Effect of Qishen decoction on dedifferentiation of sinusoidal endothelial cells by autophagy
Xu Mao , Xing-Xing Yuan , Lei Yang , Dan-Dan Li , Bing-Yu Wang ✉
1. Heilongjiang University of traditional Chinese medicine, Jiamusi, Heilongjiang Province, 154000
2. Nangang Branch of Heilongjiang Academy of traditional Chinese medicine, Harbin, Heilongjiang, 1500001
Keywords:Qishen decoction Liver sinusoidal endothelial cells Autophagy Dedifferentiation Medicated serum
ABSTRACT Objective: To observe the effect of Qishen decoction on dedifferentiation and autophagy of liver sinusoid endothelial cells (LSEC); Methods: LSEC were randomly divided into the control group, model group, Qishen Decoction low, medium, high dose group, and inhibitor group. The model was induced by 100µg/ml oxidized low-density lipoprotein (oxLDL) for 24 hours, and the corresponding drugs or medicated serum were given for intervention. The expression levels of VEGFR2 and ET1 were detected by RT-qPCR and immunofluorescence staining, the ultrastructure of LSEC was detected by transmission electron microscopy, the content of NO was detected by ELISA, the expression levels of autophagy related proteins (LC3BI, LC3BII and p62) and endothelial function related proteins (eNOS and p- eNOS) were detected by western blot; Results: The results of transmission electron microscopy showed that Qishen decoction medicated serum could increase the number of fenestra and autophagy in LSEC cells, and inhibit the formation of basement membrane under endothelium. Compared with the model group, Qishen decoction medicated serum could significantly up-regulate the expression level of VEGFR2 mRNA and protein in LSEC, down regulate the expression level of ET1 mRNA and protein, the difference was statistically significant (P<0.05). In addition, Qishen decoction medicated serum could significantly increase the expression of LC3BII, p- eNOS, eNOS protein and the ratio of LC3BII/LC3BI, p- eNOS/eNOS, and reduce the expression of LC3BI and p62 protein in LSEC, which is statistically significant compared with the model group (P<0.05). Conclusion: Qishen decoction can inhibit the dedifferentiation of LSEC by promoting the autophagy level of LSEC, and then play an anti-fibrosis role.
1. Introduction
Liver fibrosis is a key link in the development of chronic liver disease to end-stage liver disease in each group, which belongs to a pathological repair response of the body to liver injury [1]. This disease is usually manifested as diffuse excessive deposition of extracellular matrix (ECM), especially collagen type 1 α 1 (Col 1 α 1). Further development can cause disorder of liver lobule structure, nodular regeneration and formation of pseudolobule structure. Its clinical manifestations are a series of complications such as portal hypertension and liver dysfunction [2]. Considering the reversibility of liver fibrosis in histology, it is of great significance to effectively reverse LF in order to reduce the incidence of end-stage liver disease and social medical burden.
Although the previous study of the research group shows that Qishen decoction can play an anti-fibrosis role through PI3K/Akt/mTOR signal pathway and intestinal flora, its specific mechanism needs further study [3-4]. As the main source of myofibroblasts in the liver, the activation and proliferation of HSC is the central link in the formation of liver fibrosis. Previous studies of the research group showed that as one of the main active components of Qishen decoction, astragaloside can inhibit the activation of HSC through TGF-β1/Smad signal pathway, thus reversing the process of liver fibrosis [5]. However, in recent years, more and more studies have found that hepatic sinusoidal endothelial cell (LSEC) plays an important role in the process of liver fibrosis [6]. In addition, some studies have confirmed that autophagy plays an important role in maintaining the specific phenotype of LSEC, and sustained injury leads to the disappearance of the level of LSEC autophagy, thereby reducing its ability to resist oxidative stress and causing liver fibrosis [7]. Therefore, in this study, the research group intends to observe the effect of Qishen Decoction on LSEC autophagy and dedifferentiation level (also known as capillarization), so as to clarify the mechanism of Qishen Decoction on liver fibrosis.
2. Materials and methods
2.1 Animals and cell lines
Twelve SD rats of clean grade, with a body mass of 210 ± 12g, were purchased from the drug safety evaluation center of Heilongjiang University of traditional Chinese medicine, and the certificate number was SYXK (Hei) 2015010. Animal feeding conditions: the rats were raised in separate cages, drinking water freely, room temperature: 23-25 ℃, relative humidity: 30% - 45%, every 12 hours cycle of light. LSEC of rats was purchased from Beijing Beina Chuanglian Biotechnology Research Institute. LSEC was conventionally cultured in rat LSEC special complete medium containing 5% fetal bovine serum, 1% endothelial growth factor and 1% penicillin/streptomycin.
2.2 Drugs and reagents
Qishen Decoction (composition: American ginseng 10g, raw Astragalus 15g, lotus leaf 10g, Danshen 10g, Alisma 10g, raw Hawthorn 10g, Eclipta 8g, Ligustrum 8g, notoginseng 5g, Gynostemma 5g, raw licorice 3g) was purchased from the herbal medicine bureau of Heilongjiang Academy of traditional Chinese medicine. The medicine was decocted with water twice, filtered, combined and concentrated for standby (crude medicine 2g/ml); oxidized low density lipoprotein (oxidized low density) Lipoprotein, oxLDL) was purchased from Beijing solabo science and Technology Co., Ltd. before use, PBS solution was prepared into a solution with a final concentration of 100 µ g / ml, which was stored for use at 4 ℃; chloroquine was purchased from sigma company in the United States, which was prepared into a solution with a concentration of 20 µ m with double distilled water before use, which was stored for use at 4 ℃; vascular endothelial growth factor receptor 2 (vascular endothelial growth factor receptor 2, VEGFR2, endothelin 1 (ET1), microtubule associated protein 1 light chain 3 beta I (lc3bi), microtubule associated protein 1 light chain 3 beta II (lc3b II), p62, endothelial nitric oxide synthetase Synthase, eNOS, p-enos and β - actin were purchased from Abcam, USA; Goat anti rabbit IgG (H + L) labeled by Alexa fluor 350 and horseradish peroxidase was purchased from biyuntian biology; nitric oxide (no) test kit was purchased from Nanjing Jiancheng Bioengineering Research Institute.
2.3 Preparation of serum containing medicine
SD rats were randomly divided into blank group, Qishen Decoction low, middle and high dose group, 5 rats in each group. Qishen Decoction group was treated with 4.23 g/kg, 8.46 g/kg and 16.92 g/kg Qishen Decoction concentrate respectively. The blank group was treated with 10 ml / kg distilled water once a day for 7 days. After the last gavage, the rats were fasted for 12 hours and anesthetized with 10% chloral hydrate by intraperitoneal injection of 3.5 ml / kg. Blood was collected from abdominal aorta and serum was separated. The serum was inactivated by water bath, filtered by microporous membrane and frozen at - 70 ℃.
2.4 Grouping and drug intervention
The experiment was divided into blank group, model group, low dose, middle dose, high dose group and inhibitor group. LSEC with 5 × 104 cells / cell was inoculated in 96 well plates. Each group was set with 3 multiple cells and cultured in 37 ℃ and 5% CO2 incubator. In addition to the blank group, the other cells were induced by ox LDL at a concentration of 100 µ g / ml for 24 hours. After modeling, each group was given 100 µ l corresponding serum or chloroquine 20 µ m for intervention, 48 hours later for detection.
2.5 Index detection
2.5.1 Ultrastructure and autophagy of LSEC detected by transmission electron microscopy
After 48 hours of drug intervention, the cells in each group were digested and centrifuged with 0.25% trypsin, then the supernatant was discarded, fixed with 3% glutaraldehyde solution for 3 minutes, fixed with 1% frontal acid solution for 2 hours, then dehydrated with gradient ethanol, 90% acetone and 100% acetone in turn, and embedded with epoxy resin for 50-70 hours Nm ultra-thin section was placed on copper net and double stained with 3% uranyl acetate and lead citrate. The structure of LESC window and the number of autophagy were observed under transmission electron microscope.
2.5.2 Detection of VEGFR2 and ET1 mRNA expression by RT-qPCR
Take the cells of the above groups after 48 hours of drug intervention, extract RNA according to the instructions of Trizol kit, and detect the concentration of 260 nm absorbance value. According to the instructions of the kit, cDNA was synthesized by reverse transcription: VEGFR2 gene F:5’-AAGAGATTTGTTCCGGATGG-3’;R:5’-C G G C AG ATAG C T C A AT T T C A-3’; F:5’-A G A G T G T G T C T A C T T C T G C C A - 3 ’ ; R : 5 ’ -CTTCCAAGTCCATACGGAACAA-3’; - actin gene F:5’-C T G AG AG G G A A AT C G T G C G T-3’;R:5’-C C ACAGGATTCCATACCCAAG A-3’. Reaction conditions: pre denaturation at 94 ℃, 3 min; denaturation at 95 ℃, 40 s; annealing at 53 ℃, 30 s; elongation at 72 ℃, 45 s, 30 cycles; final extension at 72 ℃, 10 min. With β - actin as the internal reference gene, the expression of each group was calculated by the ratio of 2-ΔΔCTof the target gene and β - actin amplification product.
2.5.3 Expression of VEGFR2 and ET1 by immunofluorescence
After 48 hours of drug intervention, the cells in each group were placed on a slide and fixed with 4% paraformaldehyde. Add diluted primary antibody: VEGFR2 and ET1. Incubate in 4 ℃ overnight, drop add fluorescent second antibody and then incubate. DAPI was added dropwise and incubated in dark for 5 min. the samples were stained and sealed with the sealing solution containing anti fluorescence quenching agent. Then the images were observed under the fluorescence microscope.
2.5.4 Detection of cell NO level by ELISA
After 48 hours of drug intervention, the cells of the above groups were centrifuged and the supernatant was taken. The content of no was detected by ELISA. The specific steps were in strict accordance with the instructions of the kit.
2.5.5 Western blot detection of autophagy marker protein and eNOS protein expression
The total protein was extracted from each group of cells at 48 h, and then SDS-PAGE electrophoresis was carried out. After PVDF membrane transfer, LC3II、LC3I、p62、eNOS、p-eNOS and β-actin were added. Incubated overnight at 4 C, and incubated with two anti room temperature for 1 h, ECL coloration and gel image processing system were used to analyze the net absorbance value of the target band, and the target protein expression was corrected by β-actin.
2.6 Statistical analysis
The data were analyzed by spss23.0, and the data were all expressed by mean ± standard deviation(±s). Single factor analysis of variance was used for the comparison between groups, and LSD test was used for the comparison between the two groups. P < 0.05 indicated that the difference was statistically significant.
3. Result
3.1 Effect of Qishen Decoction on the ultrastructure and autophagy of LSEC
The results of transmission electron microscopy showed that there were a lot of fenestra in LSEC cells of blank group, no basement membrane formation under endothelium, and a small number of autophagy. OxLDL can significantly reduce the number of fenestra and autophagy in LSEC cells, while continuous basement membrane can be seen under local endothelium. Qishen decoction can increase the number of fenestra and autophagy in LSEC cells, inhibit the formation of subendothelial basement membrane, and show a concentration dependent manner. Compared with the model group, the number of fenestra and autophagy in LSEC cells in the inhibitor group increased significantly, and continuous basement membrane was seen under the local endothelium. See Figure 1.
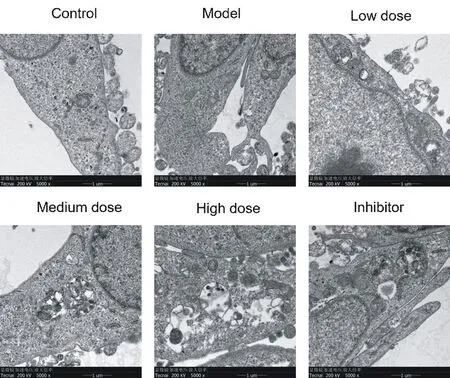
Fig. 1. Ultrastructures and autophagy of LSEC by TEM (× 5000 times)
3.2 Effect of Qishen Decoction on the expression of VEGFR2 and ET1 mRNA and protein in LSEC
100 µ g/ml oxLDL could significantly reduce the expression level of VEGFR2 mRNA and protein in LSEC, up regulate the expression level of ET1 mRNA and protein, and then promote the transformation of LSEC from differentiation type to dedifferentiation type. Compared with the blank group, the difference was statistically significant (P < 0.05). The serum containing Qishen decoction could significantly up regulate the expression level of VEGFR2 mRNA and protein in LSEC, down regulate the expression level of ET1 mRNA and protein, and then reverse the dedifferentiation of LSEC. Compared with the model group, the difference was statistically significant (P < 0.05). Chloroquine, as an autophagy inhibitor, has the same effect as oxLDL, which can significantly reduce the expression level of VEGFR2 mRNA and protein in LSEC, up regulate the expression level of ET1 mRNA and protein, and aggravate the transformation of LSEC from differentiation type to dedifferentiation type. Compared with the model group, the difference is statistically significant (P < 0.05). See Table 1-2 and Figure 2 Group n Drug concentration VEGFR2 OD ET1 OD
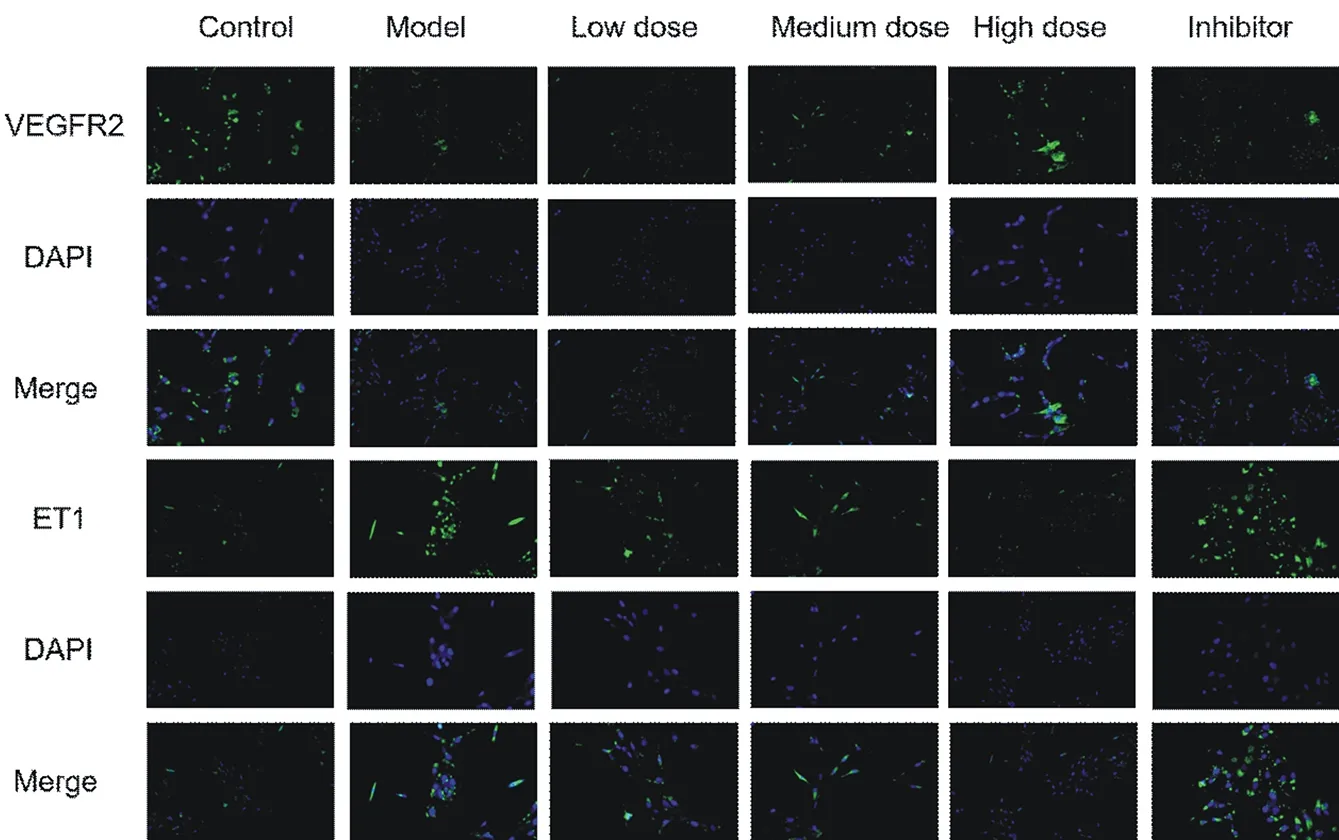
Fig. 2. Comparison of VEGFR2 and ET1 protein expression in LSEC by immunofluorescence staining (× 100 times)
Table 1. Comparison of VEGFR2 and ET1 mRNA expression in LSEC of each group(±s)
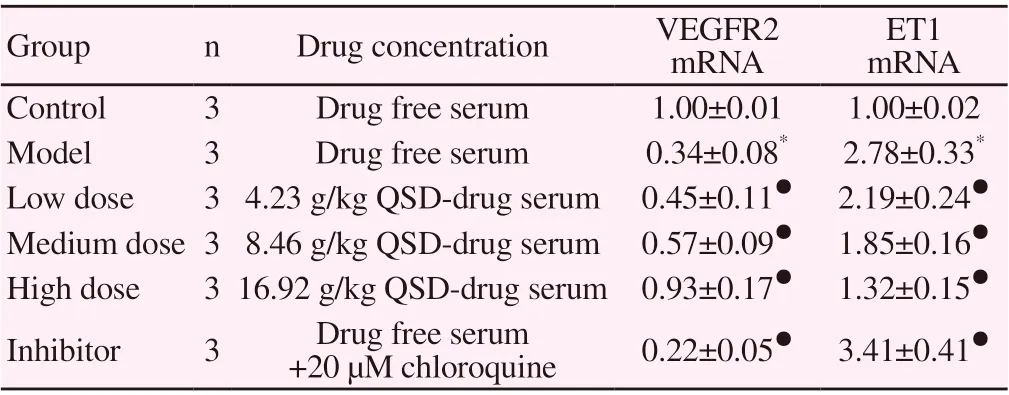
Table 1. Comparison of VEGFR2 and ET1 mRNA expression in LSEC of each group(±s)
Note: compared with the blank group, * P < 0.05, compared with the model group, ● P < 0.05.
Group n Drug concentration VEGFR2 mRNA ET1 mRNA Control 3 Drug free serum 1.00±0.01 1.00±0.02 Model 3 Drug free serum 0.34±0.08* 2.78±0.33*Low dose 3 4.23 g/kg QSD-drug serum 0.45±0.11● 2.19±0.24●Medium dose 3 8.46 g/kg QSD-drug serum 0.57±0.09● 1.85±0.16●High dose 3 16.92 g/kg QSD-drug serum 0.93±0.17● 1.32±0.15●Inhibitor 3 Drug free serum +20 µM chloroquine 0.22±0.05● 3.41±0.41●
Table 2. Comparison of VEGFR2 and ET1 protein expression in LSEC of each group(±s)

Table 2. Comparison of VEGFR2 and ET1 protein expression in LSEC of each group(±s)
Note: compared with the blank group, * P < 0.05, compared with the model group, ● P < 0.05.
Control 3 Drug free serum 0.29±0.03 0.02±0.01 Model 3 Drug free serum 0.04±0.01* 0.28±0.03*Low dose 3 4.23 g/kg QSD-drug serum 0.09±0.02● 0.23±0.04●Medium dose 3 8.46 g/kg QSD-drug serum 0.17±0.02● 0.15±0.03●High dose 3 16.92 g/kg QSD-drug serum 0.23±0.04● 0.08±0.02●Inhibitor 3 Drug free serum +20µM chloroquine 0.02±0.01● 0.41±0.05●
3.3 Effect of Qishen Decoction on NO in LSEC
Compared with the blank group, the no level in the model group was significantly lower (P < 0.05). Compared with the model group, Qishen decoction can significantly increase the content of no in LSEC, the difference is statistically significant (P < 0.05); the content of no in inhibitor group is significantly lower, the difference is statistically significant (P < 0.05). See Table 3
Table 3. Comparison of no content in LSEC of each group(±s)

Table 3. Comparison of no content in LSEC of each group(±s)
Note: compared with the blank group, * P < 0.05, compared with the model group, ● P < 0.05.
Group n Drug concentration NO(µmol/L)Control 3 Drug free serum 70.14±13.15 Model 3 Drug free serum 37.25±5.14*Low dose 3 4.23 g/kg QSD-drug serum 45.33±6.27●Medium dose 3 8.46 g/kg QSD-drug serum 52.71±7.02●High dose 3 16.92 g/kg QSD-drug serum 68.34±9.26●Inhibitor 3 Drug free serum +20µM chloroquine 25.15±3.34●
3.4 Effect of Qishen Decoction on the expression of autophagy marker protein and eNOS protein in LSEC
Compared with the blank group, the expression of LC3BII、p-eNOS、eNOS protein and the ratio of LC3BII/LC3BI、p-eNOS/eNOS in the model group decreased significantly, and the expression of LC3BI and p62 protein increased significantly (P < 0.05). Compared with the model group, Qishen decoction can significantly increase the expression of LC3BII, p-e eNOS, eNOS protein and LC3BII、p-eNOS、eNOS ratio in LSEC, reduce the expression of LC3BI and p62 protein, the difference is statistically significant (P < 0.05). Compared with the model group, chloroquine increased the ratio of LC3BII/LC3BI and the expression of p62 protein, decreased the expression of p- eNOS, eNOS protein and p- eNOS/eNOS ratio, the difference was statistically significant (P < 0.05). See table 4-5 and figure 3
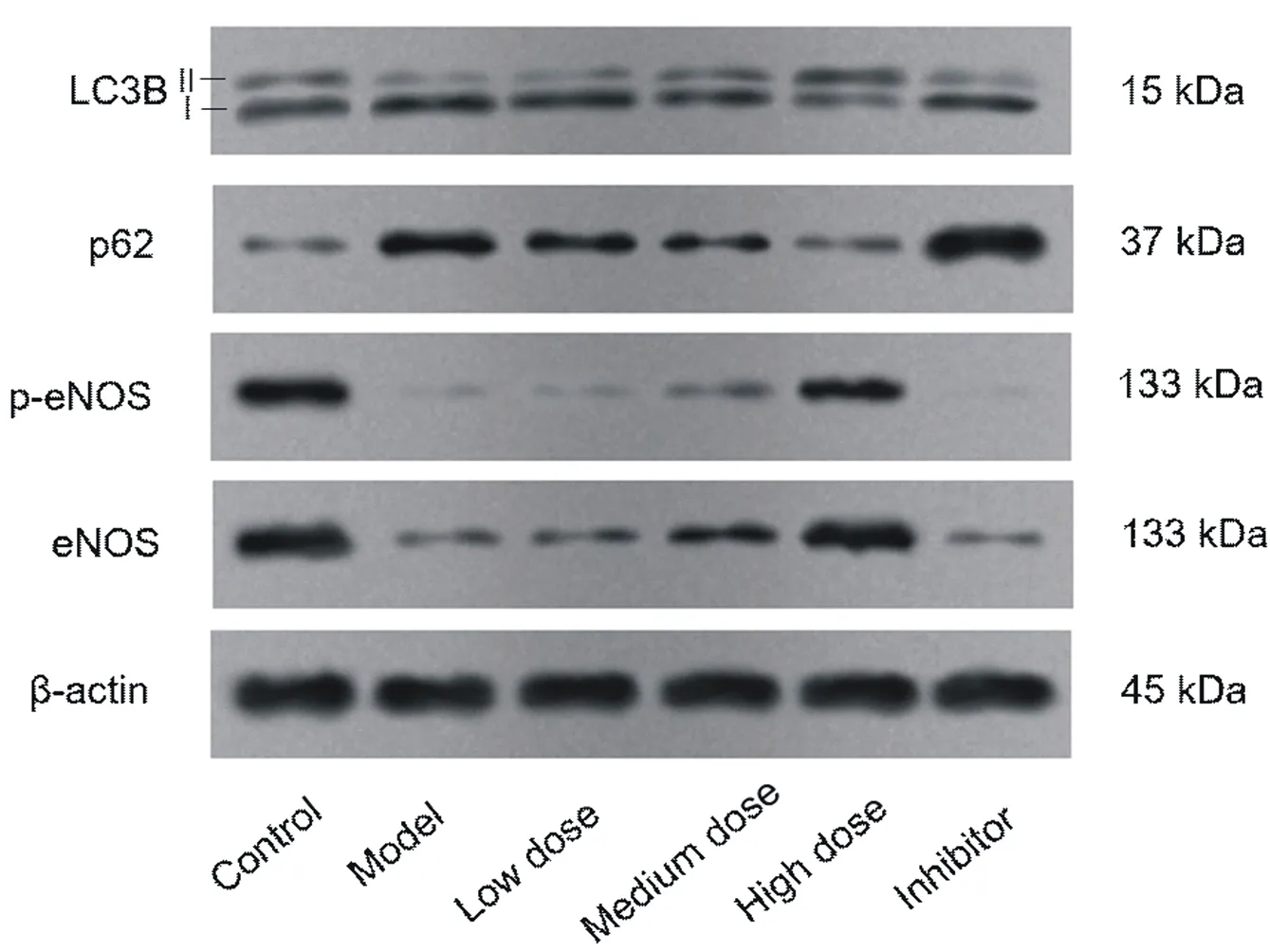
Fig3. Western blot to detect the expression of autophagy marker protein and eNOS protein in LSEC of each group
Table 4. Comparison of autophagy marker protein expression in LSEC of each group(±s)
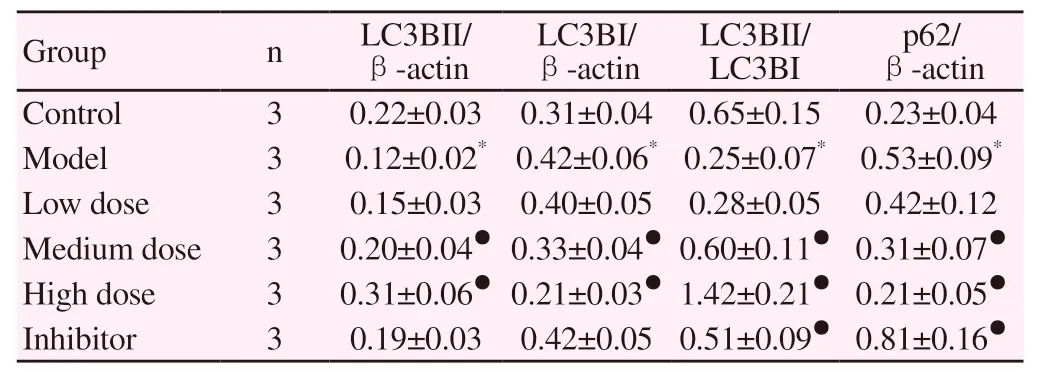
Table 4. Comparison of autophagy marker protein expression in LSEC of each group(±s)
Note: compared with the blank group, * P < 0.05, compared with the model group, ● P < 0.05.
?
Table 5. Comparison of eNOS protein and its phosphorylation expression in LSEC of each group(±s)
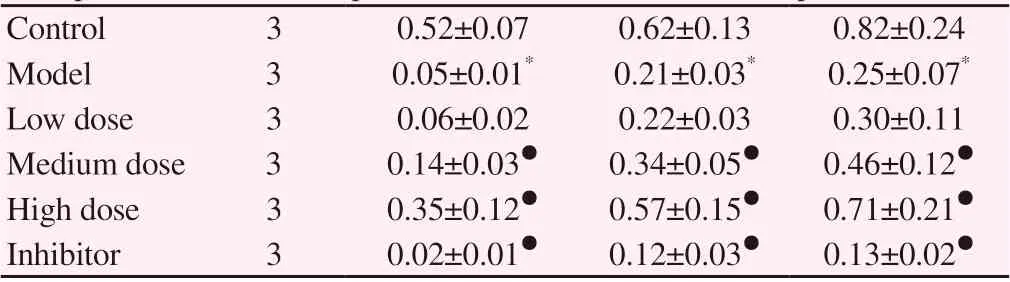
Table 5. Comparison of eNOS protein and its phosphorylation expression in LSEC of each group(±s)
Note: compared with the blank group, * P < 0.05, compared with the model group, ● P < 0.05.
4. Discussion
As an important defense line against the outside world, the liver is composed of hepatocytes and non-parenchymal cells, among which the non-parenchymal cells are composed of LSEC, HSC, dendritic cells and Kupffer cells. The surface of LSEC is rich in window pores with a diameter of about 100-150 nm, presenting a typical cribriform plate, which is the material exchange channel between the hepatic sinuses and the dise space. It is mainly involved in regulating the occurrence and development of liver diseases through the dedifferentiation of the hepatic sinuses, endocytosis and clearance function, anti-inflammatory, maintenance of HSC resting
state, secretion of angiogenic signal factors [8]. In pathological state, hepatic sinuses are affected by oxLDL, hyperglycemia, endotoxin and ethanol from intestinal tract. The diameter and quantity of LSEC fenestra become smaller, accompanied by the formation of subendothelial basement membrane, which is called LSEC dedifferentiation [9]. During the dedifferentiation of LSEC, it is characterized by the decrease of VEGFR2 expression level and the increase of ET1 expression level. The dedifferentiation of LSEC is an important pathological change in the process of liver fibrosis. Therefore, LSEC targeted therapy has become a hot spot in the research field of non-alcoholic fatty liver disease, liver fibrosis and cirrhosis [10-12]. No is a molecule with many biological activities. It can improve the microcirculation of liver, inhibit the activation of HSC and the dedifferentiation of LSEC by regulating the blood flow and the diameter of blood vessels, and then delay the development of chronic liver disease into cirrhosis [13-14]. The production of no is mediated by eNOS. After LSEC injury, eNOS activity will also be reduced, which is mainly manifested in the decrease of eNOS phosphorylation level [15]. In this study, our results show that Qishen decoction can significantly up regulate eNOS protein and its phosphorylation level in LSEC, and then increase the content of no in LSEC. On this basis, the results of this study also show that Qishen decoction can inhibit the dedifferentiation of LSEC in a concentration manner, which is manifested in increasing the level of VEGFR2 in LSEC and decreasing the level of ET1, thus inhibiting the reduction of the number of fenestra and the formation of the subendothelial basement membrane.
Autophagy is a metabolic process mediated by lysosomes to degrade damaged organelles and long-lived proteins in cells, and then maintain the balance in cells [16-18]. With the role of autophagy in liver fibrosis has been gradually interpreted, the research on the prevention and treatment of liver fibrosis targeting autophagy has become the focus of current research. In the process of autophagy, the formation of autophagic bodies with a double membrane structure surrounding the organelles to be degraded usually occurs first, which is characterized by the formation of lc3b Ⅰ I through ubiquitination modification and the combination of phosphatidylethanolamine on the membrane surface [19]. Then the autophagic lysosomes are fused with lysosomes to form autophagic lysosomes, which can degrade the contents and produce free fatty acids and amino acids for cell reuse, so as to maintain the renewal of organelles and cell homeostasis. P62, also known as the selective autophagy receptor, is the most important truck protein in the selective autophagy. After entering the autophagy by binding ubiquitin, p62 fused with lysosome. When the level of autophagy flow fossils decreased, the expression of p62 increased significantly when autophagy flow inhibited [20]. In this study, our results show that OxLDL can significantly inhibit the autophagy level of LSEC, while the concentration and high concentration of Qishen decoction can promote the autophagy of LSEC, which is manifested by the increase of the number of autophagy bodies, the expression of lc3bii protein and the proportion of lc3bii / lc3bi, and the decrease of the expression level of lc3bi and p62 protein. As a classical autophagy inhibitor, our results show that chloroquine can significantly inhibit the formation of autophagy lysosomes and then inhibit autophagy flow, showing a large accumulation of p62.
Based on the above findings, we conclude that Qishen decoction can promote the autophagy level of LSEC, and then inhibit the dedifferentiation level of LSEC through eNOS / no pathway. This may be one of the possible mechanisms of Qishen Decoction in the treatment of liver fibrosis. However, LSEC plays many roles in the evolution of chronic liver disease to cirrhosis. At the same time, the effective mechanism of Qishen Decoction in the treatment of chronic liver disease, which has multi-channel and multi-target therapeutic effect, needs further study.
 Journal of Hainan Medical College2020年16期
Journal of Hainan Medical College2020年16期
- Journal of Hainan Medical College的其它文章
- Molecular mechanism of Duhuojisheng Decoction in the treatment of knee osteoarthritis based on the network pharmacology
- Meta-analysis on clinical efficacy of Simiao Yong'an decoction in treatment of peripheral arterial occlusive diseases
- Network pharmacology-based elucidation of molecular biological mechanisms of Kanglaite injection for treatment of non-small cell lung cancer
- Research on the rules of Professor Dai Xiaohua's medication for coronary heart disease and chronic heart failure based on data mining
- Clinical study on the treatment of bronchiectasis in remission period by embedding thread combined with Jianpi Qushi Huayu plaster
- Comparative study of 99Tc-MDP and zoledronic acid in the treatment of osteoporosis
