Flexibility as a Strategy for Avoiding Call Overlap in Male Anhui Treefrogs
Jinjin SONG ,Ruolei SUN ,Ke FANG,3 ,Baowei ZHANG* ,Yezhong TANG and Guangzhan FANG*
1 School of Life Science,Anhui University,Hefei 230601,Anhui,China
2 Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu 610041,Sichuan,China
3 Institute of Bio-inspired Structure and Surface Engineering,Nanjing University of Aeronautics and Astronautics,Nanjing 210016,Jiangsu,China
Abstract Male-male vocal competition is critical for mating success in anuran species; however,it remains unknown that how males regulate their competitive strategies dynamically during competition because calling is highly time-consuming,energetically demanding and likely to increase predation risks.Since different parts of calls will encode different information for vocal communication,we hypothesized that competitive strategies of male frogs may be modulated by the temporal and spectral features of different call notes.To test this hypothesis,the natural advertisement calls (OC),its modified versions with the first call note replaced by white noise (WN) or other notes and with the fifth call note replaced by WN,were played back to the Anhui tree frogs (Rhacophorus zhoukaiyae).Results showed that 1) males produced more competitive calls in response to acoustic stimuli compared to their baseline calling during silence; and 2) males emitted more non-overlapping calls compared to overlapping calls in response to the acoustic stimuli.These results are consistent with the idea that males are flexible to acoustic signals and their competition strategies are modulated dynamically by social contexts.
Keywords acoustic stimulus,call overlap,flexibility,frog,individual recognition,male-male competition
1.Introduction
Vocal communication plays a crucial role in the survival and reproductive success in animals,such as birds,anurans and insects.Its main purpose is to maintain territories,defend resources and compete with rivals,as well as attract potential mates (Brenowitz and Rose,1994; Dyson and Passmore,1992;Harrington and Mech,1983; Roithmair,1994; Romer and Bailey,1986; Ryan,1985).Many studies focusing on anurans,reptiles and birds have shown that syllable structures and temporal-spectral features of different parts of the acoustic signals are significantly distinct among conspecific individuals,implying that these parts of acoustic signals may contribute differently in vocal communication (Fanget al.,2019; Suzukiet al.,2016; Tanget al.,2001; Williams and Staples,1992; Yueet al.,2017).For example,the advertisement calls ofGekko geckoconsist of two call phases.The first phase consists of a series of pulses for individual recognition,while the second phase consists of a series of two-note syllables for species discrimination (Yuet al.,2011).Interestingly,sexual differences in response to the same part of the advertisement call exist in some species (Bernalet al.,2007; Vicarioet al.,2001).It remains unclear,however,how animals respond to different parts of conspecific acoustic signals in a behavioral context.
In anurans,vocal communication plays a crucial role in various behavioral contexts,especially in reproductive behaviors(Gerhardt and Bee,2007; Tobiaset al.,2004).Vocalizations can be used to identify potential resources of an opponent (Beeet al.,2000; 1999),facilitate spacing between calling individuals(Brenowitz,1989; Marshallet al.,2003) and recognize territorial neighbors (Bee,2007).In other words,vocalizations contribute towards species discrimination and individual recognition for most anuran species.Generally,males emit advertisement calls,and females choose their mates through these calls (Bernalet al.,2007).Since females commonly prefer non-overlapping calls,males typically adjust the timing of their call production in relation to those of rivals (Fanget al.,2014; Reichert,2012) in order to avoid producing overlapping calls which may obscure the fine acoustic features of their own calls (Fanget al.,2019;Schwartz,1987).Males and females often respond differently to conspecific calls,implying different parts of call notes may convey separate information to males and females at the behavioral level (Wells and Schwartz,2007).This speculation is consistent with the idea that the functional differences between acoustic components of the calls in frogs may increase signal content,promote individual perception or enhance signal transmission (Preiningeret al.,2013).In addition,the auditory system may be more sensitive to the perception of the first components of conspecific acoustic signals in anurans (Fanget al.,2019; Yueet al.,2017) and birds (Jouventinet al.,1999).However,very little is known or has been hypothesized about how competitive strategies of males are modulated by the temporal and spectral features of different parts of calls.
The Anhui tree frog (Rhacophorus zhoukaiyae) was used in the present study as a model for investigating the differences in the functional role of call notes in vocal communication.The Anhui tree frog has a unique mating system (Panet al.,2017)and male calls are typically produced from inside underground nest burrows (Fanget al.,2019).The burrows are usually located at the bottom of a ridge of farmland and serve as the nest for mating,egg laying and tadpole development,similar to other species,such as the Emei music frog (Nidirana daunchina) (Cuiet al.,2012).Although advertisement calls are usually composed of 10 or more notes,the temporal and spectral features of the first call note are significantly different from those of other notes,suggesting that the first call note may play an important role in vocal communication in this species (Fanget al.,2019).Accordingly,it is logical to hypothesize that competitive strategies of males may be modulated by the temporal and spectral features of different call notes.
To test these hypotheses,the natural advertisement call and its altered versions were broadcasted to male Anhui tree frogs and that the acoustic responses of the subjects were recorded and analyzed.Since the precedence effect is the inherent characteristic response of the vertebrate auditory system(Litovskyet al.,1999; Zurek,1987),we predicted that,based on our hypotheses,males would 1) change call parameters dynamically according to social contexts; 2) avoid producing overlapping calls relative to the occurrence of acoustic stimuli.
2.Materials and Methods
2.1.Study site and subjectsThe present experiments were conducted in the Dabie Mountain area of Anhui,China (871 m above sea level,31.28° N and 115.72° E).The recordings were acquired from 12 to 22 April 2018 within a local area of about 3 600 m2.The local air temperature ranged from 5.7~16.2℃ and the relative humidity from 57.9%~92.6% during the experiments.Twenty males were monitored for the playback tests and no male was used twice for the same test.The distance between the subjects and its male neighbors was far enough so that the responses of his neighbors to the playback stimuli were relatively low.
2.2.Stimulus presentationFour stimuli were used:natural advertisement call (OC),an altered version with note 1 replaced by white noise (WN→N1),an altered version with note 5 replaced by white noise (WN→N5) and a last altered version with note 1 replaced by note 5 (N5→N1) (Figure 1).The selected OC contained sixteen notes with both temporal and frequency parameters of each note close to the average values of the population.The duration of WN was equal to the duration of the corresponding call notes,with an 8 ms rise-fall times in sinusoidal periods.These four stimuli were combined with each other to make six pairs:OC and WN→N1 (SP1); OC and WN→N5 (SP2); OC and N5→N1 (SP3); WN→N1 and WN→N5 (SP4); WN→N1 and N5→N1 (SP5); WN→N5 and N5→N1 (SP6).Because pseudoreplication can affect statistical analysis in ecological and animal behavior studies (Lazic,2010;McGregor,2000),we accounted for the possible influence of pseudoreplication in our conclusions in the present study.To do this,four advertisement calls containing 16 notes were acquired from four different individuals by random selection from our dataset.Thus,there were four sets of stimuli,each set containing one OC and its three altered versions.Each set of stimuli was played back to five subjects.
2.3.Experimental protocolThe experiments were conducted under ambient light conditions between 20:00 to 05:00 (the next day) in order to avoid the effects of visual stimulation and intense insect noise.Two speakers (SME-AFS,Saul Mineroff Electronics,Elmont,NY,USA) were placed about 1 m apart along the bottom of the ridge of the farmland,oriented toward the subject located inside his burrow.The burrow and the two speakers formed the three vertices of an isosceles triangle.Before the start of the experiments,a pure tone of 1 000 Hz was used to calibrate the peak output intensity of each speaker to 80 dB SPL (Type 2240; Bruel and Kjær,Nærum,Denmark;measured at 1 m from the speaker).Playbacks started about 10 min after the male resumed normal calling behavior following the speaker placement.The experiments consisted of seven blocks:spontaneous calls of the subjects were recorded first for 10 min (the control condition,CC),then six blocks were conducted randomly during which each of the six stimulus pairs was played back antiphonally for 10 min from either of two speakers with 2.5 s inter-stimulus interval (ISI) and 3 min inter-block intervals (Table 1).We randomly varied the speaker assignments and presentation order among blocks except for the first one in order to control for possible side biases.
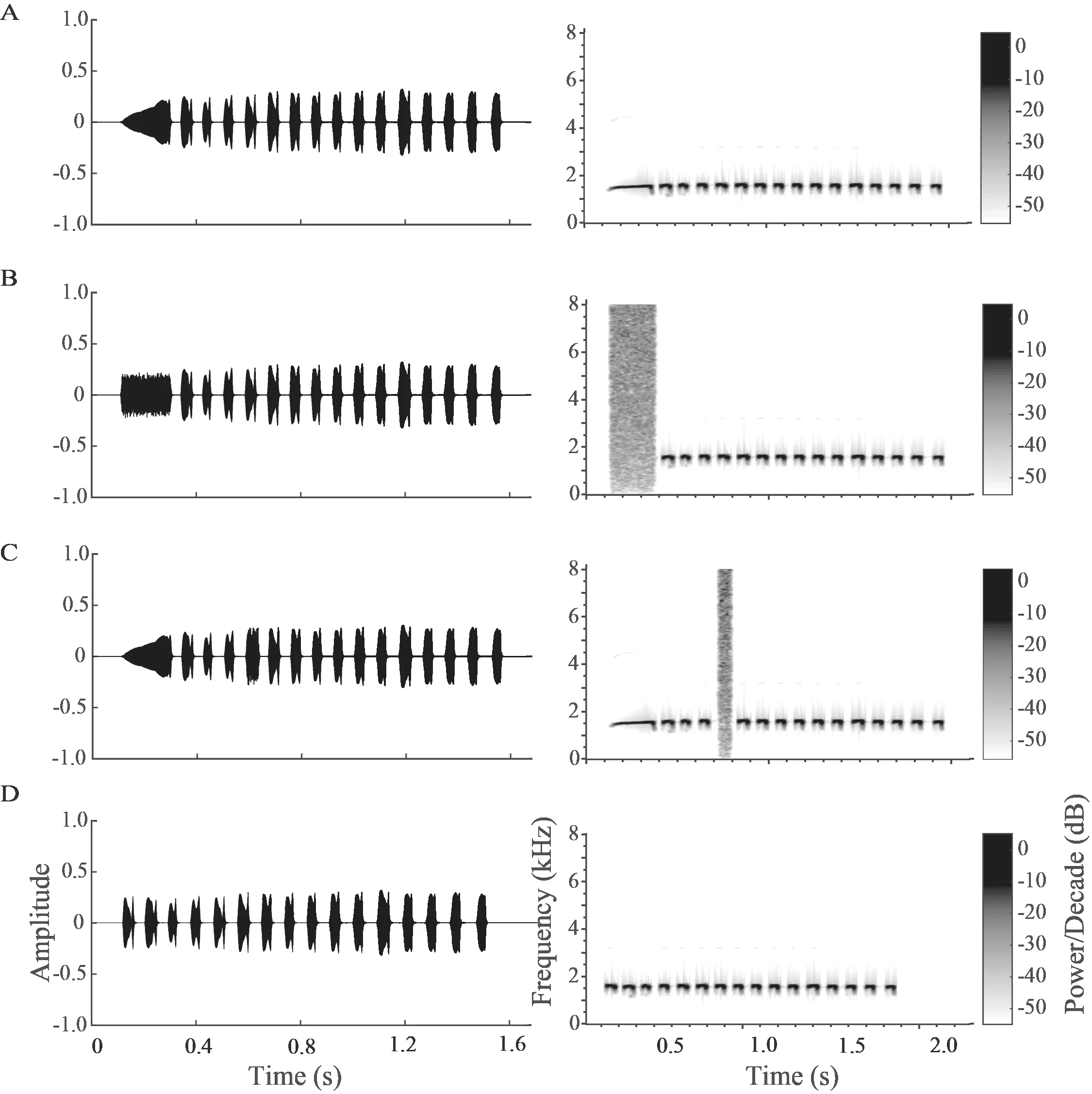
Figure 1 Waveforms and spectrograms of the four acoustic stimuli used in this study:(A) natural advertisement call (OC); (B) note 1 replaced by white noise (WN→N1); (C) note 5 replaced by white noise (WN→N5) and (D) note 1 replaced by note 5 (N5→N1).
Both the subjects’ vocal responses and the playback stimuli were simultaneously recorded with a Sennheiser ME66 microphone (Sennheiser,Wedemark,Germany),connected to a laptop (Thinkpad X201; Lenovo,China) with a sampling rate of 44.1 KHz and a 16 bit resolution.The microphone was mounted on a long bamboo rod and placed at about 0.5 m from the subject.All playback orders were randomized using custom-made software in C++and saved in txt files so that calls recorded from each subject could be correlated with each playback stimulus.After the experiments,the subjects were captured in order to measure body mass and snout-to-vent length (SVL).
2.4.Data processingThe Adobe Audition 3.0 software (San Jose,California,USA) was used to analyze the amplitudemodulated waveforms (oscillograms) and audio spectrograms of male competitive calls.Methodsfor data analysis were similar to those described previously (Fanget al.,2014; Jianget al.,2015).Briefly,because the ability of interval timing exists in frogs (Fanget al.,2014),the ISIs were divided into two equal phases for data analysis:a pre-phase defined as the period before the playback and a post-phase defined as the period after the playback (see the Appendix,Figure S1).Thus a completed“trial” consisted of two playbacks and four phases.The first phase occurred before the first playback,the second phase occurred after the first playback,the third phase occurred before the second playback and the fourth phase occurred after the second playback (see the Appendix,Figure S2A).In addition,according to the temporal relationship between the subjects’responses and the onset of the stimulus playbacks,responsive vocalizations of the animals were categorized into two classes:overlapping calls for which call onset time overlapped the ongoing stimulus and non-overlapping calls that were initiated during the ISI (Fanget al.,2014) (also see the Appendix,Figure S2B).Thus,the advertisement calls produced before,during and after a given playback were considered as response calls (Fanget al.,2014) (also see the Appendix,Figure S2C).Consequently,for each male and each block,the following acoustic parameters of male response calls were measured for each stimulus type,overlapping and non-overlapping calls,respectively:the number of advertisement calls,their onset time relative to the beginning of the ongoing or upcoming stimulus,the average number of notes and the average duration of advertisement calls.
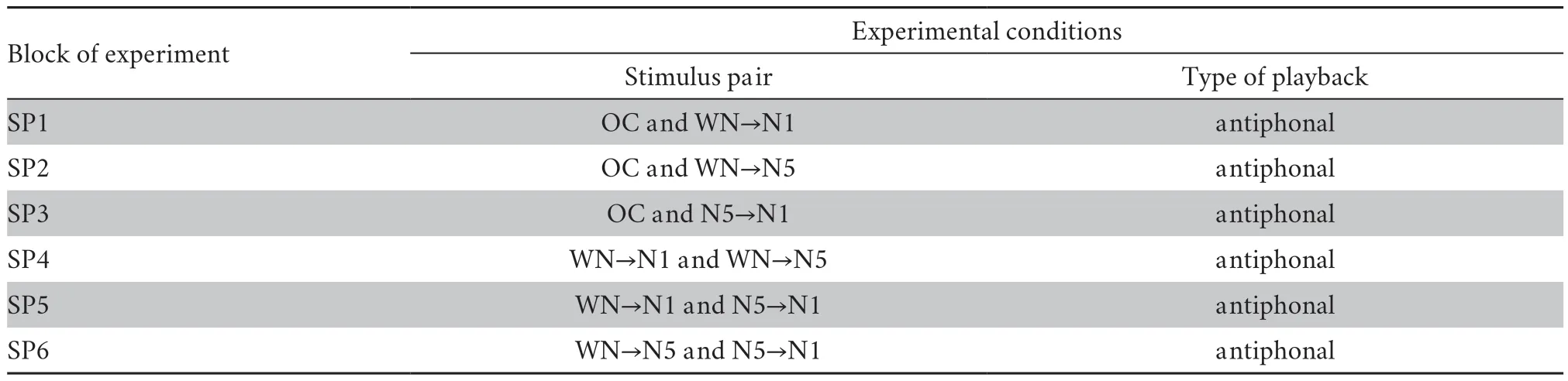
Table 1 Experimental conditions based on playback stimuli.
2.5.Statistical analysesThe normality and the homogeneity of variance for all data were estimated with the Shapiro-WilkWand Levene’s tests,respectively.In order to statistically analyze the acoustic parameters of male response calls produced during different blocks,a two-way repeated measures ANOVA was conducted with the factor of “stimulus set” (the four stimulus sets) and “block” (the seven blocks,i.e.the control condition and the six stimulus pairs),or “acoustic stimulus” (the four stimuli in each stimulus set).For analyzing the acoustic parameters of male response calls with respect to overlapping the stimulus or not,a three-way repeated measures ANOVA was conducted with the factor of “stimulus set” (the four stimulus sets),“timing of responsive call” (overlapping and non-overlapping),and“acoustic stimulus”.There was no significant main effect of the first factor for all above ANOVAs,consistent with the idea that the results of the present statistical analyses were not affected by pseudoreplication.Thus,each dataset was pooled regardless of “stimulus set” and statistically analyzed using a one-way or two-way repeated measures ANOVA,including the last one or two factors,respectively.Both main effects and interactions were examined.
Simple effects analysis was applied when the interaction was significant.For significant ANOVAs,data were further analyzed for multiple comparisons using the least-significant difference (LSD) test.Greenhouse-Geisser epsilon (ε) values were employed when the Greenhouse-Geisser correction was necessary.Estimations of effect size were determined with partialη2for ANOVAs (partialη2=0.20 is a small effect size,0.50 is a medium effect size and 0.80 is a large effect size)(Cohen,1992).SPSS software (release 23) was utilized for the statistical analysis.A significance level ofP< 0.05 was used in all comparisons.
3.Results
3.1.Acoustic stimulations evoked male responsesFor the number of male response calls produced during different blocks,the main effect was significant for the factor “block”(F6,114=2.638;ε=0.633,P=0.02,partialη2=0.122).The number of response calls during the control condition (CC) was significantly lower than those during other conditions with stimulus playbacks,although the difference between SP1 and CC was not statistically significant (P< 0.05,Figure 2A and Table 2).Similarly,the main effects were significant for the factor “block” for the number of call syllables (F6,114=13.769;ε=0.639,P=0.000,partialη2=0.42) and the call duration(F6,114=12.075;ε=0.582,P=0.000,partialη2=0.389).Multiple comparisons showed that both the number of call syllables(P< 0.001,Figure 2B and Table 2) and call duration (P< 0.001,Figure 2C and Table 2) were significantly lower or shorter for CC than those for other presentation conditions,respectively.
3.2.No significant difference of acoustic responses among the four stimuliFor the factors “acoustic stimulus”,there was no significant difference in the numbers of advertisement calls in response to the four types of stimuli (F3,57=0.251;ε=0.755,P=0.86,partialη2=0.013).There was also no significant difference between the number of syllables in response to the four types of stimuli (F3,57=0.204;ε=0.77,P=0.893,partialη2=0.011).Similarly,there was no significant difference in call duration in response to the four types of stimuli (F3,57=0.504;ε=0.747,P=0.681,partialη2=0.026; Table 2).
3.3.The differences of acoustic parameters between overlapping and non-overlapping callsFor the number of calls,the main effect was significant for the factor “timing of responsive call” (F1,19=22.758;ε=1.0,P=0.000,partialη2=0.545) but not “acoustic stimulus” (F3,57=0.251;ε=0.755,P=0.86,partialη2=0.013).The number of non-overlapping calls was significantly higher than that of overlapping calls (P< 0.001,Figure 3A and Table 3).
For the number of call syllables,there was no significant main effect for the factors “timing of responsive call” (F1,19=3.191;ε=1.0,P=0.09,partialη2=0.144) and “acoustic stimulus”(F3,57=0.861;ε=0.625,P=0.425,partialη2=0.043; Figure 3B and Table 3).Similarly,for the call duration,there was no significant main effect for the factors “timing of responsive call” (F1,19=4.12;ε=1.0,P=0.057,partialη2=0.178) and “acoustic stimulus” (F3,57=1.08;ε=0.666,P=0.35,partialη2=0.054; Figure 3C and Table 3).

Figure 2 The differences of call parameters between the control condition (CC) and other six stimulus pairs (SP) for number of calls (A),number of syllables (B) and call duration (C).CC,the control condition,i.e.no sound was played back; SP1,antiphonal playback of natural advertisement call (OC) and its altered version with note 1 replaced by WN (WN→N1); SP2,antiphonal playback of the stimulus pair OC and WN→N5; SP3,antiphonal playback of the stimulus pair OC and N5→N1; SP4,antiphonal playback of the stimulus pair WN→N1 and WN→N5; SP5,antiphonal playback of the stimulus pair WN→N1 and N5→N1; SP6,antiphonal playback of the stimulus pair WN→N5 and N5→N1.*,P < 0.05; and **,P < 0.001.

Table 2 Results of ANOVAs for the number of calls,the number of syllables and call duration in responding to stimulus playbacks as a function of the factors “block” and “acoustic stimulus”.
4.Discussion
The present study showed that when the four stimuli,consisting of natural advertisement call (OC) and its three altered versions,were presented,1) maleR.zhoukaiyaeproduced more competitive calls in response to the stimulus pairs compared to their baseline calling during silence; and 2) males invariably avoided producing competitive calls overlapping the acoustic playbacks.These results are consistent with the hypotheses that males were flexible and their competition strategies were dynamic in order to avoid overlapping.
4.1.Male competitive strategy may depend on female preferencesOverlapping is often discriminated against by females and cannot be avoided in choruses (Schwartz,1987).Although overlapping is considered as an antagonistic signal and used to indicate a willingness to escalate the vocal confrontation (Naguib,1999),overlap may obscure the fine temporal components and structures of male vocalizations(Schwartz,1987).Therefore,females of some species including frogs and birds typically prefer non-overlapped signals (Amyet al.,2008; Martínez-Rivera and Gerhardt,2008).Accordingly,males usually produced non-overlapping calls to avoid the interference of call overlap.For example,male green treefrogs place calls predominantly during silent gaps and avoid call production during short,but not long,noise segments (Höbel,2014).Therefore,how males regulate their call timing to avoid the problem of call overlaps and masking interferences which has a profound impact on mating success (Richardsonet al.,2008).The present results showed that male frogs produced more non-overlapping calls than overlapping calls and changed their call parameters during competition,consistent with the conclusion that call interaction between males is a dynamic process,and the temporal relationship of male calls from different individuals are fluid and vary with environmental acoustics or competitive levels among males (Wells and Schwartz,2007).In addition,since the latencies to call of the subjects in the present study were too short for eavesdropping(about 6 s,data not shown),the males’ vocalizations were direct responses to the playbacks perceived as competitors but not responses to the outcome of eavesdropping on potential rivals(Garciaet al.,2019).
The possibility of missed mating opportunities due to call overlap or interference may be an important factor in shaping male signal behavior,including the unique call timing interactions found in some vocal species (Schwartz,2001).In a lek or chorus,the leader-follower relationship may change among alternating,synchronized or partially overlapping(Bosch and Márquez,2001; Gerhardt and Huber,2002; Grafe,2003; Mooreet al.,1989; Wells and Schwartz,2007).Compared with the period of stimulus presentation,more leading calls were emitted by males during ISI in the present study,resulting in less of a chance of call overlap.It is also consistent with the idea that alternating calls can preserve the fine acoustic features within calls,preventing overlapping of male calls to obscure or degrade these structures (Wells and Schwartz,2007),and maintaining the acoustic space among males.For example,the overlapping calls damage the ability of males to judge the intensity of neighbors’ voices and affect the interval between males’ spacing (Grafe,1996).However,accurate alternating calls may be difficult to achieve because males in dense choruses typically produce multi-note calls and fail to exhibit call alternation.Interestingly,the current results showed that the number of calls of non-overlapping calls was significantly higher than those of overlapping calls,consistent with the idea that males can rely on increasing call production,number of notes and/or call duration to avoid the threat of call overlap in dense choruses (Caldartet al.,2016; Grafeet al.,2012).Thus,males regulate their calls in ways that increase the signal-to-noise ratio of their vocalizations (Caldartet al.,2016; Grafeet al.,2012),including increasing call production,increasing number of notes and increasing call duration,thereby making their calls more attractive to females.

Figure 3 The differences of call parameters of non-overlapping and overlapping calls in responding to the playbacks of the natural advertisement call (OC) and other altered versions for number of calls (A),number of syllables (B) and call duration (C).WN→N1,WN→N5 and N5→N1,the three altered versions of OC.**,P < 0.001.
Sexual selection,the differential success of individuals over competition for mates (Darwin,1871; Andersson,1994),led to the evolution of male ornaments or displays and female preferences for this phenotype.In addition,the evolution of male signaltiming behavior is strongly influenced by female preferences(Höbel and Gerhardt,2007).In the present study,male frogs responded to playbacks of conspecific calls by producing more non-overlapping calls and changing the call parameters against the negative effects of call overlap,consistent with the fact that females in the reproductive stage prefer non-overlapping calls.Perhaps,due to the fact that the precedence effect in many species is the inherent response characteristic of the auditory system (Litovskyet al.,1999; Zurek,1987),females have a strong preference for signals that are temporarily dominant under some conditions (Wells and Schwartz,2007).Therefore,the males actively adjust their call strategy to compete with rivals,thereby maximizing the proportion of their leading calls and reducing the neighbors’ competitive calls to an unattractive backward position,and ultimately,achieving the purpose of attracting females (Richardsonet al.,2008).
4.2.Males are flexible during male-male competitionIn the present study,males increased their vocal responses to the different versions of acoustic stimulus compared with their spontaneous calls; however,there were no significant difference among these versions,suggesting that males may be flexible in response to signal variations.Sexual selectiontheory predicts that males will be less selective in mate choices than females because of their smaller reproductive investment(Darwin,1871).Consequently,males would be expected to be more permissive than females in responding to acoustic signals(Bernalet al.,2007).Consistent with this,vocal animals typically exhibit sexual dimorphism in their responses to conspecific or heterospecific vocalizations at both behavioral and neural levels.Usually,males vocalize in response to a wide range of audible signals whereas females are much more selective when responding phonotactically (Baugh and Ryan,2010; Bernalet al.,2007; Bernalet al.,2010).For example,male túngara frogs(Physalaemus pustulosus) and male cricket frogs (Acris crepitans)are more likely than conspecific females to respond to nonspecies-typical signals (Bernalet al.,2007; Burmeisteret al.,1999).Compared with males,however,choosing mates in females are influenced by many factors such as sex steroids,reproductive costs and investment risks (Gerhardt and Bee,2007; Grafe,1997;Leary,2014; Ryser,1989; Shine,1980).Consequently,females were much more cautious in response to signal variation than males (Bernalet al.,2007).These sex differences are consistent with the fact that the cost of not responding to a potential sexual signal would be greater in males than females while the cost of responding inappropriately to sexual solicitation signals would be greater in females than males (Searcy and Brenowitz,1988; Wiley,2006).In other words,the inherent asymmetry in fitness costs associated with errors in mating signal recognition results in greater flexibility in males than in females.

Table 3 Results of ANOVAs for the number of calls,the number of syllables and call duration in responding to stimulus playbacks as a function of the factor “acoustic stimulus” and “timing of responsive call”.
AcknowledgementsWe sincerely thank Zhonglou SUN,Liu YANG,Zheng KONG,Ying LI,Xinlei LAI,Zhengbao LI,Xuyu WANG from School of Life Science,Anhui University and the members of Animal Behavior and Neural Mechanism Group of Chengdu Institute of Biology,Chinese Academy of Sciences,for their discussions and suggestions.We also thank Daniel FANG from School of Medicine,University of Utah,for his help in improving English writing.This work was supported by the grants from the National Natural Science Foundation of China (Nos.31970422,31672305 and 31372217 to Guangzhan FANG,No.31572275 to Yezhong TANG),the grants from Biodiversity Conservation Programe of Ministry of Ecology and Environment of China to Baowei ZHANG.Animal procedures were approved by the Animal Care and Use Committee of the Chengdu Institute of Biology.
Appendix

Figure S1 Schematic diagram illustrating the temporal relationships between the pre-phase and post-phase time periods during which subject calls were produced in response to stimulus playbacks.ISI:inter-stimulus interval (reproduced from Fang et al.,2014 with permission).

Figure S2 Schematic “cylindrical” diagrams depicting the temporal relationships between the stimulus playbacks and occurrences of subject male response calls.(A) A sample trial (including two stimulus playbacks from the left and right audio channels) was divided into 4 equal phases and two periods for the stimulus playback (orange and pink regions).Pre-S1/S2 is the pre-phase period before the first/second stimulus playback; Post-S1/S2 is the post-phase period after the first/second stimulus playback; (B) The time axis was divided into the overlapped period (orange regions) during which subject call onsets occurred during playbacks and the non-overlapped period (purple regions) during which subject call onsets occurred before or after playbacks; (C) Two time periods during which the subject called in response to the first (S1) and second (S2) stimulus,respectively.Note that the data of the experiments are cyclic,with the end of one cycle coinciding with the start of the next,thus the right and left edges of each subgraph coincide (reproduced from Fang et al.,2014 with permission).
 Asian Herpetological Research2020年3期
Asian Herpetological Research2020年3期
- Asian Herpetological Research的其它文章
- A New Species of Microhyla (Amphibia:Anura:Microhylidae) from Langbian Plateau,Central Vietnam
- A New Species of Hemiphyllodactylus Bleeker,1860 (Reptilia:Squamata) from Hunan Province,China
- Geographic Variation in the Skull Morphometry of Four Populations of Batrachuperus karlschmidti (Urodela:Hynobiidae)
- The Impact of Stream Landscape on Genetic Structure and Dispersal Patterns in Stream Salamander (Pachyhynobius shangchengensis)
- Age and Body Size of the Shangcheng Stout Salamander Pachyhynobius shangchengensis (Caudata:Hynobiidae) from Southeastern China
- No Evidence for the Compensation Hypothesis in the Swelled Vent Frog(Feirana quadranus)
