Ratiometric delivery of doxorubicin and berberine by liposome enables superior therapeutic index than Doxil ®
,Yongjun Wng,
a Wuya College of Innovation,Shenyang Pharmaceutical University,Shenyang 110016,China
b Department of Pharmaceutics,Shenyang Pharmaceutical University,Shenyang 110016,China
c Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education,Shenyang Pharmaceutical University,Shenyang 110016,China
Keywords:Berberine Doxorubicin Nanoliposomes Remote loading Combination therapy Cardiotoxity
ABSTRACT Although the appearance of Doxil alleviated the cardiotoxicity of DOX,the progression-free survival of patients was not prolonged compared with traditional medication regimens,and side effects such as hand-foot syndrome has occurred.In order to solve this dilemma,we have designed a novel co-delivery strategy to construct a co-loaded liposome of berberine(BER) and doxorubicin (DOX),which was called LipoBeDo.The optimal synergistic ratio of the two drugs was screened by cell cytotoxicity experiments in vitro,and the optimal attenuation ratio was further determined by in vivo cardiac H&E staining pathological sections.The optimal combination treatment caused a robust increase in apoptotic cells of 4T1,as compared to drug alone treatment.The prepared co-loaded liposome,LipoBeDo,had high encapsulation efficiency and good stability.The nanoliposome carrier controlled the biological fate of the drugs and maintained a pre-defined optimal ratio in vivo.The LipoBeDo significantly inhibited tumor growth in 4T1 murine mammary carcinoma model compared with Doxil (P <0.05),and completely overcame the myocardial rupture toxicity caused by Doxil in mice.Our co-loaded liposome delivery platform technology provided a new direction for the clinical treatment of triple-negative breast cancer and the safe application of DOX.
1.Introduction
Doxorubicin,also known as adriamycin,has been extensively applied in treating a variety of cancers,such as leukemia,lymphomas,breast carcinoma,osteogenic sarcoma,Kaposi’s sarcoma [1].However,long-term administration of DOX can induce cardiotoxicity,which is a major dose-limiting toxicity,and severe patients develop congestive heart failure(CHF) [2].The appearance of liposomal DOX (Doxil®in America or Caelyx®in Canada and Europe) was reported to significantly reduce cardiac toxicity of DOX,and raise the median cumulative dose that causes the patient to develop symptomatic CHF from 492 mg/m2to 1500 mg/m2in Doxil and 785 mg/m2in Caelyx [2—5].Although Doxil tremendously decreased cardiotoxicity compared with free drugs,clinical trials have revealed that the progressionfree survival and overall survival (OS) of liposomal DOX was not different from conventional DOX solution [6].Furthermore,there were some side effects that have not been observed in free DOX:mucosal and cutaneous toxicities,such as palmar-plantar erythrodysesthesia syndrome (PPE) or hand and foot syndrome and mild myelosuppression which were related to the dose and the interval between doses [7—9].It has been reported that DOX-induced cardiotoxicity is aggravated by the re-use of DOX liposomes or in combination with cyclophosphamide,and even some patients develop CHF in clinical application[8—12].Thus,the use of liposomal DOX is not absolutely safe,and the efficacy of Doxil needs to be increased to reduce its dosage.
Combination chemotherapy is a new trend focused on achieving a long-term prognosis and minimizing adverse side effects by simultaneously administrating two or more therapeutic agents [13,14].Unlike single drugs,combination chemotherapy can regulate different signaling pathways in tumor cells,causing a synergistic response that maximizes treatment effectiveness [15—18].Here,The combination of berberine(BER)and DOX was selected.
Berberine,an alkaloid isolated from the Coptis Chinensis,has drawn a lot of attention due to its anti-tumor activities and cardiovascular effects in the last decades [19,20].The recent studies have confirmed that BER has pro-apoptotic,anti-proliferation,anti-invasion,anti-angiogenesis and anti-metastasis effects on some tumor cells.BER has been proven to inhibit proliferation of A549 and H1299 human non-small cell lung cancer cells by decreasing mitochondrial membrane potential and the expression of Bcl-2 and Bcl-xl caused by the increased expression of a related antagonist (Bak) of Bax and Bcl-2 [21].Additionally,BER can induce apoptosis in osteosarcoma cells by leaving the cell cycle arrest in G1 and G2/M phases.G1 arrest was dependent on the presence of p53 because G1 arrest was eliminated in p53-deficient osteosarcoma cells,and G2/M was not.G1 arrest and induction of apoptosis were accompanied by p53-dependent up-regulation of p21 and pro-apoptotic genes [22].It was reported that BER could inhibit the cell proliferation and induce cell cycle arrest and apoptosis in BIU-87 and T24 bladder cancer cell lines by promoting cell cycle arrest at G0/G1 and increase the cleaved caspase-3 and caspase-9 protein expressions in a dosedependent manner [23].Moreover,BER has cardiovascular effects such as positive inotropic effects,negative chronotropic,antiarrhythmic,and vasodilator properties.Some experimental and clinical trials have shown that BER hydrochloride can effectively alleviate the symptoms of congestive heart failure[24].
It was reported that the combination of BER and DOX may be an effective treatment for tumors,they are acting on different targets in the cancer cells and affect different phases in the cell cycle.Mittal et al.reported that cell growth inhibition by BER and DOX may be accompanied by induction of cell cycle arrest with reduced number of proliferating cells in S phase,the combination of the drugs showed greater inhibitory effect on Akt phosphorylation compared with drug alone[25].Yue et al.found that BER sensitized DOX chemotherapy through the dose-orchestrated AMPK signaling pathway [26].Zhu et al.covered that BER had the ability to inhibit DOX-mediated signal transducer and activator of transcription 3(STAT3)activation and sensitized the cytotoxic effect of treatment of DOX [27].Tong et al.reported the combination of DOX and BER produced synergistic effects in A549 (CI=0.61) and HeLa (CI=0.73) cells [28].Furthermore,many studies have covered that BER can ameliorate DOXinduced cardiotoxicity.BER pretreatment inhibits DOXinduced caspase 9 and 3 activation and up-regulated Sirt3 and Sirt1 protein levels in H9c2 cardiomyoblasts.In addition,BER modulated cell death and autophagy in H9c2 cells treated with DOX [29,30].BER reduced DOX-induced cardiomyocyte apoptosis by decreasing mitochondrial membrane potential,inhibiting the increase of AMP/ATP ratio and AMPKa phosphorylation as well as increasing Bcl-2 expression[31].In another study,rats were intraperitoneally injected with DOX while continuously instilling equal doses of BER solution,and the serum creatine kinase (CK),creatine kinase isoenzyme(CK-MB)and malondialdehyde(MDA)levels were significantly increased[32].However,most of these studies have stepped at the cellular levelin vitro.In vivostudies have also been limited to oral or intraperitoneal administration,causing intravenous administration of BER solution was complicated by lethal cardiac suppression,hypotension and vasodilation [33],which cannot truly reflect the combined effects of BER and DOX.
Major challenge in the optimization of drug combination therapy is the ability to transfer the benefits of synergistic effects observedin vitroto thein vivosetting [27].In our study,the nanocarrier of liposome was applied to deliver a predefined ratio of BER and DOX,which can efficiently and stably co-remotely load two drugs.The fixed ratio was jointly evaluated by the synergistic anti-tumor effectin vitroand the reduction of cardiac toxicityin vivo.
Applying a 4T1 mouse tumor model,the efficacy of the co-loaded liposomesin vivoshowed the following characteristics:a strong synergistic inhibition of tumor growth was achieved,and the efficacy was greatly improved compared to the commercial Doxil effect;histopathological sections showed that co-encapsulated drug liposomes can completely overcome the DOX-induced myocardial rupture and dissolution;this novel combined drug delivery system provides new ways for safe application of DOX.
2.Materials and methods
2.1.Drugs,lipids and reagents
DOX hydrochloride (DOX) and BER hydrochloride (BER)were purchased from Dalian Meilun Biotechnology Co.,LTD.(Dalian,China).Hydrogenated Soybean Phospholipids(HSPC),and cholesterol and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000] (DSPE-MPEG2000) were purchased from Shanghai A.V.T Pharmaceutical Co.,Ltd.(Shanghai,China).Sepharose CL-4B and Sephadex G-50 were obtained from Sigma-Aldrich(St.Louis,MO).Polycarbonate filters obtained from Poretics(Livermore,USA.).
2.2.Cell culture
The murine breast cancer cells 4T1 and human breast cancer cells MDA-MB-231 were provided by the cell bank of Chinese Academy of Sciences (Beijng,China).4T1 cells were maintained in RPMI-1640 medium(Gibco,USA)supplemented with 10% fetal bovine serum (FBS,GEMINI FoundationTM)and MDA-MB-231 cells were cultured in RPMI-1640 medium(HyClone,USA) supplemented with 10% fetal bovine serum(FBS,BI),the two kinds of cells were cultured in the presence of 30 mg/l penicillin and 100 mg/l streptomycin at 37°C in a humidified atmosphere containing 5%CO2.
2.3.Synergistic effect between BER and DOX at the cellular level
The Chou-Talalay method was employed to ascertain ratios of DOX and BER which synergistically inhibit cancer cell proliferationin vitro.The MTT method was used to assess the cell viability.Briefly,the logarithmic growth 4T1 or MDA-MB-231 cells were collected and seeded in 96-well plates at a density of 1000 or 4000 cells/well.After 12 h of attachment,cells were exposed to different fixed molar ratios of DOX and BER solution at 37°C for 48 h.Then,20 μl of 5 mg/ml MTT was added to each well and incubated for 4 h at 37°C.Afterwards,the media was replaced with 150 μl of DMSO to dissolve the formazan crystals formed by living cells.Measurement of absorbance at 570 nm was carried out with a microplate reader (Thermo,USA).The cell viability was calculated from the following formula:
All tests were performed in three replicates.The CompuSyn®software was used to compute the combination index (CI),a quantitative indicator of evaluating the level of antagonism or synergism,and fitted the Fa—CI graphs (Fa:fraction of affected cells).The CI value was determined by using the Median-drug effect analysis with the following principle:
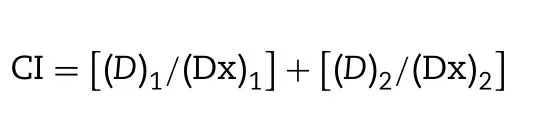
where (D)1and (D)2represent doses of DOX and BER,respectively,used in the combination to produce a certain effect (e.g.50% cell survival).(Dx)1and (Dx)2represent the doses of DOX and BER,respectively,requested the two drugs given as single agents to have the same effect.CI=1.0 indicates additive activity,and CI>1 or CI<1.0 means antagonism or synergy,respectively.
2.4.Liposome preparation
The liposomes were prepared using the method of ammonium sulfate gradient.The HSPC,cholesterol,DSPEMPEG2000 in the weight ratio of 95.8:31.9:31.9(same as Doxil)were used.Liposomes manufactured by the established thin film hydration method.Briefly,lipids were dissolved in ethanol,and the resultant solution was removed by rotary evaporation at 60°C to form a thin lipid film.The lipid sample was then placed under vacuum for 12 h to remove residual solvent.The dried lipid films were hydrated at 70°C for 30 min using 250 mM ammonium sulfate solution ((NH4)2SO4) to form multilamellar vesicles (MLV).Then the MLV was extruded through 0.4 μm,0.2 μm and 0.1 μm polycarbonate membranes stepwise.Each extrusion step was performed 9—11 times at 70°C using the highpressure extrusion device.To establish the ion gradients between intra-liposomal and extra-liposomal,the extruded liposome samples were passed through a Sepharose CL-4B gel column pre-equilibrated with PBGS buffer,pH 7.0(300 mM glucose,3.5 mM Na2HPO4and 3.5 mM NaH2PO4·2H2O).
2.5.Drug remote loading
Prior to drug loading,the pH of blank liposomes were adjusted to 8.5 by using 800 mM Na2HPO4.The diluted liposomes were measured for the phospholipid concentration.Then the liposomal solution and 5 mg/ml BER solution were mixed and incubated for 30 min and subsequently added 8 mg/ml DOX solution incubated for 15 min at 60°C.The ratio of BER to phospholipids is fixed at 1:5 (w/w) and the dose of DOX depends on the ratio of BER to DOX.After drug loading,gel filtration method was applied to determine encapsulation efficiency (EE%).Briefly,200 μl liposome samples were loaded on a PBGS pre-saturated Sephadex G-50 gel column and methanol was used as a demulsifier.The concentrations of the two drugs were determined by HPLC simultaneously (Waters e2695 Separations Module and Waters 2489 UV-vis Detector on a reverse ODS XB-C18column (4.6 mm×250 mm,5 μm)with solvent ratio:55% ACN and 45% 10 mM NH4H2PO4used phosphoric acid to adjust the pH to 3.0;at a flow rate of 1.0 ml/min.The UV detector was kept at 232 nm.The encapsulation efficiency(EE%)and drug loading capacity(DL%)of drugs were calculated according to the following equation:whereCfinalof drug is the encapsulated drug concentration inside the liposome,Cinitialof drug is the drug concentration of loading solution for preparing liposome.W1,W2,andW3 represent the amount of BER or DOX encapsulated in the liposome,the total amount of BER or DOX added,and the amount of lipids,respectively.Each experiment was repeated in triplicate,and the results were expressed by mean±SD.
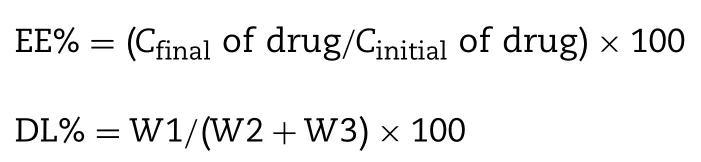
2.6.Liposome characterization
The sizes,polydispersity indexes (PDI) and zeta potentials of liposomes were determined utilizing a Malvern ZetaSizer NanoZS.Prior to characterization,the samples were diluted 100×with distilled de-ionized water,and each measurement was performed in three replicates.The prepared co-loaded liposomes were passed through a sterile filter and stored at 4°C.The particle size,zeta potential and EE% of liposomes were measured for six months with interval of 1 month.
2.7.Animals
Female BALB/c mice (18—22 g body weight) and Sprague—Dawley (SD) rats (200—250 g) were provided by Animal Experimental Center of Shenyang Pharmaceutical University.Breeding conditions alternated light and darkness for 12 h and animals were given a standard diet.All the experiments were executed in compliance with Animal Management Rules of the Ministry of Health of the People’s Republic of China(document number 55,2001) and the Animal Experiment Ethics Review of Shenyang Pharmaceutical University.
2.8.Hematoxylin and eosin(H&E)staining of hearts to screen out the best attenuation ratio
Eighteen healthy female BALB/c mice were randomly divided into 6 groups:(1)5%glucose,(2)liposomal DOX,(3)liposomal co-encapsulated BER/DOX combination,the molar ratio of BER and DOX was 7.8,(4)liposomal co-encapsulated BER/DOX combination,the molar ratio of BER and DOX was 15.60,(5)liposomal BER,(6) liposomal BER.The DOX doses of group 2,3 and 4 were fixed at 1.2 mg/kg.The doses of BER in the group of 3 and 4 were based on the molar ratio changes between the two drugs.After four consecutive intravenous administration,the mice were sacrificed and the hearts were collected and fixed with 10%buffered formalin,then paraffin embedded,and cut into 4 μm sections subsequently for H&E staining.The stained tissue sections were evaluated by a board-certified veterinary pathologist who was blinded with respect to treatment conditions.
2.9.Cryo-transmission electron microscopy(cryo-TEM)
The morphology of the single drug-loaded liposomes and the co-loaded liposomes was examined through cryo-TEM.Briefly,3.5 μl of each liposome sample was loaded onto a negative glow discharged R1.2/1.3 100 porous carbon membranes grid (Cu 200 mesh) (Quantifoil,Jena,Germany) and blotted to remove excess.The sample was flash frozen in a liquid ethane-propane mixture and the grid holder was transferred to a FEI Talos F200C electron microscope at an operating voltage of 200 kV to examine.Serial EM software with a nominal defocus value of-5 μm was utilized to collect data.
2.10.Apoptosis assay
Apoptosis detection kit (Vazyme,China) and flow cytometer(Becton Dickinson,USA) were applied to evaluate cell apoptosis.Briefly,4T1 cells in six-well plates were incubated with 400 ng/ml BER,40 ng/ml DOX or BER and DOX combination (400 ng/ml for BER and 40 ng/ml for DOX) at 37°C for 48 h.Then,cells were stained with AV and PI for 10 min in the dark.Finally,cells were diluted with binding buffer and detected by flow cytometry.
2.11.Pharmacokinetics and bio-distribution study
Twenty SD rats were randomly divided into 4 groups and fasted for 12 h before administration.Drugs were given intravenously with the following groups:(1)BER(12 mg BER/Kg B.W.) and DOX (1.2 mg DOX/kg B.W.) solution combination(2) liposomal BER (12 mg BER/Kg B.W.) (3) liposomal DOX(1.2 mg DOX/kg B.W.)(4)liposomal co-encapsulated BER(12 mg BER/Kg B.W.)and DOX(1.2 mg DOX/kg B.W.)combination.After drug administration,0.4 ml of blood was withdrawn at a predetermined time:0.08,0.25,0.5,1,2,4,8,12,24,48 and 72 h.Plasma samples were obtained by immediate centrifugation at 13 000 rpm for 10 min and stored at-20°C.
Approximately 3.5 million 4T1 cells in 200 μl PBS(pH=7.4)were subcutaneously inoculated in the right flank of female BALB/c mice.When the tumor volume reached 200 mm3,mice were randomly divided into 4 groups:(1) liposomal BER (12 mg BER/Kg B.W.),(2) liposomal DOX (1.2 mg DOX/kg B.W.),(3) liposomal co-encapsulated BER (12 mg BER/Kg B.W.)and DOX (1.2 mg DOX/kg B.W.) combination 4) Doxil (1.2 mg DOX/kg B.W.).At different time points (4,8,12 and 24 h) post intravenous injection,mice were sacrificed(n=3)and organs(heart,liver,spleen,lung,kidney and tumor) were harvested at 6,24 and 48 h.Tissues were weighed and diluted 1:1 by saline and homogenized with saline carefully in an ice bath and stored at-80°C until analysis.
The concentrations of BER and DOX in rat plasma and tissues were determined by a validated UPLC—MS—MS method after liquid-liquid extraction method using gliclazide as internal standard.The quantification of DOX and BER in plasma was carried out using ACQUITY UPLCTM system(Waters Co.,Ltd.,Milford,MA,USA) with an ACQUITY UPLC BEH C18column (50 mm×2.1 mm,1.7 μm; Waters Corp,Milford,MA,USA).The mobile phase consisted of methanol(A) and water contained 0.1% formic acid (B) at a flow rate of 0.2 ml/min.The gradient elution was performed:0—0.5 min,20% A; 0.5—1.8 min,80% A; 1.8—1.9 min,92% A; 1.9—2.0 min,70% A; 2.0—4.6 min,20% A.Pharmacokinetic parameters were computed using DAS 2.0 software.
2.12.Antitumor efficacy evaluation
Mice bearing 4T1 tumor were utilized to evaluate thein vivoantitumor activity.Approximately 3.5 million 4T1 cells in 200 μl PBS (pH=7.4) were subcutaneously inoculated in the rightflank of female BALB/c mice.When the tumor volume reached 100 mm3,mice were randomly divided into 6 groups (n=5):(1) 5% glucose aqueous solution,(2) liposomal BER (12 mg BER/Kg B.W.),(3) liposomal DOX (1.2 mg DOX/kg B.W.),(4)liposomal co-encapsulated BER (12 mg BER/Kg B.W.) and DOX(1.2 mg DOX/kg B.W.) combination,(5) DOX solution (1.2 mg DOX/kg B.W.),(6) Doxil (1.2 mg DOX/kg B.W.).Each group was administered intravenously on day 4,7,10,13,16 post tumor inoculation.Tumor volumes were calculated as
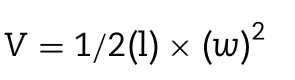
where l andwmean the longest and the shortest diameters of tumor,respectively.Body weights were also weighed per day.Mice were sacrificed on the 19th d after tumor inoculation,then the tumors were taken out for weighing and photographing,and the main organs were collected for hematoxylin and eosin(H&E)staining.
2.13.Liposomal toxicity assessment in rats
Thirty-six healthy female BALB/c mice were randomly divided into six groups (n=6),(1) Doxil (1.2 mg DOX/kg B.W.),(2)DOX solution (1.2 mg DOX/kg B.W.),(3) liposomal BER (12 mg BER/Kg),(4)liposomal DOX(1.2 mg DOX/kg B.W.),(5)liposomal co-encapsulated BER (12 mg BER/Kg B.W.) and DOX (1.2 mg DOX/kg B.W.) combination (6) 5% glucose aqueous solution.The drugs were administered once every two days for a total of five doses,the dosing interval was the same as the antitumor efficacy test.After the fifth administration,the whole blood was collected from cardiac puncture and used for blood analysis.Three of them were used to count white blood cells (WBC),red blood cells (RBC),hemoglobin (Hb),platelets (PLT),and plateletcrit (PCT).The other three were used for serum enzyme analysis,including serum alanine aminotransferase (ALT),serum aspartate aminotransferase(AST),serum urea test (UREA),serum creatinine test (CREA),serum phosphocreatine kinase (CK),serum phosphocreatine kinase isozyme(CK-MB),serum lactate dehydrogenase(LDH).The myocardial enzyme was used as a quantitative index for evaluating the safety of the hearts of mice after administration.(Myocardial enzyme is a general term for various enzymes present in the myocardium,including AST,LDH,CK,CK-MB).
2.14.Statistical analysis
All the quantitative data were expressed as mean±standard deviation(SD).A one-way ANOVA was used to determine the significance in the experiments,P <0.05 was regarded as statistical difference andP <0.01 was considered statistically significant difference.
3.Results and discussion
3.1.Ascertaining the optimal synergistic molar ratio of BER and DOX in breast cancer cell lines
Before preparing the drug-loaded liposomes,we performed a cytotoxicity experiments on 4T1 and MDA-MB-231 cellsto determine the optimal ratio of synergy between the two drugs.Chou and Talalay analyses,which is widely used to assess the interaction of two or more drugs,were utilized to assess synergy or antagonism.Cytotoxicity evaluation was performed to determine the IC50of the two drugs against 4T1 and MDA-MB-231 cells.It was found that,DOX was 8 times more toxic than BER on 4T1,and for MDA-MB-231,DOX was 64 times more toxic than BER(Table 1).Therefore,an equivalent mixture of BER and DOX requires a BER/ DOX molar ratio bigger than one.
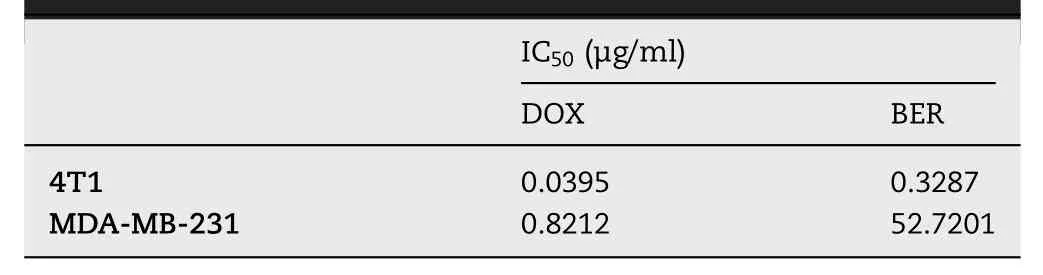
Table 1-IC50 of BER and DOX on 4T1 and MDA-MB-231 cells.
As a result,we fixed the BER/DOX molar ratios of 0.78,3.12,6.24,7.8,12.48 and 15.6 to explore synergies.The results of the interaction between the two drugs were shown in Fig.1.When the BER/DOX molar ratios were 0.78,3.12 and 6.24,the two drugs showed additive or antagonistic effects in both cells.At a molar ratio of BER/DOX of 12.48,there was a weak synergistic effect on 4T1,and a strong antagonistic effect on MDA-MB-231 cells.Fortunately,when the molar ratios of BER/DOX were 7.8 and 15.6,the combined drugs showed synergistic or strong synergistic effects in both cells.Based on the above,we considered the ratios of 7.8 and 15.6 as the better synergistic ratios(Fig.1A and 1B).
3.2.BER and DOX co-encapsulation in liposomes
According to thein vitrocytotoxicity assays,we prepared double-loaded liposomes with BER/DOX molar ratios of 7.8 and 15.6.The Doxil’s formulation (HSPC:CHO:DSPEPEG2000=95.8:31.9:31.9,w/w) were applied to load both drugs.Regrettably,the formulation of Doxil (30 mM sucrose and 20 mM histidine with pH=7.0 in the external medium)resulted in the EE% of BER was less than 30%,and therefore the replacement of the external medium was considered.The effect of different external medium types on the EE% of the two drugs was shown in Fig.2A.When the external medium was replaced with PBGS,the EE% of BER exceeded 80%,so PBGS was considered to be the best external medium.Then,the molar ratio of BER to phospholipid was fixed at 0.14,and the effects of the loading sequence of the two drugs and the pH of liposome on the EE% were investigated.Fig.2 showed that different conditions had almost no effect on the EE% of DOX,probably because the dose of DOX was little,it was a planar structure,and the pKa value was lower than BER and it was more easily contained.When the pH of the liposome was adjusted to 7.50—8.50,the EE% of BER was over 90%.Further,different BER/PL ratios were studied and we noticed that when the BER/PL ratio was increased to 0.41 (m/m),only the pH of the liposomes was adjusted to 8.50,the EE% of the two drugs could reach to 95%,the drug loading capacity (DL%)could reach to 11.67%.A possible cause of this result was that when the pH reached 8.50,the gradient magnitude was above 1000 and the driving force for the BER to enter the liposomes was stronger.

Fig.1-In cell level screening of the ratio of BER and DOX for synergistic effect,and the molar ratios of BER/DOX were fixed at 0.78,3.12,6.24,7.80,12.48,15.60.CI-FA curves of BER and DOX to 4T1 cells(A)and MDA-MB-231 cells(B).
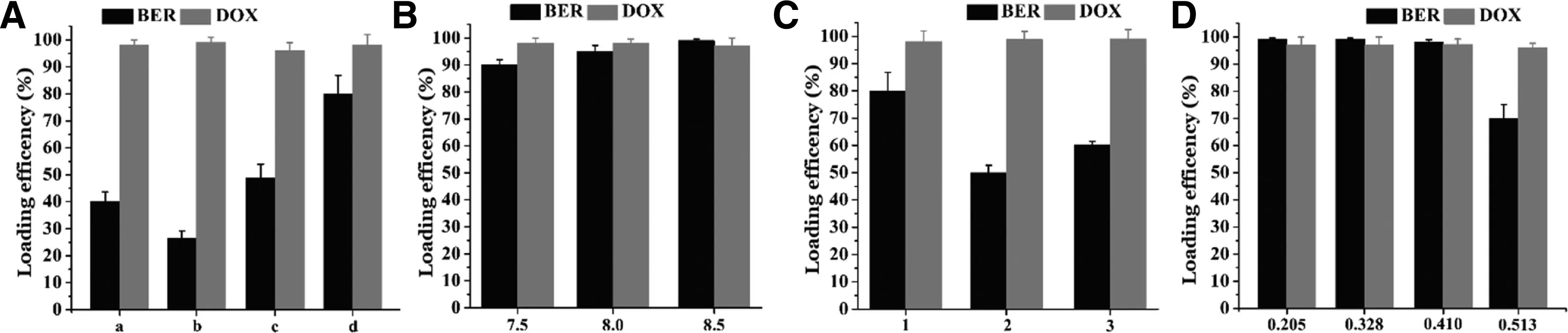
Fig.2-(A)Different extra liposome medium types resulted in different EE%,and“a”represented 250 mM sucrose+50 mM glycine;“b”represented 30 mM sucrose+20 mM histidine and pH was adjusted to 7.0,same as Doxil;“c”represented 17 mM Hepes+144 mM NaCl,“d”represented PBGS.Effects of liposomal pH(B)and dosing order,“1”represented loading BER for 30 min and then DOX for 15 min;“2”represented simultaneous loading of two drugs for 45 min;“3”represented pre-loaded DOX for 15 min,followed by BER for 30 min(C)on EE%of two drugs when the ratio of BER to lipid was fixed at 0.14.(D)Effects of different BER to lipid ratio on EE%.
3.3.Determine the optimal proportion of synergies and attenuation in vivo
As can be seen from Fig.3,the liposomal DOX group caused severe myocardial rupture and myocardial lysis,and the degree of myocardial fragmentation in mice with a BER/DOX ratio of 7.8 was essentially the same.The myocardium of the co-loaded drug liposome with a BER/DOX ratio of 15.6 was intact.There was no significant difference in myocardial health between the liposomal BER group and the control group,and no myocardial rupture and myocardial lysis occurred.Based on the above results,the BER/DOX molar ratio of 15.6 was selected as the optimum ratio in the subsequent studies.
3.4.Characterization and storage stability of the liposomes
We already knew that the commercially available Doxil has gelation precipitate formed by DOX [34],so we are interested in exploring whether the low DL ratio of liposomal DOX will form precipitate and whether BER interfere with the precipitated form of DOX,because the formation of precipitate was associated with drug release.Fig.4 showed the internal morphology of liposomal DOX (LipoDOX),liposomal BER(LipoBER) and co-loaded liposome combination (LipoBeDo),respectively.It can be seen from the graphs that the LipoBER had no obvious precipitation crystal form,and the liposomal DOX has colloidal precipitate,but the precipitation volume was smaller than that of Doxil,because the liposomal DOX we prepared (DL ratio=0.027) had a lower DL ratio than Doxil(DL ratio=0.354).The morphology of the double-loaded liposomes LipoBeDo was a superposition of two separate liposomes and BER did not interfere with the precipitated form of DOX.
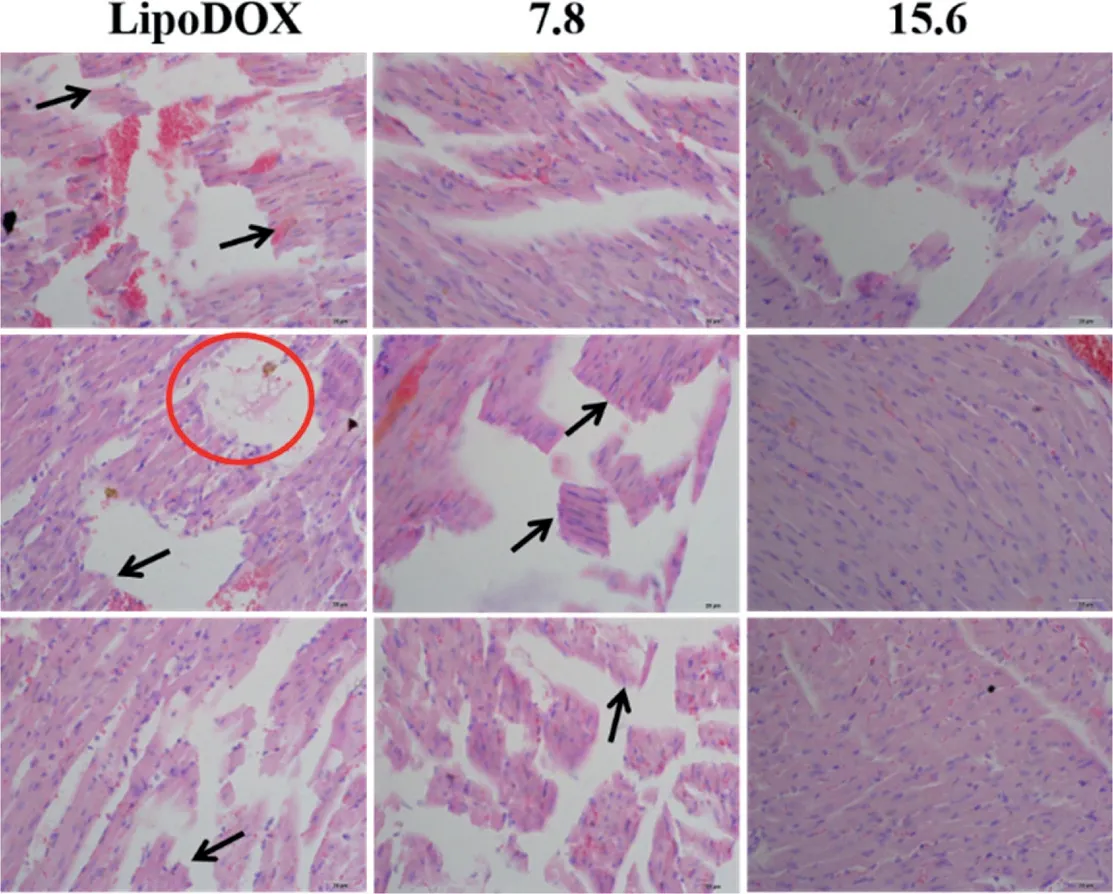
Fig.3-Evaluating the cardiotoxicity of mice after three consecutive doses of different mole ratios co-loaded liposomes.Arrows represented myocardial breaks and circles represented myocardial lysis(n=3).The scale bar represents 25 μm.
The particle size of the liposome was about 100 nm,PDI<0.1,and the zeta potential was about-13 mV.The particle size and potential images showed in Supplementary information Fig.5A&B.Changes in particle size,Zeta potential and EE%of LipoBeDo during storage were shown in Fig.5C&D.
3.5.Apoptosis
Fluorescence-activated cell sorting (FACS) analysis was applied to detect cell apoptosis (Fig.6).BER and DOX combination treatment (18.1%) caused a robust increase in the late apoptotic cells,as compared to BER (0.769%)and DOX (3.83%) treatment and a modest increase in the early apoptotic cells,5.78% with BER and DOX combination treatment,0.846% with BER and 1.57% with DOX.Therefore,the combination of BER and DOX can increase the proportion of apoptotic cells and improve thein vitroanti-tumor effect.
3.6.The synergistic ratio of DOX and BER can be maintained in plasma via liposome co-encapsulation
A schematic diagram of thein vivopharmacokinetic behaviors of the different drug delivery groups were presented in Fig.7C.The specific pharmacokinetic parameters were displayed in Table 2.It can be concluded that the solution was rapidly eliminated,the half-life (T1/2) value of the free BER and free DOX were 2.209 h and 0.498 h,and compared with free solution,theT1/2of the LipoBER and LipoDOX were extended by 2.679 and 23.327 times,respectively.It was worth noting thatT1/2of the LipoBER was 8.126 h,while the half-life of the BER component in the LipoBeDo was prolonged to 12.281 h(P<0.05),which was substantially the same asT1/2of LipoDOX(12.115 h) and DOX in LipoBeDo (12.058 h).The co-loaded liposome extended the time of BER in the body.
The concentration ratio of the two drugs in plasma was showed in Fig.7A&B.The pharmacokinetic behaviors of BER and DOX in the co-loaded liposome were generally same,which was reflected in the half-life,MRT values of the two drugs.As a result,the ratio of the two drugsin vivowas maintained within 72 h after administration.The ratio of BER and DOX in the free drug combination group fluctuated vigorously in 2 h post injection,deviating from the initial dose ratio 15.6.This was due to nanoliposome carriers provide an ability to govern the pharmacokinetic behaviors of encapsulated drugs.
AUC,MRT,TmaxandCmaxof LipoBER and LipoDOX were observably higher than those of the solution group,while CLz was significantly lower.Compared with free BER and free DOX,AUCof LipoDOX (271.719 μg·h/ml) and LipoBER(12.508 μg·h/ml) increased by 2696.742 and 4406.651 times,respectively.
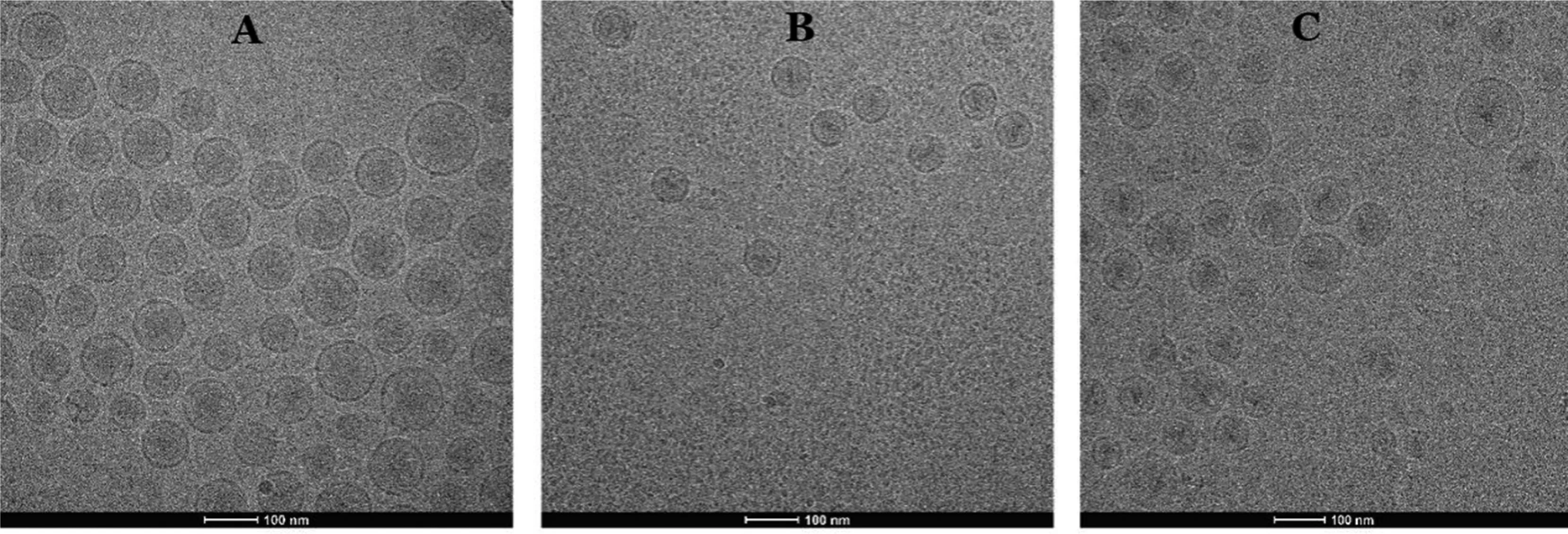
Fig.4-Cryo-TEM graphs of LipoBER(A),LipoDOX(B)and LipoBeDo(C).All of them at drug to lipid ratio(m/m)of 0.41 for BER and 0.027 for DOX.The scale bars represented 100 nm.
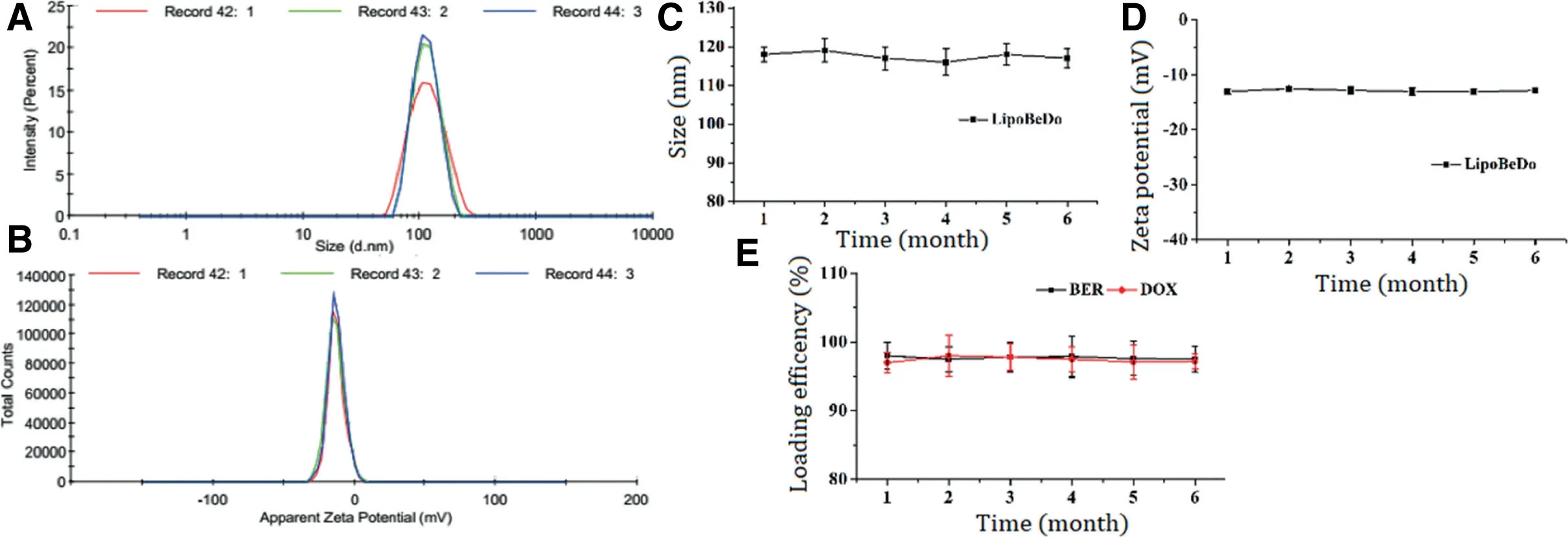
Fig.5-The particle size of the co-loaded liposome LipoBeDo(A)and zeta potential(B).The changes of particle size(C),zeta potential(D)and EE%of(E)LipoBeDo in triplicate during storage among six months.
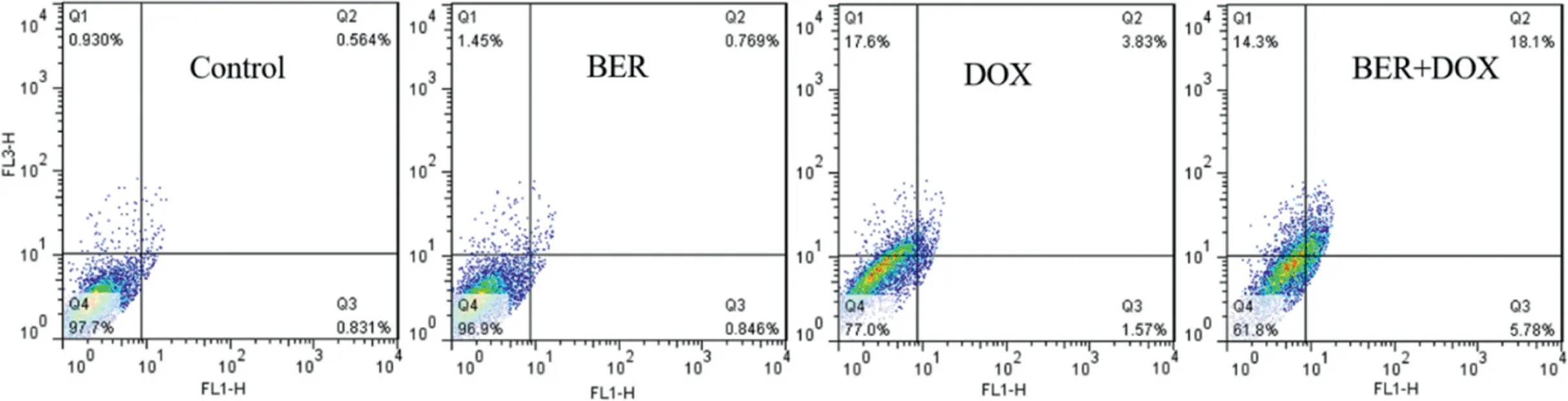
Fig.6-Apoptosis rates of BER,DOX,BER and DOX combination(BER:DOX=15.6:1;m/m).
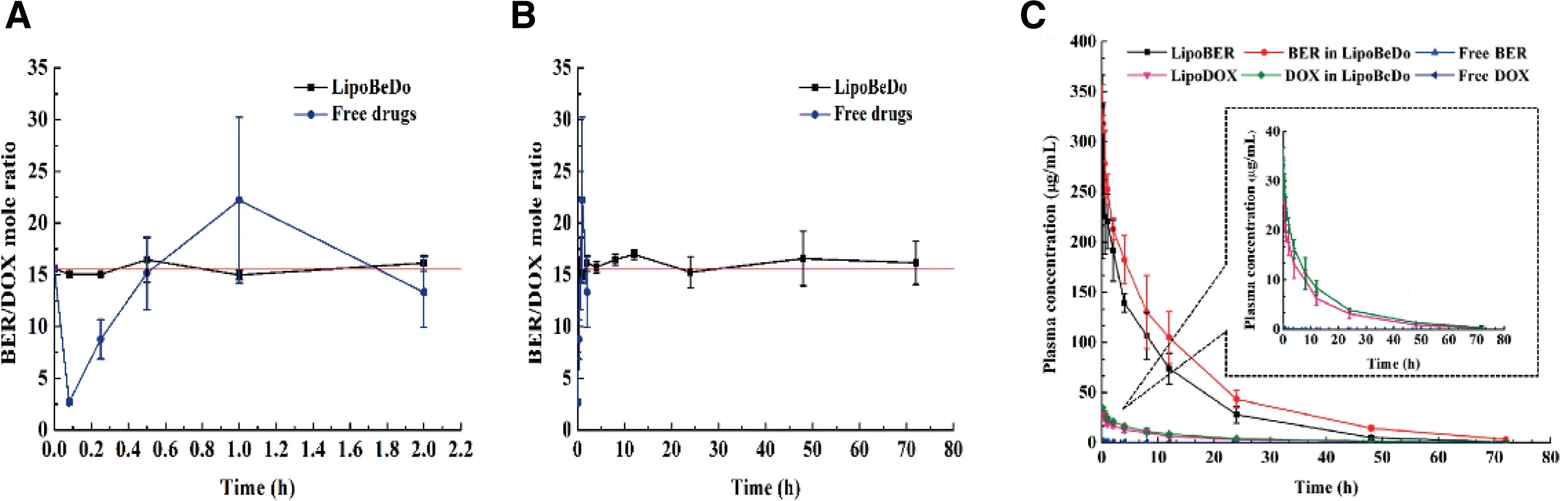
Fig.7-The change of BER and DOX molar ratio after i.v.administration different preparations within 2 h(A)and 72 h(B).Plasma elimination curves of BER and DOX in different groups(C).
The release profiles of BER in LipoBeDo were different from that of LipoBER,and its half-life,MRTwere significantly extended (P <0.05),while the DOX release rates from LipoDOX were nearly identical to DOX in LipoBeDo,andMRTvalues were slightly prolonged.The reason why the halflife of BER in LipoBeDo was longer than that of LipoBER’s may be speculated two.One is that due to the addition of DOX,it will cause damage to the MPS system [35],so less liposome is removed by the MPS,which results in the phenomenon that the half-life of BER in LipoBeDo is longer than LipoBER’s;another possible reason is that the addition of DOX affects the polarity,ionic strength,pH,etc.of the internalaqueous phase,thus affecting the solubility and release rate of BER.
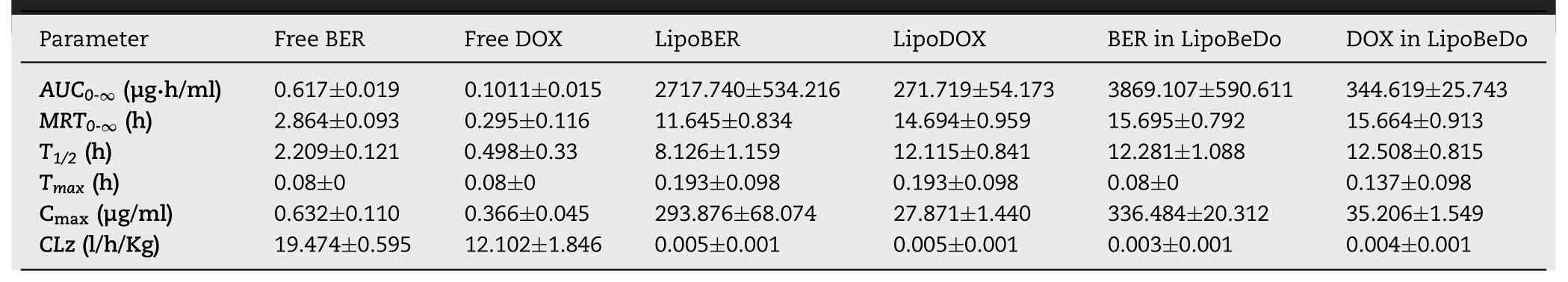
Table 2-Pharmacokinetic parameters for BER and DOX in each group.
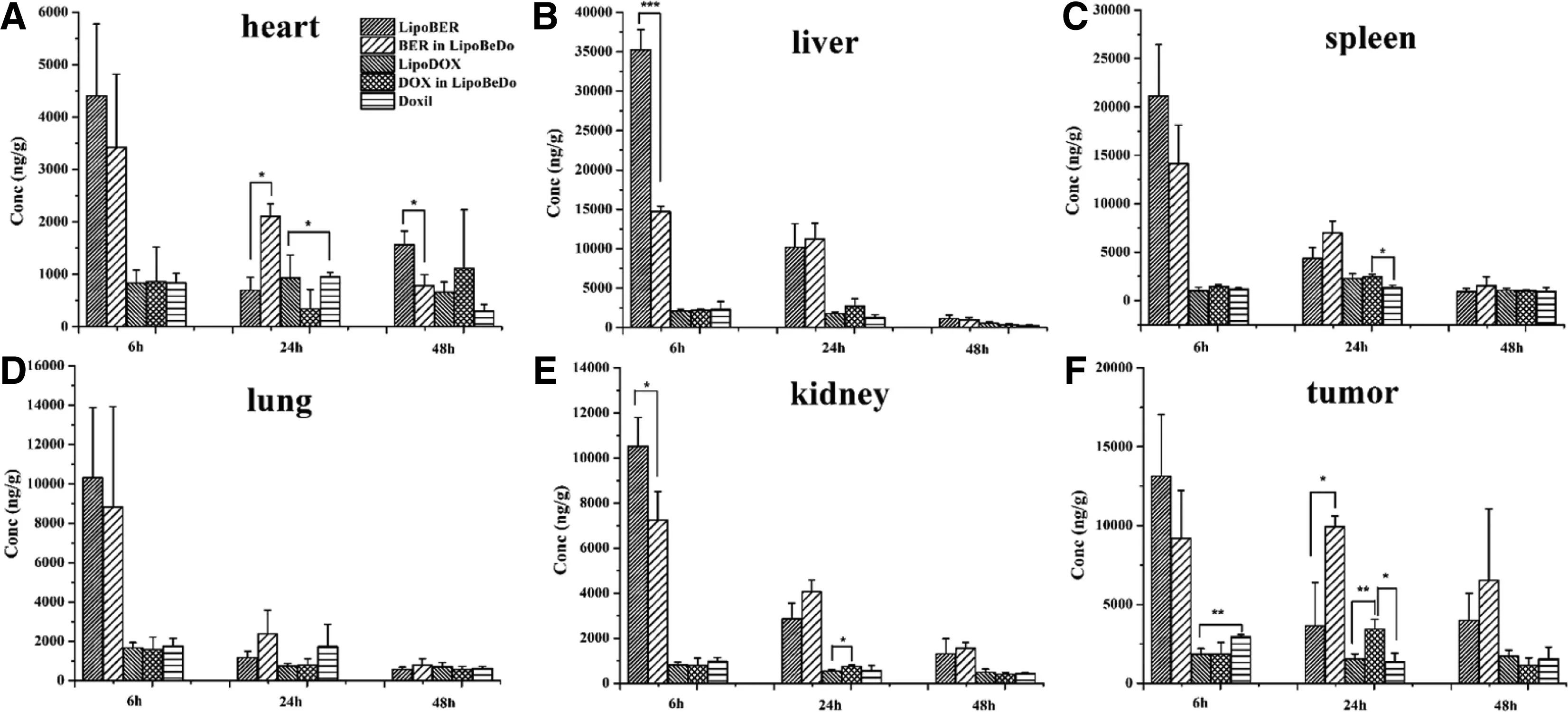
Fig.8-Bio-distribution of BER and DOX levels in main tissues including heart(A),spleen(B),liver(C),lung(D),kidney(E)and tumor(F)in BALB/C mice.The data are shown as mean ±SD(n=3).
3.7.Bio-distribution study
The time course of BER and DOX biodistributions to various organs after the administration of the liposomal drug formulations and Doxil®are given in Fig.8.The accumulation amount in the liver and spleen was the highest at 6 h after administration of the liposome.Among them,LipoBER accumulated significantly more in tissues than LipoBeDo at 6 h,and the difference between liver (P <0.001) and kidney(P <0.05)was significant,while at 24 h,LipoBER accumulated significantly less than LipoBeDo,the difference between heart(P <0.001) and tumor (P <0.05) was obvious.The results of this experiment showed that LipoBER was taken up faster after administration than the co-loaded liposome LipoBeDo,and the pharmacokinetic parameters also showed thatT1/2(8.216 h) was shorter than LipoBeDo (12.281 h).At 24 h,the accumulation of DOX in tumor in the LipoBeDo group was significantly higher than LipoDOX (P <0.01) and the Doxil (P<0.05),and the antitumor effect was predicted to be superior to LipoDOX.
3.8.Inhibition of tumor growth by co-encapsulated liposome
The liposomes were selected to challenge highly metastatic and highly invasive 4T1 tumor-bearing mice.This model was also chosen because of its ability to form in immunocompetent BALB/C mice,and its efficacy evaluationin vivois more accurate and reliable than immunodeficient mice.The tumor-bearing mice were randomly divided into 6 groups (n=5):5% glucose aqueous solution,liposomal BER (LipoBER),liposomal DOX (LipoDOX),co-encapsulated liposome(LipoBeDo),Free DOX and Doxil.
Direct intravenous injection of BER hydrochloride caused severe cardiac suppression in mice to die,so only the DOX solution was used as a control.The co-loaded liposomes significantly inhibited tumor growth with a tumor inhibition rate up to 74.7%.Its antitumor activity was prominently superior the commercially available Doxil(P <0.05)and single drug liposomes (P <0.001).The anti-tumor effects of the LipoBER and LipoDOX groups were notably more effective than the Free DOX group and the 5% glucose solution group.The reasons for the LipoDOX group was not good as Doxil was that the drug-to-lipid(DL)ratio of the liposomal DOX prepared by us was 0.027,lower than the DL ratio of Doxil (0.354),causing faster released compared with the same dose of Doxil and fewer drugs were taken into tumor cells.The results of thein vivopharmacokinetic experiments also confirmed this conclusion.The half-life of LipoDOX was about 12 h,which was much lower than the Doxil lasting for 55 h.
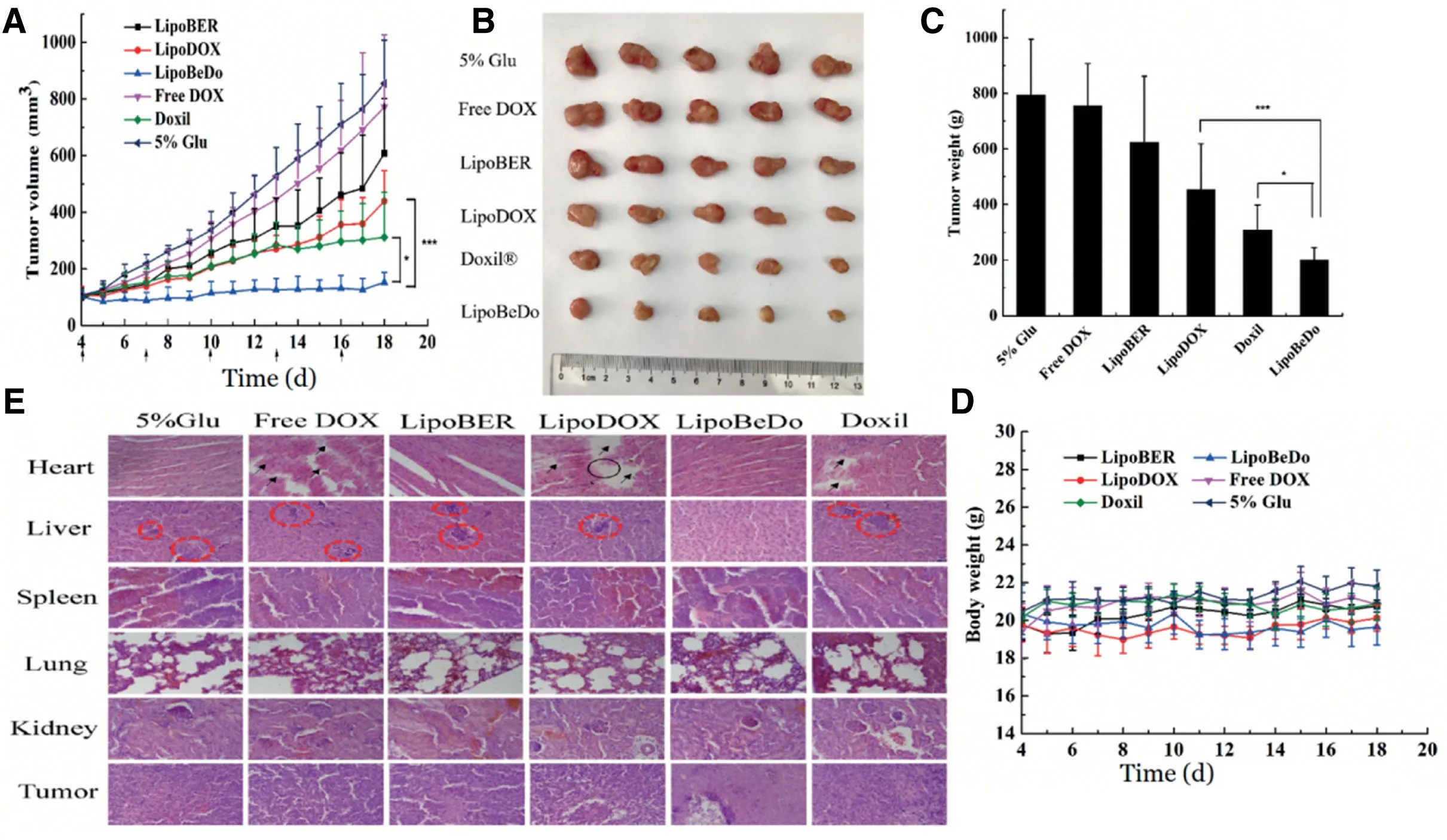
Fig.9-Tumor volume growth inhibition curve(A)Solid tumor photograph at the last day of the 15-day treatment(B)Tumor weights(C)Mice body weight change curve after administration(D)H&E stained histological sections of mice,the scale bar represents 25 μm(E).
The body weight of all animals was stable during the administration period.It could be seen in H&E staining that the myocardial tissue of the mice administrated coencapsulated liposome group LipoBeDo was intact,while the mice administrated DOX solution,DOX-loaded liposome and Doxil mice showed different degrees of myocardial damage.It can be revealed from the results of tumor section that there was a large amount of necrosis in the tumor of the coloaded liposome group,while the tumor growth of the other drug-administered group was vigorous.In addition,the 4T1 tumor model is common in liver metastasis.Our pharmacodynamic results displayed that all but the co-loaded liposomal combination generated tumor metastasis,further demonstrating the anti-tumor advantage of the co-loaded liposomes.The spleen,lung and kidney of each group were healthy,and the staining results were shown in Fig.9E.Tumor volume growth curve inhibition map,mice body weight change map,tumor weights and solid tumor photograph during administration were shown in Fig.9A-D.
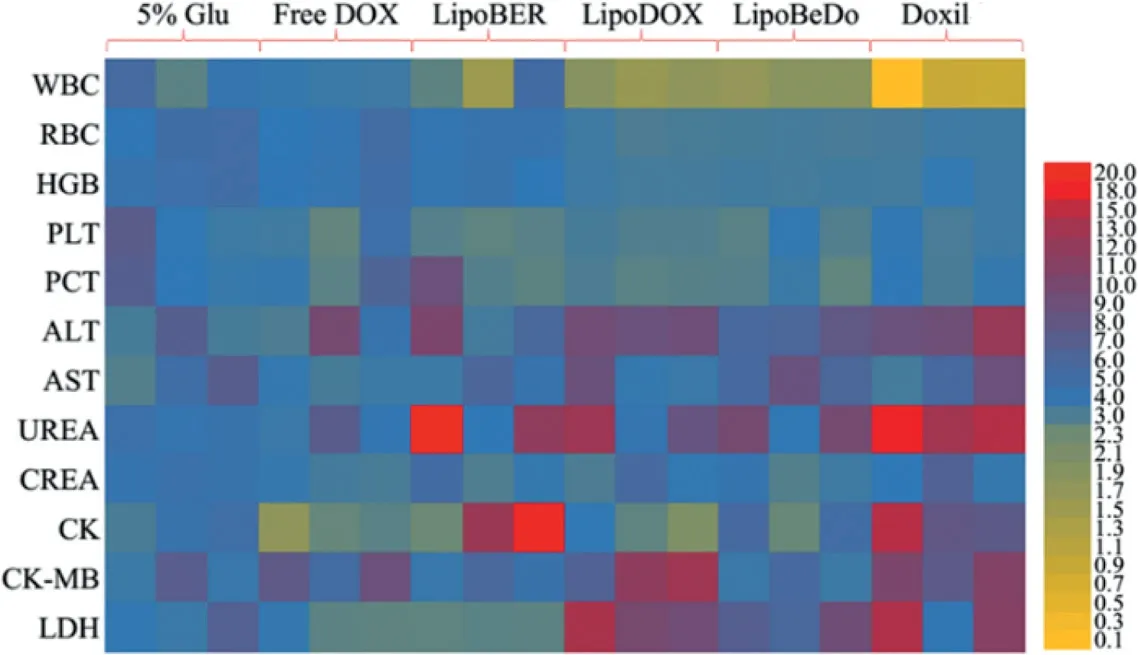
Fig.10-Routine blood test,liver and kidney function and myocardial zymography tests of each group.
3.9.Toxicity evaluation
The health status of mice in each administration group was displayed in Fig.10.Taken together,the biochemical parameters of mice administrated low-dose Doxil were significantly different from those of normal mice.The biochemical parameters of our co-loaded liposome group were normal.Moreover,AST,CK,CK-MB and LDH in the cardiac index were no different from normal mice,further demonstrating the safety of the co-loaded liposome for intravenous injection.
4.Conclusions
In this study,berberine and doxorubicin hydrochloride coencapsulated liposomes were prepared.Co-loaded liposomes demonstrated superiorin vivoanti-tumor activity and inferior cardiac toxicity,caused by DOX,compared with single drug liposomes and Doxil,which is concerned with the properties on keeping the optimized synergistic effect of the two drugs.All these presented results revealed that berberine and doxorubicin hydrochloride co-loaded liposomes can be an efficient formulation for enhanced breast cancer therapy with great clinical application prospects.This platform technology for co-loaded liposomes can also be applied to other combination therapies.
Conflicts of interest
The authors declare that there is no conflicts of interest.
Acknowledgment
This work was financially supported by the Career Development Program for Yong and Middle-aged Teachers in Shenyang Pharmaceutical University.
 Asian Journal of Pharmacentical Sciences2020年3期
Asian Journal of Pharmacentical Sciences2020年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- Microsponges for dermatological applications:Perspectives and challenges
- Sprinkle formulations-A review of commercially available products
- Redox dual-stimuli responsive drug delivery systems for improving tumor-targeting ability and reducing adverse side effects
- Intranasal delivery of paeoniflorin nanocrystals for brain targeting
- Enhanced intestinal lymphatic absorption of saquinavir through supersaturated self-microemulsifying drug delivery systems
- Development of composite PLGA microspheres containing exenatide-encapsulated lecithin nanoparticles for sustained drug release
