMR (600 MHz,CDCl3):δH7.83 (1H,d,J=8.7 Hz,H-5),6.91 (1H,m,H-6),7.64 (1H,dd,J=8.3,1.8 Hz,H-6'),7.57(1H,d,J=1.8 Hz,H-2'),7.12 (1H,d,J=8.3 Hz,H-5'),6.16 (2H,s,3',4'-OCH2O-),3.79 (3H,s,3-OCH3),6.91 (1H,m,H-4"),5.95 (1H,d,J=10.0 Hz,H-5"),1.46 (6H,s,H-7",8").Based on the above NMR data and compared with the known compound in reference [10],compound 22 was identified as pongachromene.
Compound 23 was isolated as a yellow crystal(CH2Cl2-MeOH).1H-NMR (400 MHz,DMSO-d6):δH7.90 (1H,d,J=15.3 Hz,H-β),7.80 (1H,d,J=15.3 Hz,H-α),13.66 (1H,s,-OH),8.31 (1H,d,J=8.4 Hz,H-6'),7.58 (1H,d,J=2.0 Hz,H-3'),7.42(1H,dd,J=8.4,2.0 Hz,H-5'),7.04 (1H,d,J=8.8 Hz,H-5),6.57 (1H,dd,J=8.8,2.4 Hz,H-6),6.52 (1H,d,J=2.4 Hz,H-2),3.87 (3H,s,3-OCH3),3.85 (3H,s,4-OCH3),3.83 (3H,s,4'-OCH3).13C-NMR (150 MHz,DMSO-d6) can be seen in Table 1.Based on the above NMR data and compared with the known compound in reference [29],compound 23 was identified as 2'-hydroxy-3,4,4'-trimethyoxy-chalcone.
Compound 24 was isolated as a colorless prismatic crystal (CH2Cl2-MeOH).1H-NMR(600 MHz,DMSO-d6):δH8.08 (1H,d,J=2.2 Hz,H-5''),7.37 (1H,brs,H-4''),7.80 (1H,d,J=8.7 Hz,H-6'),7.42 (1H,d,J=8.7 Hz,H-5'),8.01 (2H,m,H-2,6),7.56 (3H,m,H-3,4,5),7.22 (1H,s,H-8),4.22 (3H,s,2'-OCH3).Based on the above NMR data and compared with the known compound in reference [30],compound 24 was identified as pongamol.
Compound 25 was isolated as a yellow amorphous powder (MeOH).1H-NMR (600 MHz,DMSO-d6):δH7.67 (2H,d,J=8.8 Hz,H-2,6),7.05(2H,d,J=8.8 Hz,H-3,5),7.62 (1H,d,J=16.0 Hz,H-7),6.52 (1H,d,J=16.0 Hz,H-8),4.94 (1H,d,J=7.4 Hz,H-1'),3.68 (1H,m,H-6'α),3.45 (1H,m,H-6'β),3.37 (1H,m,H-5'),3.26 (2H,m,H-4',2'),3.16 (1H,m,H-3'),3.70 (3H,s,9-OCH3),5.35(1H,s,-OH),5.12 (1H,s,-OH),5.04 (1H,s,-OH),4.58 (1H,s,-OH).13C-NMR (150 MHz,DMSO-d6)can be seen in Table 1.Based on the above NMR data and compared with the known compound in reference [31],compound 25 was identified as linocinnamarin.
Compound 26 was isolated as a white needle crystal (MeOH).1H-NMR (600 MHz,DMSO-d6):δH8.28 (2H,s,4',4''-OH),6.59 (4H,s,H-2',2'',6',6''),4.61 (2H,d,J=3.8 Hz,H-2,6),4.15 (2H,dd,J=6.8,8.8 Hz,H-4a,8a),3.77 (2H,m,H-4b,8b),3.75(12H,s,-OCH3),3.04 (2H,m,H-1,5).Based on the above NMR data and compared with the known compound in reference [32],compound 26 was identified as syringaresinol.
Compound 27 was isolated as a colorless prismatic crystal (MeOH).1H-NMR (600 MHz,D2O):δH4.14 (1H,s,H-4),3.69 (1H,d,J=10.1 Hz,H-2),3.29 (2H,m,H-6),2.83 (3H,s,-CH3),2.13(1H,d,J=14.6 Hz,H-3),1.91 (3H,m,H-3,5).13C-NMR (150 MHz,D2O):δC173.5 (C=O),63.6(C-4),60.5 (C-6),48.5 (CH3),42.1 (C-2),33.8(C-5),28.5 (C-3).In CD spectrum,there was a positive Cotton effect at 250 nm.According to the reference [33],the absolute configuration was (2S,4R).Based on the above NMR data and compared with the known compound in reference [32],compound 27 was identified as (2S,4R)-4-hydroxy-N-methylpipecolic acid.
Compound 28 (EI-MSm/z:256[M]+) was isolated as a white needle crystal (MeOH-CH2Cl2).1H-NMR (600 MHz,CDCl3):δH2.34 (2H,t,J=7.4 Hz),1.63 (2H,m),1.27 (24H,s),0.88 (3H,t,J=6.8 Hz).13C-NMR (150 MHz,CDCl3):δC180.3(C-16),34.2 (C-15),32.1 (C-3),29.9 (C-13),29.9(C-12),29.8 (C-11),29.8 (C-10),29.8 (C-9),29.7(C-8),29.6 (C-7),29.5 (C-6),29.4 (C-5),29.2 (C-4),24.8 (C-14),22.8 (C-2),14.3 (C-1).Based on the above NMR data and compared with the known compound in reference [34],compound 28 was identified as palmitic acid.
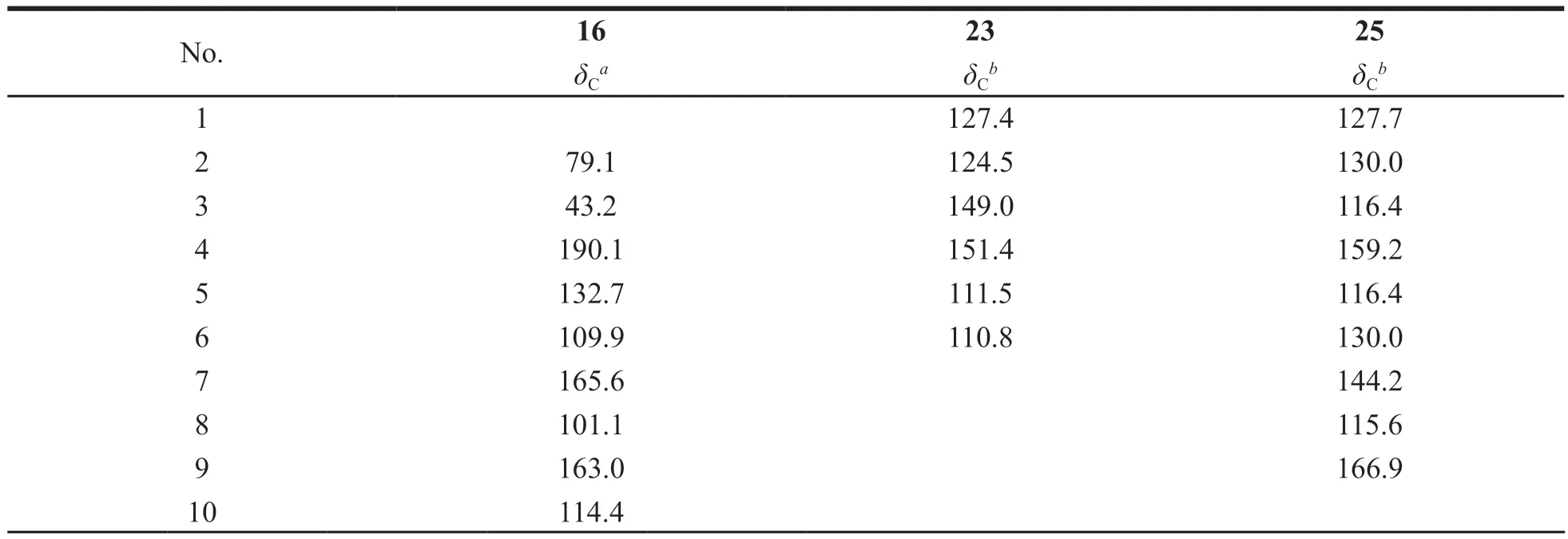
Table 1 The 13C-NMR data of compounds 16,23,and 25
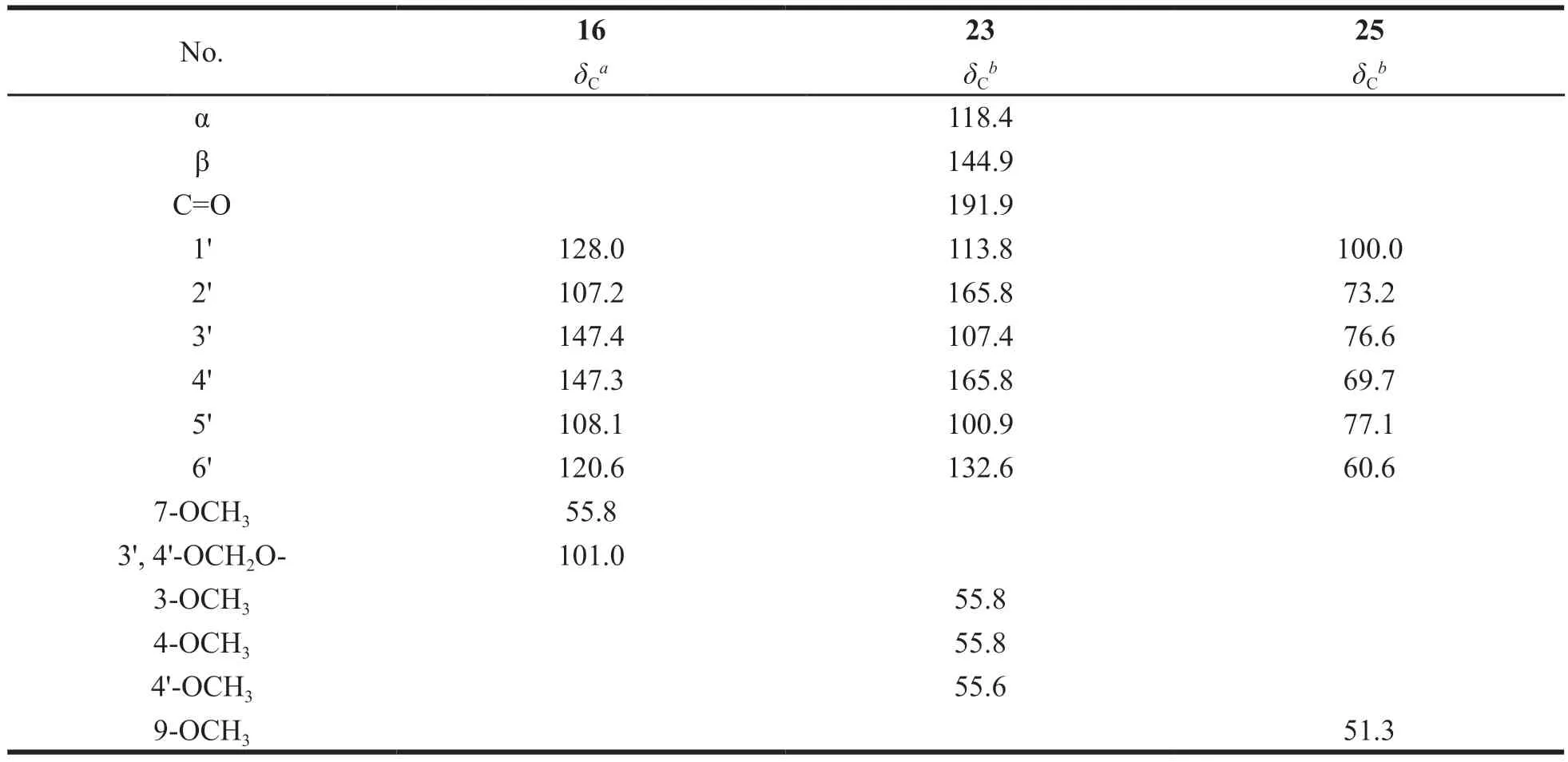
Continued table 1
3.2 Cytotoxic activities
The cytotoxic activities of sixteen flavonoids isolated from the seeds ofP.pinnataagainst H292 cells were evaluated by using MTT assays.Compounds 14 (IC50,50.0μM) and 15 (IC50,61.5μM) showed moderate cytotoxic activities,while compounds 5 (IC50,158.5μM),20 (IC50,293.8μM) and 22 (IC50,100.0μM) exhibited weakly cytotoxic activities.
4 Conclusion
The detailed investigation on the 95%ethanol extract of the seeds ofP.pinnataled to the isolation and structural elucidation of twenty-eight compounds.Among them,compounds 16,18,21,23,25 and 26 were obtained from thePongamiaVent.for the first time.
Their cytotoxic activities were further evaluated by MTT assays.The results showed that compounds 14 and 15 had moderate cytotoxic activities,while compounds 5,20 and 22 had weak cytotoxic activities.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Grant No.81872768,81673323,U1903122),Liaoning Revitalization Talents Program (XLYC1807118),and Liaoning BaiQianWan Talents Program (2018).
 Asian Journal of Traditional Medicines2020年4期
Asian Journal of Traditional Medicines2020年4期
- Asian Journal of Traditional Medicines的其它文章
- Contribution Regulations for Asian Journal of Traditional Medicines
- A review of phytochemistry and pharmacology perspectives of Gentiana rhodantha Franch.ex Hemsl.
- A review of research on coumarins from Radix Glehniae
- Phenolic compounds from Peanut testa
- Study on chemical composition of Viscum coloratum and its HPLC fingerprint in different harvest periods
- Impact of polyherbal formulations MEF-4 and MEF-8 on high fat diet induced obesity in SD rats
