Preparation of Clay/Biochar Composite Adsorption Particle and Performance for Ammonia Nitrogen Removal from Aqueous Solution
HUANG Xiao, BAI Jie, LI Kuiran, ZHAO Yangguo, TIAN Weijun,and HU Chunhui
Preparation of Clay/Biochar Composite Adsorption Particle and Performance for Ammonia Nitrogen Removal from Aqueous Solution
HUANG Xiao1), 3), BAI Jie1), LI Kuiran2),*, ZHAO Yangguo1), TIAN Weijun1),and HU Chunhui1)
1)College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, China 2) College of Marine Life Science, Ocean University of China, Qingdao 266003, China 3) Nanjing University of Information Science and Technology, Collaborative Innovation Center of Atmospheric Environment and Equipment Technology, Jiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control, Nanjing 210044, China
This study aimed to present a novel clay/biochar composite adsorption particle, which made from abandoned reed straw and clay to remove ammonia nitrogen (NH4+-N) from micro-contaminated water. The removal performance of NH4+-N by composite adsorption particle was monitored under different raw material proportions and initial NH4+-N concentration. Besides, adsorption kinetics and adsorption isotherms were investigated to reveal the adsorption mechanisms. The results showed that NH4+-N was effectively removed under optimal proportion of biochar, foaming agent and crosslinker with 20%, 3%, and 3%, respectively. The optimal contact time was 150min and the best removal efficiency was 88.6% at initial NH4+-N concentration of 20mgL−1. The adsorption performance was well described by the second order kinetic model and Freundlich model. The novel clay/biochar composite adsorption particle in this study demonstrated a high potential for NH4+-N removal from surface water.
clay; biochar; composite adsorption particle; ammonia nitrogen removal; adsorption mechanism
1 Introduction
Liaohe Estuarine Wetland (LEW) is a unique ecosystem, which plays an important role in regulating the climate, dispersing floods, providing habitat for wild life, and conserving biological diversity (Lin., 2016). However, it has been seriously polluted by ammonia nitrogen (NH4+-N) during human activities,.., crab breeding and oil drilling over the past few decades. High NH4+-N led to the reduce of dissolved oxygen, deterioration of water quality, and over reproduction of algae and some microorganisms, even water eutrophication (Hina., 2015). Thus, NH4+-N removal from LEW becomes crucial issue for controlling water eutrophication.
However, removing NH4+-N from surface water is different from waste water treatment plant since in-situ remediation is harder than ex-situ remediation. Up to now, many studies have focused on removing the NH4+-N pollution from contaminated water by the methods such as break point chlorination (Park., 2015), selective ion exchange (Thornton., 2007), air stripping (Bonmati., 2003), and biological treatment (Huang., 2016). NH4+-N removal by chemical and biological methods is effective, but these methods pose threat to water ecological system, even introduce new pollutants. By comparison, adsorption is simple and effective due to its easy operation, low cost, repeated use, and fewer secondary products. Hence selection of environment friendly and inexpensive materials is particularly necessary.
The porous materials usually used as NH4+-N adsorbents predominantly include zeolite (Zheng., 2008), activated carbon (Zhu., 2012), coal cinder ball (Wang., 2015), clay (Rožić., 2000) and biochar (Kizito., 2000). Sometimes, they were modified by inorganic acid, hydrogen peroxide and some metal salts in order to increase their adsorption capacity. Biochar has been widely used in agriculture and pollutants removal due to its perfect adsorption performance, positive effect on harvest growth and carbon holding (Kasozi., 2010; Sparrevik., 2013; Wang., 2013), and has been conducted NH4+-N adsorption in soil and water (Taghizadeh-Toosi., 2012; Yao., 2012; Gai., 2014). At present, however, there were no reports on the application of biochar in surface water (river or lake) pollution control. Most of them were limited to the laboratory scale owing to its feature of light quality, and it is difficult to be recycled. Therefore, it is necessary to explore a kind of biochar application method in surface water for NH4+-N removal.
Clay is another adsorption material used in adsorbing organic compounds, inorganic compounds and heavy metal (Boufatit., 2007; Ji., 2015; Wu., 2015). Whether biochar and clay are considered to prepare composite adsorption materials to solve the application problem of biochar? Yao. (2014) developed a low-cost adsorbent clay-biochar composite comprising of biochar and clay. The clay particles distributed on biochar surfaces and used for adsorbing methylene blue. To our knowledge, it was the first study to attempt to combine biochar and clay into new engineered material. However, more characteristics and details need to be investigated to optimize the performance of new engineering material.
This study aimed to develop a method to prepare a novel clay/biochar composite adsorption particle that was made from local reed straw and clay for environmental remediation. In addition, for evaluating the effect of raw material proportions on the performance of NH4+-N removal from water, different biochar proportions, foaming agent concentrations, and crosslinker content were conducted. This work would develop a novel clay/biochar composite adsorption particle by using biomass waste and provide insight into the adsorption characteristics of clay/biochar composite particle. Finally, the results will help us to develop applicable technologies for NH4+-N removal from LEW surface water.
2 Materials and Methods
2.1 Preparation of Biochar
Reed straw and clay were obtained from LEW and cleaned by deionized water for several times to remove surface impurities, and then dried in open crucibles for 24h at 105℃. The reed straw was crushed by a micro plant grinding machine (FZ102, Weiye, Beijing) and sifted out 0.85mm powder for using. The reed straw powder was charred in a pyrolyzer under a constant oxygen-limited condition with heating rate of 10℃min−1, holding a temperature of 600℃ for 3h. Biochar and clay samples were then crushed and sieved yielding a uniform 0.15mm size fraction. The biochar samples were dried for 24h at 105 ℃ and sealed in a brown container for later using after rinsing with deionized water several times to remove ash. Detailed information about the biochar characteristics were shown in Table 1.

Table 1 Characteristics of biochar
2.2 Preparation of Clay/Biochar Composite Particle
Clay/biochar composite particle was prepared by using clay, biochar, Na2SiO3, and NaHCO3. Different proportions of biochar, Na2SiO3, and NaHCO3were mixed with clay and formed 8-10mm composite particle by disk balling machine (BY-300, Tianzhuo, Zhengzhou). Afterwards, the composite particle samples were oven-dried for 24h at 45℃ to remove moisture, and then charred as above mentioned under an oxygen-limited condition. Finally, the composite particle samples were cooled at room temperature and stored in a brown container. The whole process of preparing clay/biochar composite adsorption particle was shown in Fig.1.
The proportions of biochar, Na2SiO3, and NaHCO3affect the adsorption property of clay/biochar composite particle. Three experimental groups were named as E1, E2, and E3, respectively, to discuss the effect biochar, Na2SiO3, and NaHCO3on clay/biochar composite particle adsorption performance of NH4+-N (Thornton., 2007). The detail experimental groups were listed in Table 2.
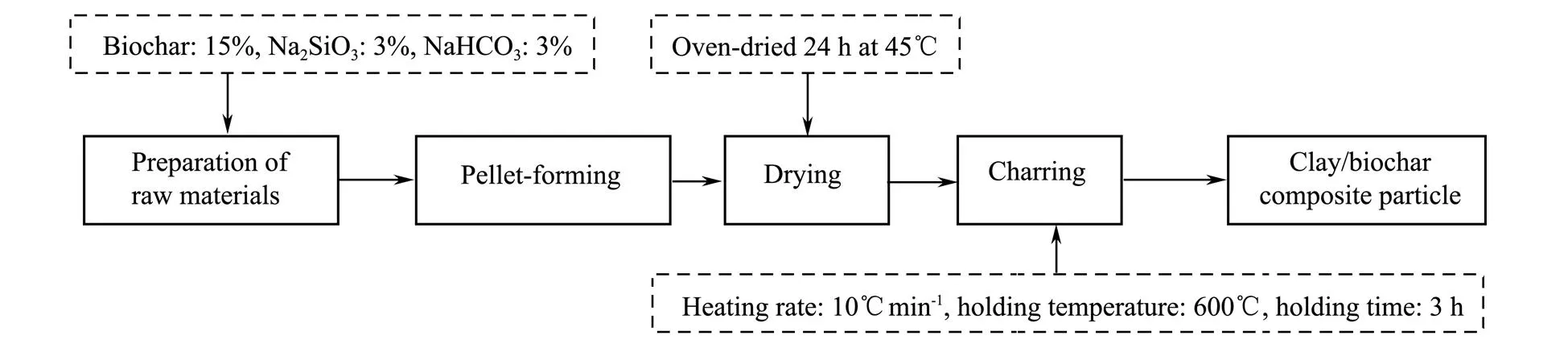
Fig.1 The whole process of preparing clay/biochar composite adsorption particle.

Table 2 Proportions of biochar, Na2SiO3, and NaHCO3 of clay/biochar composite particle
2.3 Experimental Setup for Batch Adsorption
In this study, adsorption was conducted in pure NH4+-N solution. Meanwhile, 1.0g clay/biochar composite particles were put into 50mL flask with 25mL NH4+-N solution, and shaken at 150rmin−1for a certain contact time at 25℃. Samples were collected at different time intervals.., 5, 10, 20, 40, 60, 90, 120, 150, 180, 240, and 300 min.
To obtain adsorption isotherms, 1.0g of the clay/biochar composite particles was put into 25mL of aqueous solution with initial NH4+-N concentrations from 4 to 100 mgL−1. The mixtures were agitated by a reciprocating shaker with 150rmin−1at 25℃ for 4h, and then filtrated by 0.45µm RC-membrane to determine NH4+-N concentration.
2.4 Analysis Method
The elemental composition (C, H, O, and N) of reed biochar sample was determined by an elemental analyzer (MicroCube, Elementar, Germany). Ash content of the biochar was measured by heating the biochar samples in a muffle furnace at 750℃ for 4h. The Fourier transform infrared spectroscopy (FTIR) spectra for the clay, biochar and clay/biochar composite adsorption particle were performed with wavenumbers from 400 to 4000cm−1and resolution value was 1.0cm−1(Tensor 27, Bruker, Germany). Scanning electron microscopy (SEM) micrographs were obtained on FEG environmental scanning electron microscope (FEI, Quanta 450, USA). The biochar sample was dried at 45℃ for 24h and coated with a thin carbon layer to increase conductivity. An Everhart- Thornley Detector (ETD) and a solid state Back Scattered Electron detector (BSED) were used to accelerate a 20kV voltage to take SEM images in a high vacuum mode.
The samples were filtrated by 0.45µm RC-membrane (Minisart RC 15), and then measured according to Nes- sler’s reagent colorimetric method. Each sample was measured 3 times and the average value was analyzed.
2.5 Data Analysis
2.5.1 Adsorption capacity
In the NH4+-N adsorption experiment, the adsorption capacity of the clay/biochar composite particle at equilibrium period was calculated by using the following equation (1) and the adsorption capacity during the adsorption period was equation (2):


where,qandqis the amount of NH4+-N adsorbed by the clay/biochar composite particle at equilibrium time and during the adsorption time (mgkg−1);0andC(mgL−1) are the initial and equilibrium concentrations of NH4+-N in the solution phase, respectively;is the solution volume (L), andis the mass of clay/biochar composite particle (g).
2.5.2 Adsorption kinetics
For the pseudo first and second order models, the following expressions were used
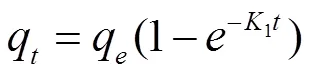

where,q(mgg−1) is the adsorbed amounts of NH4+-N by the clay/biochar composite particle at equilibrium time.qis the adsorbed amount at a given time interval ().1and2are the rate constants for the pseudo-first and second order models, respectively.
The following expression was used for the intrapartical model
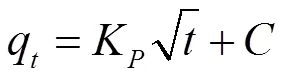
where,Kis the intrapartical diffusion rate constant (mg g−1min1/2),(mgg−1) is a constant that reflects the boun- dary layer effect. A plot ofqagainst1/2gave a linear relationship from which theKvalue was determined from the slope andas the intercept.
2.5.3 Adsorption isotherms
The data of adsorption isotherms were fitted with Fre- undlich model and Langmuir equation. The Freundlich equation is given by:

The Langmuir equation is given by:

where,q(mgg−1) is the the amount of NH4+-N adsorbed by the clay/biochar composite particle at equilibrium time,max(mgg−1) andK(Lmg−1) are Langmuir constants that indicate the maximum adsorption and relative binding energy of the clay/biochar composite particle, respectively.Kandare Freundlich constants that measure the relative NH4+-N adsorption capacity and adsorption intensity of the clay/biochar composite particle respectively; whileC(mgL−1) denotes the equilibrium concentration of NH4+-N remaining in solution after adsorption is completed.
3 Results and Discussion
3.1 Characteristics of Clay/Biochar Composite Particle
The preparation of clay/biochar composite particle is shown in Fig.2a. The particle was approximately 8-10 mm in diameter with grey black and rich interspace. Each composite particle is about 0.2g.
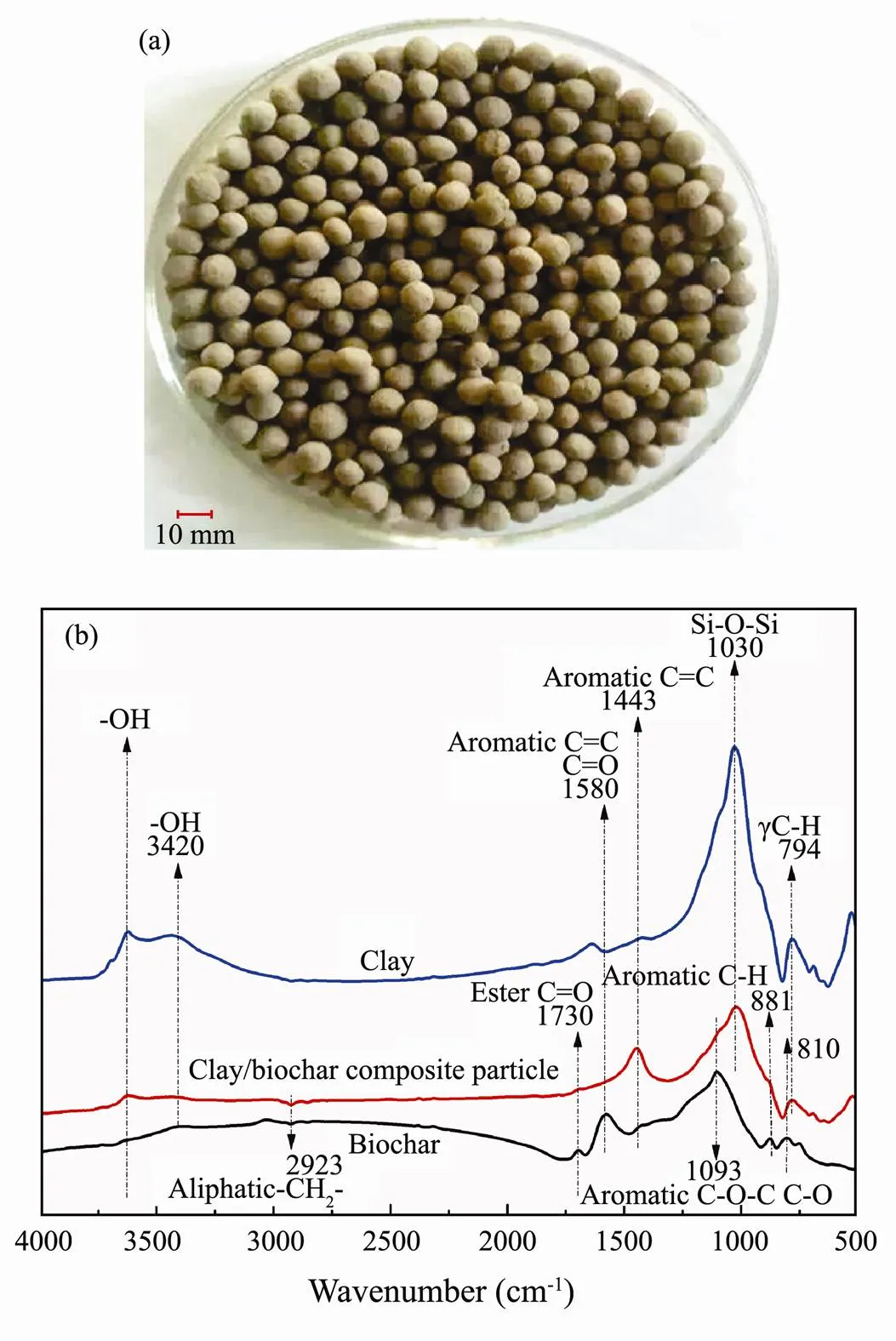
Fig.2 Characteristics of clay/biochar composite adsorption particle (a) Picture of clay/biochar composite adsorption particle, and (b) FTIR analysis of biochar, clay, and clay/biochar composite adsorption particle.
FTIR spectrum analysis of biochar, clay, and clay/ biochar composite particle are shown in Fig.2b. An evident difference of FTIR spectrum characteristics was observed between them. The wide band at 3600cm−1and 3420cm−1were related to -OH vibration and larger fluctuation of clay was appeared than biochar and clay/biochar composite particle. This phenomenon indicated that -OH obviously decreased after synthesis of clay/biochar composite adsorption particle. The peak at 1730, 1580, and 1093cm−1were associated with stretching vibration of ester C=O, aromatic C=C/C=O, and aliphatic C-O-C/ C-O groups in biochar, respectively. However, these groups, in clay and clay/biochar composite particle, were diminished or disappeared. Meanwhile, Si-O-Si group, which appeared at 1030cm−1, weakened from clay to clay/biochar composite particle. The peak at 794cm−1of clay/biochar composite adsorption particle was diminished and C=C group achieved a reinforcement at the peak of 1443cm−1comparing with clay.
The adsorption-desorption isotherms of N2exhibited H3 hysteresis loops with the relative pressure ranging from 0.20 to 0.99, which indicated that there were many mesopores in the clay/biochar composite particle. The existence of mesopores was beneficial to the diffusion of NH4+-N molecules to the surface and interior of the composite particle. The average aperture was 6.38 nm, and BET specific surface area was 41.22m2g−1. Fig.3 shows the SEM images of biochar, clay, and clay/biochar composite adsorption particle. Because of the high temperature burning, different surface morphology was identified among three materials by SEM analysis. More flaky textures were observed on the surface of lay/biochar composite adsorption particle than biochar, clay. It can be inferred that more interspace exists in clay/biochar composite adsorption particle (Wang., 2017).

Fig.3 SEM images of (A) biochar, (B) clay, and (C) clay/biochar composite adsorption particle.
3.2 NH4+-NAbsorbed by Clay/Biochar Composite Adsorption Particle
Clay/biochar composite particle is porous adsorbent material. Previous studies (Rožić., 2000; Kizito., 2015) reported that biochar and clay could effectively removed NH4+-N. HaHCO3, as a foaming agent was used to increase porosity and Na2SiO3was adopted as inorganic bonder to enhance the strength of clay/biochar composite particle.
Fig.4 showed NH4+-N adsorption by clay/biochar composite particle. At the first ten minutes, NH4+-N declined from 50 to 34.68mgL−1rapidly, and nearly half of NH4+-N was adsorbed at 60 min and the rest of NH4+-N in solution was 19.98mgL−1when adsorption time was 300 min. The maximum adsorbed amount and removal efficiency from pure solution by clay/biochar composite particle were 0.75mgL−1and 60.04%. From the change of NH4+-N concentration, it can be found that the adsorption equilibrium time is 180 min.

Fig.4 NH4+-N adsorption by clay/biochar composite adsorption particle.
It is obvious that the increase of contact time from 0 to 300 min enhanced the adsorption of NH4+-N significantly. The concentration of NH4+-N in aqueous solution decreased to 20-30mgL−1from 50mgL−1and the adsorption gradually becomes slower until equilibration. Half amount of the NH4+-N was adsorbed by clay/biochar composite particle after increasing contact time up to 150-180 min. Hence, the optimum contact time for the adsorption of NH4+-N by clay/biochar composite particle was fixed as 150-180min. This trend could be explained in three ways (Mohan., 2002; Qu., 2008; Kizito., 2015),.., 1) The initial NH4+-N is rapidly adsorb- ed through mass transfer and the process is based on NH4+-N concentration gradient between the solid and liquid phases; 2) Slow physical adsorption process that is slower than first process and ionic balance between the solid and liquid phase emerged with slight desorption occurring for the physically bound NH4+-N; 3) Chemisorption and some extent intrapartical diffusion process is the final step. This process is much slower than above two processes. A similar trend has been reported in other studies (Boopathy., 2013; Yao., 2014).
Similar results were also reported in previous literatures (Table 3). Overall, similar adsorption trends were observed and this result was better than fly ash, sepiolite (Uğurlu., 2011), and coal cinder ball (Wang., 2015). However, it is specifically worth mentioning that the adsorption capacity of this composite particle was lower than the previous studies, which might be caused by two reasons. On the one hands, biochar owned more adsorption capacity than clay. Adsorption capacity of composite particle was less than biochar, due to less biochar and more clay were added in it. On the other hands, many researches (Rožić., 2000; Boopathy., 2013; Kizito., 2015) were conducted in powder form, more specific surface and adsorption sites were exposed in solution to react with NH4+-N. In this study, the adsorbent material was granular, possibly due to the absence of the adsorption sites and less specific surface, which resulted in less adsorption capacity (Boopathy., 2013).

Table 3 Similar results about NH4+-N adsorption of porous materials
3.3 Effect of Raw Material Proportions on NH4+-N Adsorption
3.3.1 The effect of biochar proportion
The effect of biochar proportion on NH4+-N adsorption is presented in Fig.5. The results indicated that increasing the adsorption time and the proportion of the biochar from 0% to 20% improved NH4+-N removal and equilibrium adsorbed amount (Fig.5a). The clay/biochar composite particles, which added reed biochar, presented higher absorption capacities by comparison with clay particle. The equilibrium concentrations of NH4+-N were 24.01 and 19.98mgL−1and removal efficiencies were 51.83% and 60.04% when the proportions of biochar were 15% and 20%, respectively. However, there is no obvious increase of NH4+-N removal efficiency when the proportions of biochar were lower than 50%.
Biochar is a kind of porous material, and the larger specific surface area and adsorption capacity were achieved if more biochar was added. Fig.5b shows the effect of biochar proportion on NH4+-N adsorption capacity of clay/biochar composite particle. As shown in this figure, adsorption capacity raised with the increase of biochar proportion and adsorption time. The adsorption capacities were 0.55, 0.58, 0.56, 0.60, 0.64 and 0.75mg g−1, respectively, with biochar proportion increasing. At the initial stage of contact time, it was found that the adsorption capacity of clay/biochar composite particle for removing NH4+-N was rapid, and then reached to equilibrium within 150-180min. This phenomenon might be due to the presence of a great many active sites on clay/ biochar composite particle for the adsorption of NH4+-N. Besides, the adsorption behavior of NH4+-N greatly depended on the quantity and the surface properties of reed biochar and clay.

Fig.5 Effect of biochar proportion on NH4+-N adsorption (a) NH4+-N concentration and removal efficiency, (b) NH4+-N adsorption capacity.
3.3.2 The effect of foaming agent content
In order to increase the porosity of the clay/biochar composite particle, the content of foaming agent (NaHCO3) was increased from 0 to 3.0% (w/w). The effect of foaming agent dosage on NH4+-N adsorption performance is shown in Fig.6. NH4+-N adsorption performance of clay/biochar composite particle was largely depended on the account of NaHCO3, and the removal efficiency presented better with the increase of NaHCO3with concentrations of NH4+-N fluctuated in a certain range from 25.90 to 29.76mgL−1after 300min. Besides, with the increase of NaHCO3proportion, a better linear relation (2=0.99) was fitted (Fig.6a). Adsorption capacity kept rising trend with the increase of NaHCO3proportion and adsorption time. The best adsorption capacity and adsorption time were 0.60mgg−1at NaHCO3proportion 3.0% and 250 min, respectively.
NaHCO3, as an endothermic foaming agent, plays an important role for clay/biochar composite particle in form of pore structure and affects NH4+-N adsorption performance of clay/biochar composite particle. With increase in foaming agent content, more gas bubbles form, which lead to well-connected channel. In addition, the cross- linking efficiency of Na2SiO3changed with increase in foaming agent content. More foaming agent content will lead to the variation of the network ability that resisted the pressure from the environment (Qi., 2007). During the process of calcination, NaHCO3was decomposed into Na2CO3and CO2(Shown in Eq.8), which enhanced the interspace of composite particle and slowed down excessive bonding. Na2CO3dissolved easily in aqueous solution when clay/biochar composite particle was added into NH4+-N solution and more expansive adsorption channels and specific surface area were provided to the contact of adsorbate and adsorbent. It might be the reason why more adsorption capacity was obtained with the increasing of NaHCO3.
2NaHCO3®Na2CO3+H2O+CO2(=400℃). (8)
Yan. (2007) compounded a kind of monolithic macroporous polymers using NaHCO3as foaming agent to increase the pore volume and a gradual increase in level of porosity was obtained when the NaHCO3was raised from 6 to 12 (w/w). Qi. (2007) researched the effect of amount of NaHCO3on macroporous superabsorbent composite and found that swelling rate had maximums at 3.571% (W/W) of amount of NaHCO3.

Fig.6 Effect of foaming agent content on NH4+-N adsorption (a) NH4+-N concentration and removal efficiency, (b) NH4+-N adsorption capacity.
3.3.3 The effect of crosslinker content
Na2SiO3is an ideal crosslinker, which has some advantages,.., low softening temperature, producing medium viscosity coatings, moderate ability to suspend powders, and cost-effectiveness and applicability (Chen., 2012). In this study, to obtain high rigidity and mechanical stability clay/biochar composite particle, Na2SiO3was used as crosslinker to provides a highly crosslinked particle. Fig.7 shows the effect of crosslinker content (Na2SiO3) on NH4+-N adsorption performance of clay/biochar composite particle. The results reflect that all of experimental groups remained the similar adsorption trend and no significant difference was observed among them. Therefore, the addition of crosslinker did not present a serious impact on NH4+-N, which was adsorbed effectively from 50 to 25-26mgL−1, and 0.58-0.60mgg−1adsorption equilibrium capacity was obtained after 300 min. Some research has confirmed that the cross- linker content affected the crosslinking degree, which subsequently affected the pore structure of the composite particle (Yan., 2007).
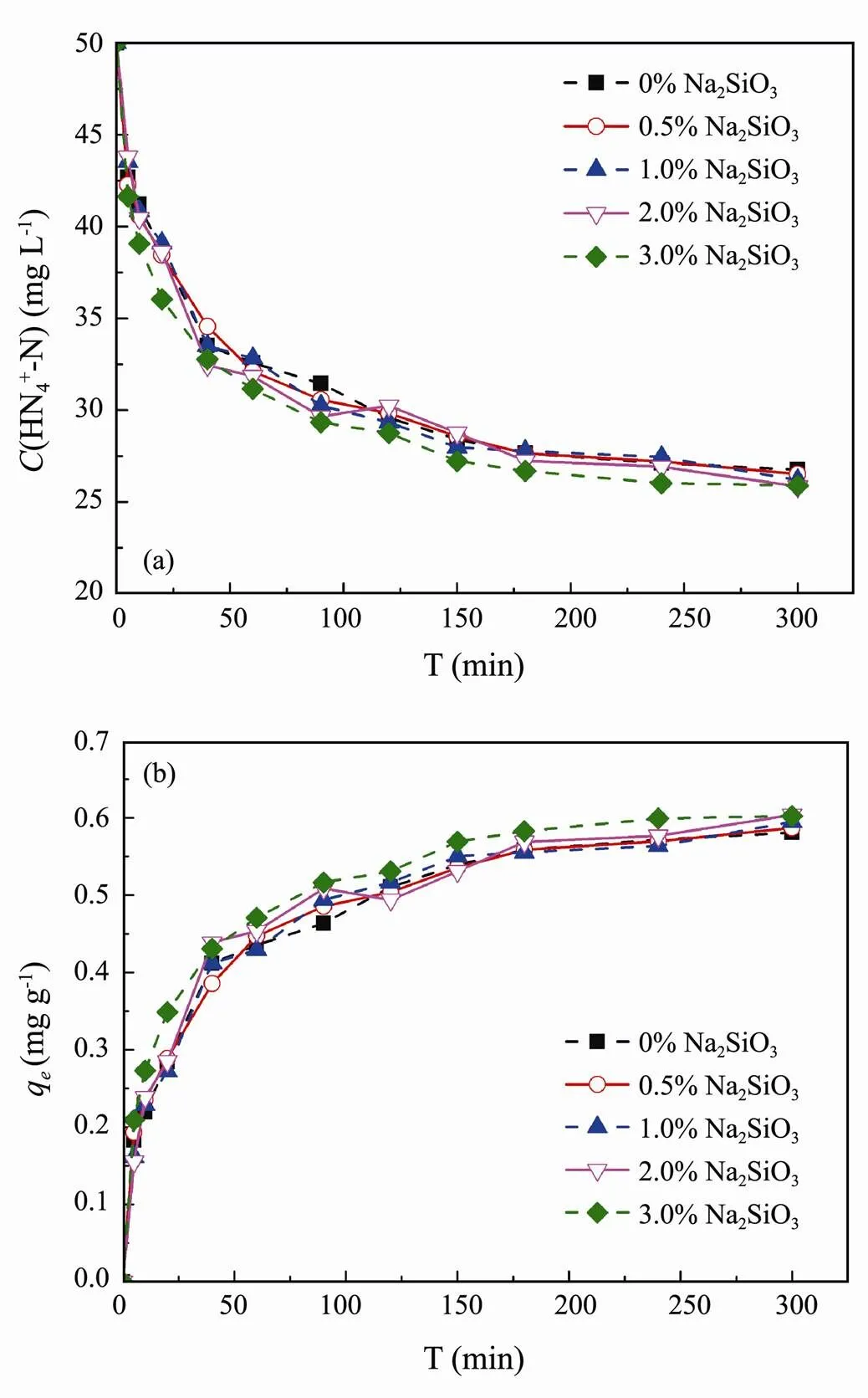
Fig.7 Effect of crosslinker content on NH4+-N adsorption (a) NH4+-N concentration, (b) NH4+-N adsorption capacity.
3.4 Effect of Initial NH4+-N Concentration
The adsorption performance largely depended on initial NH4+-N concentration as well as raw material proportions. Different removal efficiencies and adsorption equilibrium time were achieved due to the existence of concentration gradient. The effect of initial concentration on NH4+-N adsorption performance was shown in Fig.8. With the contact time extended, NH4+-N concentration decreased (Fig.8a) and adsorption equilibrium capacity increased (Fig.8b) at different concentration gradient tests. Besides, adsorption equilibrium time was prolonged when the initial concentration increased, and the adsorption equilibrium time was 180 to 250 min. From Fig.8c, the results indicated that the NH4+-N removal efficiency increased from 53.20% to 88.65% when the initial concentration was from 4 to 20mgL−1. However, as the initial concentration increased from 20 to 100mgL−1, the removal efficiency decreased to 28.89%. It is different from removal efficiency, the NH4+-N adsorption equilibrium capacity (q) maintained rising trend significantly (from 0.05 to 0.71mgg−1) during the whole adsorption process. The op- timum initial concentration for clay/biochar composite particle was 20mgL−1and the removal efficiency was 88.65%. Kizito. (2015) obtained the same results and they found that the removal efficiency increased firstly and then decreased with increasing NH4+-N concentration and the adsorption equilibrium maintained rising trend. Long. (2008) reported that the increasing of initial NH4+-N concentration in the aqueous solution led to the increase of mass transfer between dsorbent and adsorbate. The adsorption capacities of different initial concentrations were fitted by nonlinear equation (Fig.8d), the adsorption capacity could be calculated by following non- linear equation.
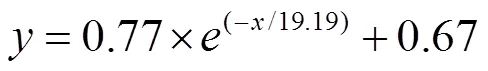
The reasons that affect the NH4+-N adsorption efficiency were the process of surface mass transfer and internal diffusion process (An et al., 2016). The higher concentration led to the faster adsorption rate at the beginning stage. This might be the fact that there was high mass flow of NH4+-N at high concentration and more ions were transferred from liquid phase to clay/biochar composite particle active sites. Hence, an increasing adsorbed amount was obtained as the initial concentration of ions increases. Meanwhile, surface adsorption resistance blocked NH4+-N adsorption and slowed the surface mass transfer when adsorption reached a certain time. There is a correspondingly low mass flow of NH4+-N to the clay/ biochar composite particle due to excess unoccupied active sites on the particle surface when the concentration of ions decreased. Therefore, the adsorption efficiency and equilibrium capacity depended on the initial concentration (Nidheesh et al., 2012).
3.5 Adsorption Mechanism
Schematic representation for the NH4+-N adsorption process of clay/biochar composite particle was shown in Fig.9. It could be well explained the adsorption process. Besides, adsorption kinetics and isotherms were fitted in Fig.10 and Fig.11.

Fig.9 Schematic representation for the NH4+-N adsorption process of clay/biochar composite adsorption particle.
3.5.1 Adsorption kinetics
Adsorption kinetics is an critical method for investigating the mechanism of adsorption. In order to understand the adsorption process of NH4+-N by clay/biochar composite particle, adsorption kinetics was studied. The process of the clay/biochar composite particle adsorbing NH4+-N was fitted by first order, second order, and intraparticle diffusion model. The kinetics experiment data and modeling were shown in Fig.10 and Table 4. Three kinetic models could well fit the process of adsorbing NH4+-N by clay/biochar composite particle under low concentration (4mgL−1). It is worth noting that first order and second order kinetics models showed a better result (2=0.945 and 0.998, respectively) for high concentration NH4+-N (50mgL−1), but intraparticle diffusion model was not reasonable (2=0.799). According to Table 4, compared to first order kinetics model and intraparticle diffusion model, the second order kinetics model was more suitable for describing the adsorption of NH4+-N by clay/biochar composite particle. This observation agreed well with the finding by Uğurlu. (2011) and Sharifnia. (2012). The second order kinetic model is usually used to describe a chemical adsorption process, which includes ion exchange happened between chemical bonds and the adsorption. The interaction between two reagent particles becomes the rate-limiting step (Ho and McKay, 1999; Özcan., 2007). Thus the reaction between chemical bonds occurred in NH4+-N adsorption process by Si-O functional group on clay/biochar com- posite particle plays an important role for NH4+-N adsorption.

Fig.10 Adsorption kinetics and modeling for NH4+-N absorbed by clay/biochar composite adsorption particle (a) First order kinetic modeling, (b) Second order kinetic modeling and (c) Intraparticle diffusion modeling. Symbols are experimental data and lines are model results.
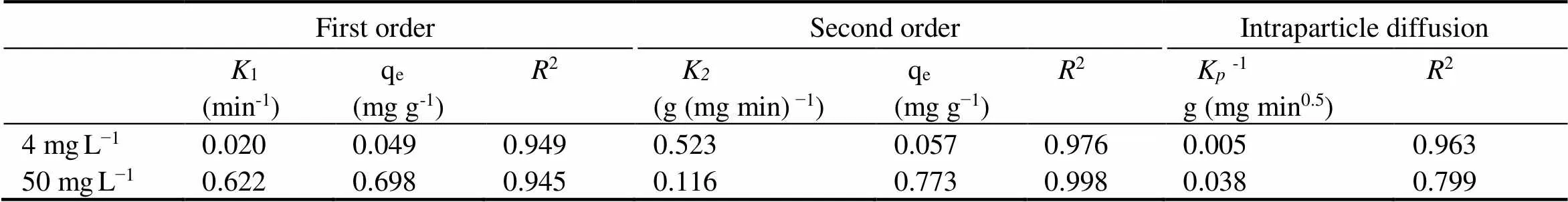
Table 4 The adsorption kinetics of biochar ball clay/biochar composite adsorption particle

Fig.11 Adsorption isotherms date and modeling for NH4+-N onto clay/biochar composite adsorption particle.
Interestingly, adsorption of high concentration NH4+-N by clay/biochar composite particle fits well with intraparticle diffusion model. Two-steps diffusion process could give a better explanation than one-step diffusion process. The fitting equation in Fig.10c was obvious different with correlation coefficient. The correlation coefficient of two-step diffusion model (first-step2=0.926; second- step2=0.874) was better than one-step diffusion model (2=0.799). Wu. (2009) summarized a multi-linearity plot betweenqand0.5and they found that two or three steps were involved in the whole intraparticle diffusion process. Hence, external diffusion (a fast adsorption), internal diffusion and adsorption reaction stages (a rate- limiting step) are involved before reaching adsorption equilibrium (Bruemmer., 1988; Ayoob., 2008). Above theory could well explain the phenomenon in this study and conclude that internal diffusion is the rate-limiting step. However, the fitting curve of high concentration (50mgL−1) was not through the origin of coordinates in this work and demonstrated that internal diffusion was not the only rate-limiting step for clay/biochar composite particle adsorbing NH4+-N (Ayoob., 2008). Comparably, it was the rate-limiting control step for low concentration NH4+-N (4mgL−1).
3.5.2 Adsorption isotherms
The adsorption equilibrium isotherm is used to describe the distribution of adsorbate molecules between the liquid and the solid phases under an equilibrium state (Almeida., 2009). In this study, it showed that the maximum absorption capacity of clay/biochar composite particle was 4.264mgg−1. The adsorption isotherms of NH4+-N on clay/biochar composite particle was shown in Fig.11 and the fitting parameters were listed in Table 5. The adsorption isotherms could be well fitted by Freundlich model with2=0.979, which was better than Langmuir model (2=0.964). The experimental result was consistent with Yao. (2014) whose results indicated that Freundlich model had slightly better fitting performance than Langmuir and Langmuir-Freundlich models. For Freundlich model,reflects the curvature in the isotherm and the energy distribution characteristic of adsorption site. Adsorption constantKreflects the adsorption capacity or adsorption power of the adsorption material (Xing., 1996; Mohan., 2002). On the other hand,=1.56 in this study was between 1 and 10, which means that the adsorption process was preferential adsorption.

Table 5 Langmuir and Freundlich adsorption isotherm of clay/biochar composite adsorption particle
4 Conclusions
This study demonstrated that clay/biochar composite adsorption particle effectively removed NH4+-N from aqueous solution. The clay/biochar composite adsorption particle adsorption performance was significantly affected by proportion of biochar and foaming agent content. The addition of crosslinker did not seriously impact on NH4+-N adsorption of clay/biochar composite adsorption particle. In addition, the NH4+-N initial concentration affected the adsorption capacity of composite particle and the removal efficiency increased firstly and then decreased with the increase of initial concentration. Second order kinetics model was more suitable for describing the adsorption of NH4+-N by clay/biochar composite adsorption particle, and the composite adsorption particle adsorbing NH4+-N could be well fitted by Freundlich model. This study developed a novel clay/biochar composite adsorption particle applying for environmental remediation and thus offered a strategy for practical application of biochar.
Acknowledgements
This work was supported by the National Major Project of Water Pollution Control and Management Technology in China (No. 2013ZX07202-007), the Shenzhen Science and Technology Project (No. GRCK2017042116092660), and the National Natural Science Foundation of China (No. 51308066).
Almeida, C. A. P., Debacher, N. A., Downs, A. J., Cottet, L., and Mello, C. A. D., 2009. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay., 332 (1): 46-53.
An, S. W., Jeong, Y. C., Cho, H. H., and Park, J. W., 2016. Adsorption of NH4+-N andonto Mg2+-modified zeolites., 75 (5): 1-11.
Ayoob, S., Gupta, A. K., Bhakat, P. B., and Bhat, V. T., 2008. Investigations on the kinetics and mechanisms of sorptive removal of fluoride from water using alumina cement granules., 140 (1): 6-14.
Bonmati, A., and Flotats, X., 2003. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre-or post-treatment to mesophilic anaerobic digestion., 23 (3): 261-272.
Boopathy, R., Karthikeyan, S., Mandal, A. B., and Sekaran, G., 2013. Adsorption of ammonium ion by coconut shell-activated carbon from aqueous solution: Kinetic, isotherm, and thermodynamic studies., 20 (1): 533-542.
Boufatit, M., Ait-Amar, H., and McWhinnie, W. R., 2007. Development of an Algerian material montmorillonite clay. Adsorption of phenol, 2-dichlorophenol and 2, 4, 6-trichloro- phenol from aqueous solutions onto montmorillonite exchanged with transition metal complexes., 206 (1): 394-406.
Bruemmer, G. W., Gerth, J., and Tiller, K. G., 1988. Reaction kinetics of the adsorption and desorption of nickel, zinc and cadmium by goethite. I. Adsorption and diffusion of metals., 39 (1): 37-52.
Chen, G. Q., Li, N. N., Fu, X. S., and Zhou, W. L., 2012. Preparation and characterization of a sodium polyacrylate/sodium silicate binder used in oxidation resistant coating for titanium alloy at high temperature., 230: 134-138.
Gai, X., Wang, H., Liu, J., Zhai, L., Liu, S., Ren, T., and Liu, H., 2014. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate., 9 (12): e113888.
Hina, K., Hedley, M., Camps-Arbestain, M., and Hanly, J., 2015. Comparison of pine bark, biochar and zeolite as sorbents for NH4+-N removal from water., 43: 86-91.
Ho, Y. S., and McKay, G., 1999. Pseudo-second order model for sorption processes., 34 (5): 451-465.
Huang, X., Bai, J., Li, K., Zhao, Y. G., Tian, W. J., and Dang, J. J., 2017. Characteristics of two novel cold-and salt-tolerant ammonia-oxidizing bacteria from Liaohe Estuarine Wetland., 114 (1): 192-200.
Ji, M., Su, X., Zhao, Y., Qi, W., Wang, Y., Chen, G., and Zhang, Z., 2015. Effective adsorption of Cr(VI) on mesoporous Fe-functionalized Akadama clay: Optimization, selectivity, and mechanism., 344: 128-136.
Kasozi, G. N., Zimmerman, A. R., Nkedi-Kizza, P., and Gao, B., 2010. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars)., 44 (16): 6189-6195.
Kizito, S., Wu, S., Kirui, W. K., Lei, M., Lu, Q., Bah, H., and Dong, R., 2015. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry., 505: 102-112.
Lin, Q., Ishikawa, T., Akoh, R., Yang, F., and Zhang, S., 2016. Soil salinity reduction by river water irrigation in a reed field: A case study in Shuangtai Estuary Wetland, northeast China., 89: 32-39.
Long, X. L., Cheng, H., Xin, Z. L., Xiao, W. D., Li, W., and Yuan, W. K., 2008. Adsorption of ammonia on activated carbon from aqueous solutions., 27 (2): 225-233.
Mohan, D., and Singh, K. P., 2002. Single and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse-An agricultural waste., 36 (9): 2304-2318.
Nidheese, P. V., Gandhimathi, R., Ramesh, S. T., and Singh, T. S. A., 2012. Kinetic analysis of crystal violet adsorption on to bottom ash., 36 (3): 249-262.
Özcan, A., Ömeroğlu, C., Erdoğan, Y., and Özcan, A. S., 2007. Modification of bentonite with a cationic surfactant: An adsorption study of textile dye Reactive Blue 19., 140 (1): 173-179.
Park, S. H., Padhye, L. P., Wang, P., Cho, M., Kim, J. H., and Huang, C. H., 2015. N-nitrosodimethylamine (NDMA) formation potential of amine-based water treatment polymers: Effects of in situ chloramination, breakpoint chlorination, and pre-oxidation., 282: 133-140.
Qi, X., Liu, M., Chen, Z., Zhang, F., and Zhao, L., 2010. Preparation and properties of macroporous superabsorbent composite., 21 (3): 196- 204.
Qu, B. C., Zhou, J. T., Xiang, X. M., Zheng, C. L., Zhao, H. X., and Zhou, X. B., 2008. Adsorption behavior of Azo Dye CI Acid Red 14 in aqueous solution on surface soils., 20 (6): 704-709.
Rožić, M., Cerjan-Stefanović, Š., Kurajica, S., Vančina, V., and Hodžić, E., 2000. Ammoniacal nitrogen removal from water by treatment with clays and zeolites., 34 (14): 3675-3681.
Sharifnia, S., Khadivi, M. A., Shojaeimehr, T., and Shavisi, Y., 2016. Characterization, isotherm and kinetic studies for ammonium ion adsorption by light expanded clay aggregate (LECA)., 20: S342-S351.
Sparrevik, M., Field, J. L., Martinsen, V., Breedveld, G. D., and Cornelissen, G., 2013. Life cycle assessment to evaluate the environmental impact of biochar implementation in conservation agriculture in Zambia., 47 (3): 1206-1215.
Taghizadeh-Toosi, A., Clough, T. J., Sherlock, R. R., and Condron, L. M., 2012. Biochar adsorbed ammonia is bioavailable., 350 (1-2): 57-69.
Thornton, A., Pearce, P., and Parsons, S. A., 2007. Ammonium removal from digested sludge liquors using ion exchange., 41 (2): 433-439.
Uğurlu, M., and Karaoğlu, M. H., 2011. Adsorption of ammonium from an aqueous solution by fly ash and sepiolite: Isotherm, kinetic and thermodynamic analysis., 139 (1): 173-178.
Wang, C., Lu, H., Dong, D., Deng, H., Strong, P. J., Wang, H., and Wu, W., 2013. Insight into the effects of biochar on manure composting: Evidence supporting the relationship between N2O emission and denitrifying community., 47 (13): 7341-7349.
Wang, N., Wang, Y. F., Omer, A. M., and Ouyang, X. K., 2017. Fabrication of novel surface-imprinted magnetic graphene oxide-grafted cellulose nanocrystals for selective extraction and fast adsorption of fluoroquinolones from water., 409 (28): 6643-6653.
Wang, Y., Tian, W., Wu, C., Bai, J., and Zhao, Y., 2016. Synthesis of coal cinder balls and its application for CODCrand ammonia nitrogen removal from aqueous solution., 57 (46): 21781-21793.
Wu, F. C., Tseng, R. L., and Juang, R. S., 2009. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics., 153 (1): 1- 8.
Wu, Y., Si, Y., Zhou, D., and Gao, J., 2015. Adsorption of diethyl phthalate ester to clay minerals., 119: 690-696.
Xing, B., Pignatello, J. J., and Gigliotti, B., 1996. Competitive sorption between atrazine and other organic compounds in soils and model sorbents., 30 (8): 2432-2440.
Yan, Q. Z., Lu, G. D., Zhang, W. F., Ma, X. H., and Ge, C. C., 2007. Frontal polymerization synthesis of monolithic macroporous polymers., 17 (16): 3355-3362.
Yao, Y., Gao, B., Fang, J., Zhang, M., Chen, H., Zhou, Y. M., Creamer, A. E., Sun, Y. N., and Yang, L. Y., 2014. Characterization and environmental applications of clay-biochar composites., 242: 136-143.
Yao, Y., Gao, B., Zhang, M., Inyang, M., and Zimmerman, A. R., 2012. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil., 89 (11): 1467-1471.
Zheng, H., Han, L., Ma, H., Zheng, Y., Zhang, H., Liu, D., and Liang, S., 2008. Adsorption characteristics of ammonium ion by zeolite 13X., 158 (2): 577-584.
Zhu, K., Fu, H., Zhang, J., Lv, X., Tang, J., and Xu, X., 2012. Studies on removal of NH4+-N from aqueous solution by using the activated carbons derived from rice husk., 43: 18-25.
. Tel: 0086-532-66782758E-mail: likr@ouc.edu.cn
February 28, 2019;
August 20, 2019;
November 13, 2019
(Edited by Ji Dechun)
 Journal of Ocean University of China2020年3期
Journal of Ocean University of China2020年3期
- Journal of Ocean University of China的其它文章
- Simulation of the Power Take-off System for a Heaving Buoy Wave Energy Converter
- Biochemical Factors Affecting the Quality of Products and the Technology of Processing Deep-Sea Fish,the Giant Grenadier Albatrossia pectoralis
- Study on Wave Added Resistance of a Deep-V Hybrid Monohull Based on Panel Method
- Air Temperature and Emersion Time Can Affect the Survival Rate and Ammonium Loading of Swimming Crab Portunus trituberculatus Exposed to Air
- Estimation of the Reflection of Internal Tides on a Slope
- Propulsion Performance of Spanwise Flexible Wing Using Unsteady Panel Method
