Laboratory Investigation into the Evaporation of Natural-Gas Condensate Oils: Hints for the Sanchi Oil Spill
ZHANG Yong, YANG Tao, ZHANG Junbo, LV Baoyi, CHENG Xiangsheng,and FANG Yin,
Laboratory Investigation into the Evaporation of Natural-Gas Condensate Oils: Hints for the Sanchi Oil Spill
ZHANG Yong1), YANG Tao1), ZHANG Junbo2),*, LV Baoyi3), CHENG Xiangsheng1),and FANG Yin3),*
1)East China Sea Environmental Monitoring Center, State Oceanic Administration, Shanghai 201206, China 2) College of Marine Sciences, Shanghai Ocean University, Shanghai 201306, China 3) College of Ocean Science and Engineering, Shanghai Maritime University, Shanghai 201306, China
The Sanchi oil tanker collision in the East China Sea on January 6th, 2018 has caused worldwide attention due to its uniqueness. A considerable amount of highly volatile natural-gas condensate oil was spilled, burned and sank with the Sanchi tanker, this entirely new kind of maritime disaster has posed massive unknowns to the public. In this study, for better understanding of the evaporative behavior of condensate oils, two condensate oils were investigated under various laboratory conditions. The overall result demonstrates that the evaporation of condensate oils is highly dependent on the air-exposed time and the total loss of condensate oils could be more than 90% within a short time. However, a certain amount of the high-molecular weight and toxic oil contents such as phenanthrenes still highly remain in the aquatic system even after a long evaporation process, indicating their detrimental potentials to the aquatic organisms. Based on these data, for the Sanchi oil spill accident, it is assumed that although the evaporation weathering of the total condensate oil mass is probably tremendous, the long-term ecological risks of the remaining oil components in the marine environment are strongly recommended to be carefully evaluated.
condensate oil; Sanchi; oil spills; oil evaporation; n-alkanes; polycyclic aromatic hydrocarbons
1 Introduction
Around 8:00 pm on January 6th, 2018, the Panama- registered oil tanker, Sanchi, was carrying approximately 111300 metric tons of natural-gas condensate oil (Carswell, 2018; Yin., 2018) and 1900 tons of heavy fuel oil from Iran to South Korea, collided with the Hong Kong-registered CF Crystal freighter in the East China Sea, about 300 kilometers (160 nautical miles) off the coastline of Shanghai, China. Subsequently, the Sanchi experienced a catastrophic burning and drifting for more than a week and finally sank on January 14, 2018 in about 115m deep below the sea surface. None of 32 crew members on the Sanchi survived. During the accident period, hundreds of square kilometers (km2) of oil slicks had been continually discovered around the sunken location of Sanchi, the colors of the oil slicks appeared as sliver white, sliver grey, iridescence or scattered dark brown. It is the first time of natural-gas condensate oil spill accident in the world history. In consideration of the oil type and various oceanographic parameters in terms of oil physical properties, chemical compositions, local weather conditions and various marine environmental conditions, the subsequent influences caused by the Sanchi burning, drifting and sinking for the local environment and ecosystem have brought us massive unknowns.
Unlike the conventional crude oils, the natural-gas con- densates, often namely as ‘condensates’, are highly volatile and ultra-light crude oils. Condensates are mixtures of light hydrocarbons in the liquid state that are produced from the raw natural gas when the recovering temperature is reduced to below the hydrocarbon dew point temperature at a normal pressure. Typically, condensates are composed of light hydrocarbons that include paraffins, aromatics, olefins, and other small molecules, such as propane, butane, pentane,. (Thompson, 1987; Dzou and Hughes, 1993; Speight, 2015). Due to their ultra-light density, low viscosity and high content of volatile hydrocarbons, evaporation can be assumed as the main weathering process for condensate oil spills relative to the water-in-oil emulsification, photo-oxidation, dissolution and biodegradation.
According to the previous studies, within several hours to a few days, light or medium crude oils can be easily evaporated to lose up to 40% to 70% in terms of volume (NRC, 2003). Fingas. (1998, 1999, 2004, 2012) found that the turbulence level, exposed oil area, thickness of oil layer do not completely control the oil evaporation processes. Based on their studies, it is summarized that the evaporation efficiency of spilled oils, as well as the evaporation rate, are able to be changed proportionately to the environmental temperature and air exposure time. Meanwhile, other studies showed that the evaporation is positively correlated with the oil mass or volume loss but no effects on high molecular weight hydrocarbons in the oils. For example, the environmental weather- ing of heavy n-alkanes with >13 carbon atoms and poly- cyclic aromatic hydrocarbons with fused >3 aromatic rings are proved to be recalcitrant to evaporation weather- ing (Liu., 2012; Yin., 2015a, 2015b).
Currently, the scientific results of various weathering processes for condensate oils are still poorly characterized, especially for their evaporation pathway. In this study, two condensate oils were used as the representatives to evaluate their evaporative data under different environmental conditions in the laboratory. The evaporative extents of the constituent hydrocarbons include the normal alkanes and light polycyclic aromatic hydrocarbons. The results are expected to be particularly useful for better understanding the Sanchi oil spill, and also to provide valuable information for proper emergency response operations of future possible condensate oil spills.
2 Materials and Methods
2.1 Samples
Two condensate oils were referred as condensate oil 1 and oil 2. The physical and chemical properties of the two condensates are listed in Table 1. The collected seawater samples were stored at 4℃. The annual temperature of seawater at the sampling location ranges from 9.5 to 30.7 ℃ and the average salinity is about 32‰.

Table 1 Basic properties of condensate oil 1 and oil 2
2.2 Materials
The petroleum oil standard was purchased from the Institute for Environmental Reference Materials (IERM), China. The tetrachloromethane (spectrum pure reagent), anhydrous sodium sulfate (ACS grade) and magnesium silicate (60-100mesh size) were purchased from Sinopharm Chemical Reagent Co., Ltd., China. The used organic solvents of hexane were HPLC grade and purchased from Merck KGaA, Germany.
Prior to use, the magnesium silicate and sodium sulfate were prepared by heating at 550℃ in a muffle furnace for 4h. Then the cooled magnesium silicate were activated by 6% (w/w) deionized water. Both activated magnesium silicate and anhydrous sodium sulfate were stored in tightly sealed glass containers.
2.3 Experimental Set Up and Sampling Time
Experiments were conducted to determine if condensate oil evaporation is regulated by various environmental factors in the ocean that includes turbulence, temperature and salinity. To ensure the feasibility of experimental operations, a small amount of condensate oil was used in the evaporation experiment, for which 0.1mL of condensate oil was added onto the surface of 500mL of seawater in 1L of glass beaker. The entire seawater and oil mixtures were collected and prepared at each time node. It should be noticed that the seawater evaporation was not quantified separately, thus the experiment data of condensate oil in the seawater could be treated as the total evaporation of oil and seawater.
The turbulence experiment was conducted by using a magnetic rotor in the glass beaker, 500rmin−1was set for simulating the marine dynamics. The controlled sample was conducted in a static condition. The evaporation data were measured at 0, 1, 2, 5, 10, 60, 120 and 240min, respectively. The effects of salinity for oil evaporation were determined between deionized water and natural seawater (32‰) at room temperature. Also, their oil contents were measured at 0, 1, 2, 5, 10, 60, 120 and 240min, respectively.
Based on the annual seawater temperature of the oil sampling region, the temperature experiments were conducted at 10, 20, 30℃ in water baths for condensate oil 1 and oil 2. The total evaporation portion of both oil samples at three temperature levels were quantified after four hours.
Also, a time series of basic evaporation experiments with seawater were conducted for eight days at room temperature for better understanding the total loss of condensate oil over time. The evaporated oil samplings were collected at 1, 2, 24, 48, 96 and 192h, respectively.
To estimate the area and thickness effects of evaporation on weathering of condensate oils, the mass changes of condensate oil 2 with varying evaporation areas were monitored, and also its loss differences with varying oil thicknesses were tested. The oil evaporation data were directly quantified based on the weight loss using an electronic balance. Two different petri dishes with defined areas (18cm2and 11cm2) were used for the evaporation area comparisons. The volumes of 5, 7, 10 and 13mL of condensate oil 2 were loaded in two sizes of petri dishes without seawater for four days (96h). The four different volumes of condensate oil 2 were used in each petri dish with a constant area for monitoring the evaporation effects of oil thickness.
To ensure the completion of evaporation process, 5mL of each condensate oil was loaded in an 18 cm2petri dish for 30d to differentiate their chemical changes before and after evaporation.
2.4 Sample Preparation and Instrumental Analysis
The mixed oil/water samples were fully transferred into a 1L of separating funnel for twice liquid-liquid extractions by 10mL of tetrachloromethane. Combined organic phases were collected in an Erlenmeyer flask and 3g of anhydrous sodium sulfate was added for dryness. The dried extracts were mixed with 3g of purified magnesium silicate and vortexed for 20min at 200rmin−1for removing the possible animal and vegetable oils. The supernates were filtered by glass sand cored funnel and analyzed by infrared photometer.
The petroleum hydrocarbons were analyzed based on the pure condensate oil. 5mL of each condensate oil was directly measured and centrifuged at 10000rmin−1for ten minutes. 1mL of the supernate was collected in the GC vial and analyzed by an Agilent 7890A-5975 GC/MS instrument. The sample injection was 1μL in the pulsed split mode (200:1) and other detailed instrumental parameters were same as summarized below.
The oil samples were separately extracted by 10mL of hexane, then transferred into 10mL of centrifuge tube for centrifugation at 10000rmin−1for 10min, 1mL of the supernatants were taken for GC/MS analysis.
The oil extracts were analyzed by using the Agilent GC/MS equipped with a HP-5MS capillary column (30 m × 0.25mm× 0.25μm). The full-scan mode was used to detect the oil hydrocarbons of which m/z ranging from 50 to 550. The single ion monitoring (SIM) mode was used for the analysis with the m/z value of 57 for n-alkanes, m/z of 128 for naphthalene, m/z of 142 for C1-naphthal- enes, m/z of 178 for phenanthrene and m/z of 192 for C1-phenanthrenes. The GC oven temperature was programmed from 50℃ (2min hold) and ramped at 6℃min−1to 300℃ (16min hold). Inlet temperature was set at 280℃ and the electron ionization (EI) source temperature was maintained at 230℃. Helium was used as a carrier gas. 1μL of sample injection was performed in the pulsed split mode (40:1).
3 Results and Discussion
3.1 Basic Properties of Condensate Oils
As shown in Table 1, the typical physical and chemical parameters of two condensate oils are presented in detail. Light colors can be easily identified for the two condensates, which are greatly different from other conventional crudes that may have brownish or dark colors. The density and viscosity of condensate oils are much lower when compare to other light sweet crudes and heavy bunker oils (Yin., 2015a). This may explain the quick surface diffusion (low density and viscosity) of Sanchi condensate oil at the incident location. The extremely low viscosity of the condensate oils indicates their possible low probability of emulsification, which may drive the oil travels lower in the water column and slow their evaporation rate (NRC, 2003). Also, as normal light crude oils, high contents of saturates and low contents of asphaltenes are shown in both oil samples. In common, condensate oils can be classified as ultra-light crudes with tinged color, high fluidity and great volatility.
3.2 The Evaporation of Condensate Oils with Various Physical Factors
3.2.1 Turbulence
As shown in Fig.1, four hours of evaporation data with and without stirring are compared. The values show the final evaporations of two condensate oils are approximately 80% and above, especially about 90% of the con- densate oil 1 is able to be weathered through the evapora- tion pathway. For both condensates, more than 40% of oil contents evaporate within one minute then about 60% can be removed within 10min. After that, the oil evaporation rates decline significantly. The overall evaporation of the condensate oils presents no sensitive changes to the studied turbulence. In Fig.1b, it is assumed that the lower evaporation ratio of condensate oil 2 is attributed to its higher viscosity, which may cause more reactions with water molecules (emulsification) or the suspended matters (adsorption) in the seawater, thus reduce the weathering degree of evaporation. Based on the chromatographic profiles shown in Fig.6, the slightly higher portion of higher-molecular-weight components in condensate oil 2 may also lead to its lower evaporation rate and total removal.
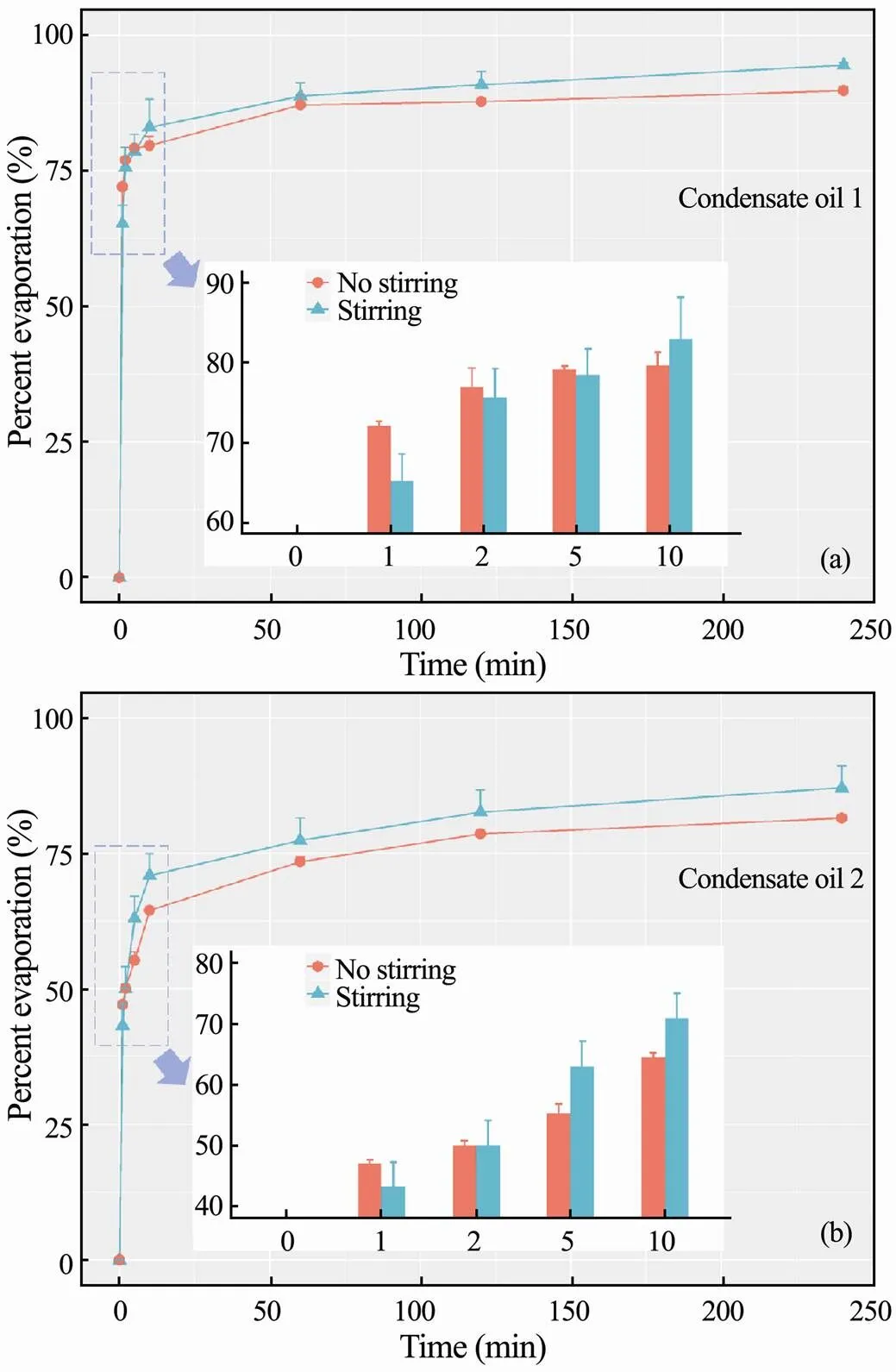
Fig.1 Percent evaporation of condensate oil 1 (a) and oil 2 (b) with and without stirring.
3.2.2 Salinity
The effects of salinity for oil evaporation were studied since the high occurrences of oil spill in the marine environment. Experiments were conducted to describe the evaporation changes of different condensate oils with salinity as the major variant. Fig.2 illustrates the correlation between the condensate oil evaporation and water salinity. The oil evaporation rates and extents are higher with seawater for condensate oil 1in Fig.2abut lower for condensate oil 2 in Fig.2b. The previous research (Wang., 1995) showed that the oil evaporation is relatively positive to the changes of water salinity. However, our results are not fully consistent with this finding, it is probably caused by the different seawater preparation in this study. More designed experiments are required to better describe the mechanisms of salinity effects on the condensate oil evaporation.
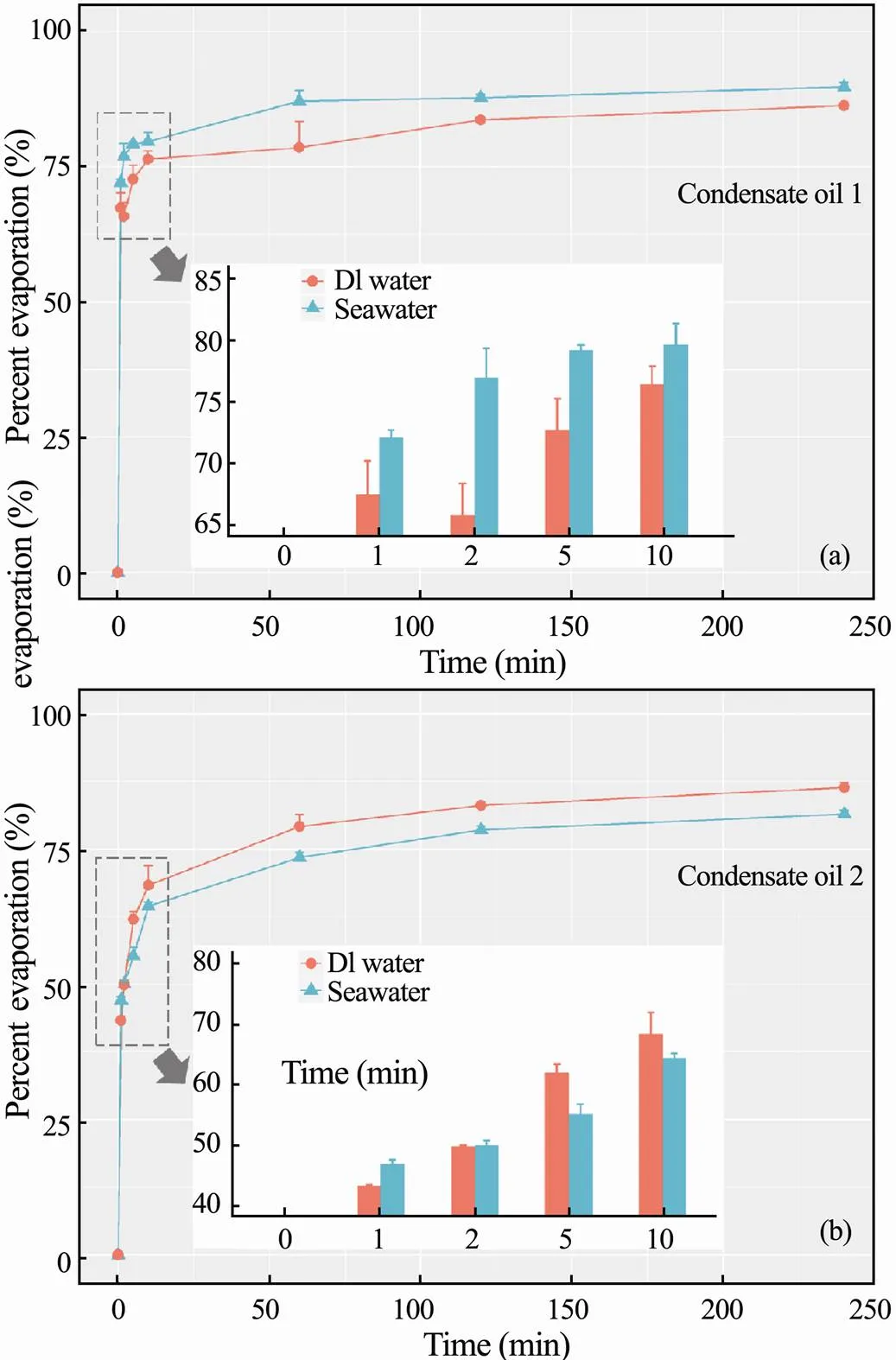
Fig.2 Percent evaporation of condensate oil 1 (a) and oil 2 (b) in seawater and deionized water.
3.2.3 Temperature
Two condensate oils were also used to conduct a series of experiments with varying evaporation temperatures. Experimental results show that close evaporation extents are presented for both oils at three temperature levels (Fig.3). The evaporated content is slight higher at 30℃, which is approximately 91% for condensate oil 1, followed by 90% at 20℃ and 87% at 10℃. For condensate oil 2, around 79% of oil mass is evaporated when the temperature is 30℃, followed by 77% at 20℃ and 79% at 10℃. Although the differences of the evaporation loss are not significant, the evaporation of condensate oils increases with increasing temperature. This finding is consistent with previous studies (Wang., 1995; Fingas, 1996), which stated that the environmental temperature and oil evaporation extent are proportionally correlated.

Fig.3 Percent evaporation of condensate oil 1 and oil 2 with varying temperature levels.
3.2.4 Time
To quantify the general evaporative profiles of condensates, long-term of evaporation experiments were conduct for eight days to demonstrate the correlation between evaporation and time.Fig.4 shows that the volatile contents of condensate oil 1 and oil 2 are about 95% and 91%, respectively. About 80% of the oil 1 mass is volatilized within one hour, and about 88% within a day, in which the total loss of the oil 1 mass is close to 95%. If combined with the above data of the no-stirring experiments, the full evaporative process of condensate oil 1 can be easily summarized. The fastest evaporation rate happens in the first minute, which is about 70%, and 78% of oil loss after ten minutes. The rate of evaporation declines considerably after about 10min, then reaches around 80% in an hour and 95% after eight days. Similarly, in combination with the no-stirring experiment of the condensate oil 2, the results show that about 47% of the total oil mass is removed after one minute, about 64% after ten minutes, and 75% after one hour. The evaporation rate declines considerably after about an hour, which shows about 89% within a day and 91% depletion after eight days. These results demonstrate that time is a major influence factor for condensate oil evaporation.
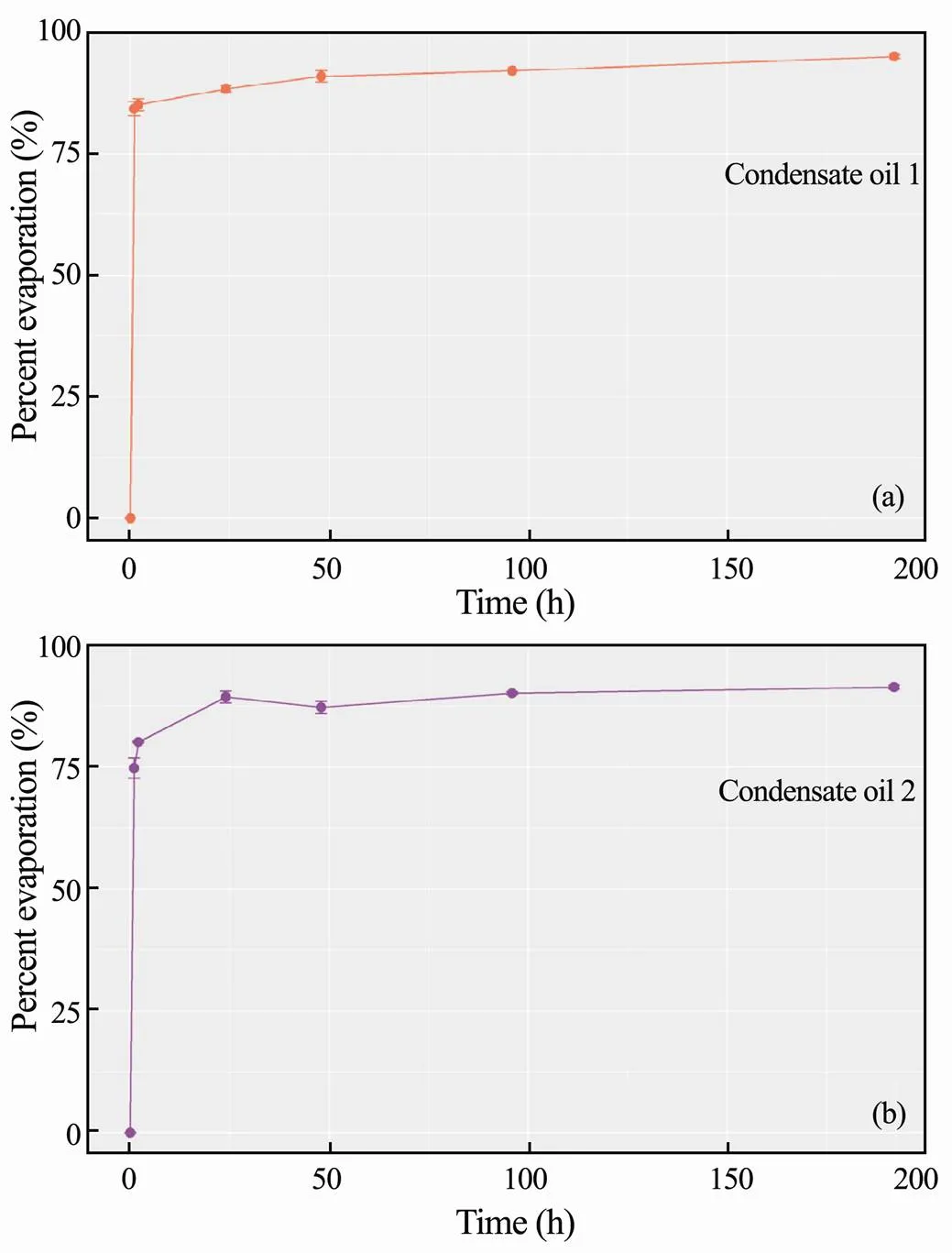
Fig.4 Percent evaporation of condensate oil 1 (a) and oil 2 (b) after eight days.
3.2.5 Area and thickness
Fig.5 describes the evaporation conditions of different volumes (to form different thicknesses) of condensate oil 2 with 18 and 11cm2evaporation areas. In the petri dishes with 18cm2, slight correlations have been observed between the evaporation rates and oil volumes. The final mass loss of 5, 7, 10 and 13mL of oil 2 are approximately 72%, 70%, 69% and 67%, respectively. In comparison, the oil evaporation with smaller area (11cm2) shows strong correlation between the oil volume (or thickness) and evaporation rate. The highest oil depletion and fastest evaporation rate have been observed by using 5mL (thinnest) of oil as the evaporative material, followed by 7, 10 and 13mL of used condensate oil. And the final mass loss of 5, 7, 10 and 13mL of oil 2 with 11cm2areaare around 78%, 70%, 69% and 63%, respectively. The oil evaporation rate and total loss is correlated with the area and thickness when the oil volumes are identical. The obvious differences of evaporation rate and total losses have been observed in the 11cm2of petri dish, as opposed to the observations in the 18cm2one, indicating the evaporation would be significant as long as the oil area is large and the thickness is thin. Similar to other light crude oils (Hollebone, 2017), the high volatile feature of condensate oils in the ocean environment can be determined because of the formation of large surface area and thin oil slicks by their high fluidity.

Fig.5 Percent evaporation of condensate oil 2 with 18cm2 (a) and 11cm2 (b) evaporative areas.
3.3 Chemical Changes of Condensate Oil Evaporation
3.3.1 Petroleum hydrocarbons
Fig.6 shows that the chromatographic data of condensate oil 1 and oil 2 under full-scan mode. As shown in the figure, apparent chromatographic responses can be observed after one minute, and the most abundant chromatographic peaks are presented between one and five minutes for all three oils. With this respect, the full chromatogram was divided into two fractions to highlight the volatile contents, thus the chromatographic peak areas were quantified from one to five minutes and from five to the end, respectively.
In Fig.6a, the peaks of condensate oil 1 are qualitative analyzed between one to five minutes, the compounds of 4 to 7 carbon atoms are the dominance, including cyc- loalkanes (.., cyclohexane, methyl cyclohexane, dimethyl cyclohexane and cyclopentane), isoalkanes (isopentane, isohexane,.), and aromatic hydrocarbons such as benzene, toluene, xylene and ethylbenzene. The area of chromatographic peaks from one to five minutes has been measured as 52% of the total peak area, indicating more than half of evaporative composition in condensate oil 1. Fig.6b describes the full-scann chromatographic peaks of condensate oil 2. Similarly, the dominant component is light hydrocarbons with carbon number of 4 to 7. Approximately 40% of total chromatogra- phic peak area is observed from one to five minutes. The lower content of volatile composition of condensate oil 2 indicates its smaller evaporation level comparing with oil 1, this is also consistent with the evaporation results that shown above.
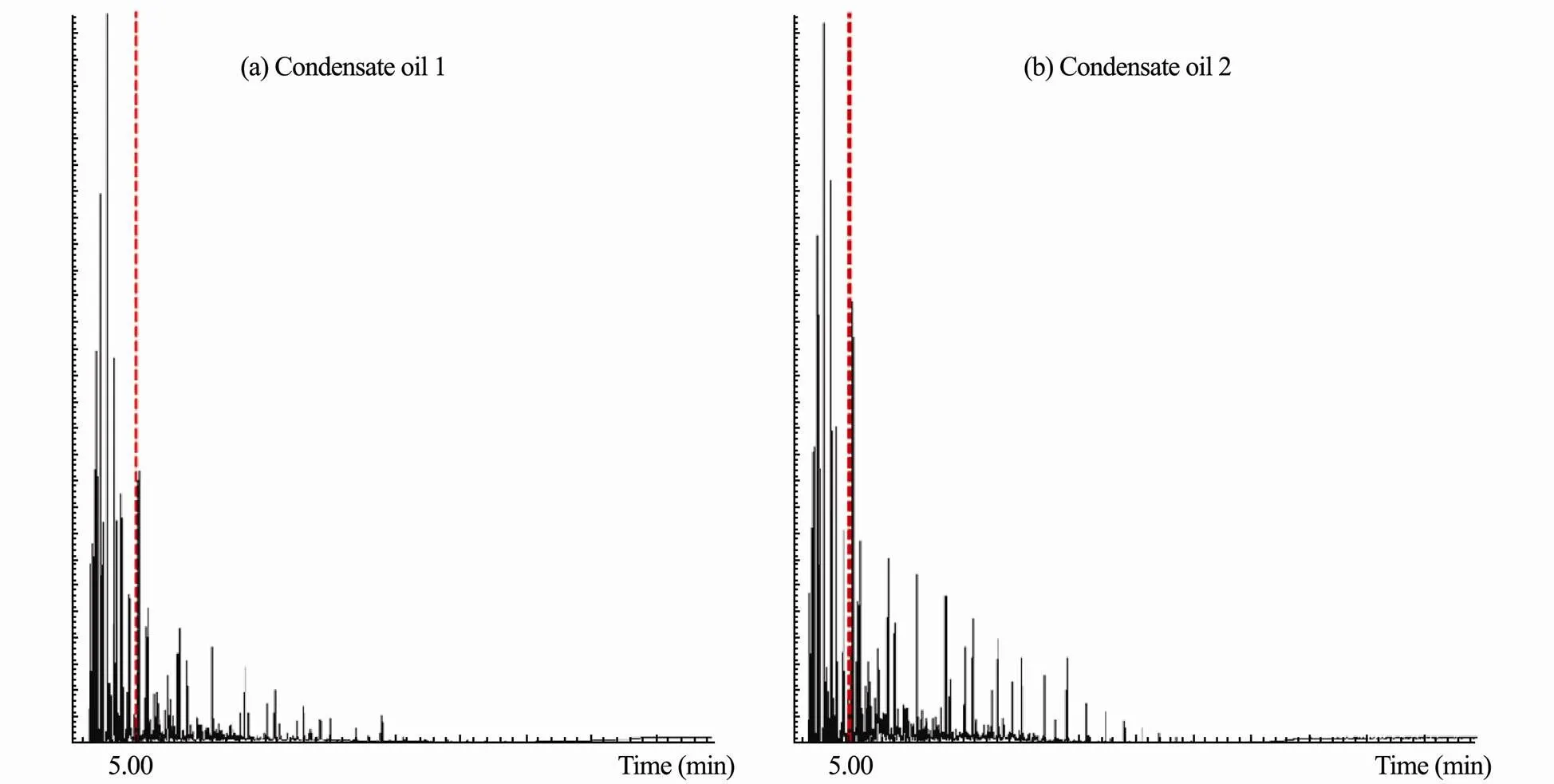
Fig.6 The chromatographic data of condensate oil 1 (a) and oil 2 (b) under full-scan mode (m/z ranging from 50 to 550).
3.3.2 Normal alkanes
The chromatographic features of n-alkanes in condensate oil 1 and oil 2 before and after evaporation experi- ments are investigated and presented in Figs.7 and 8, respectively. The chromatogram for condensate oil 1 indi- cates the presence of n-alkane compounds ranging from C9to C30before evaporation (Fig.7). In comparison, after 30d evaporation, the C9to C12n-alkanes are found to be removed since their volatility. Meanwhile, the chroma- tographic peaks of the evaporated oil present that the C13to C33n-alkanes are more highlighted due to their low volatility, indicating their potentials for oil forensic fin- gerprinting. The peak responses of the pristane, phytane, C17and C18n-alkanes, whose ratios are often used for oil source identification. The ratios of pristane/phytane, C17/ pristane and C18/phytane are calculated as: 9.167, 0.378 and 2.805 for non-evaporated oil samples, and 5.037, 0.575 and 2.513 for evaporated oil samples.
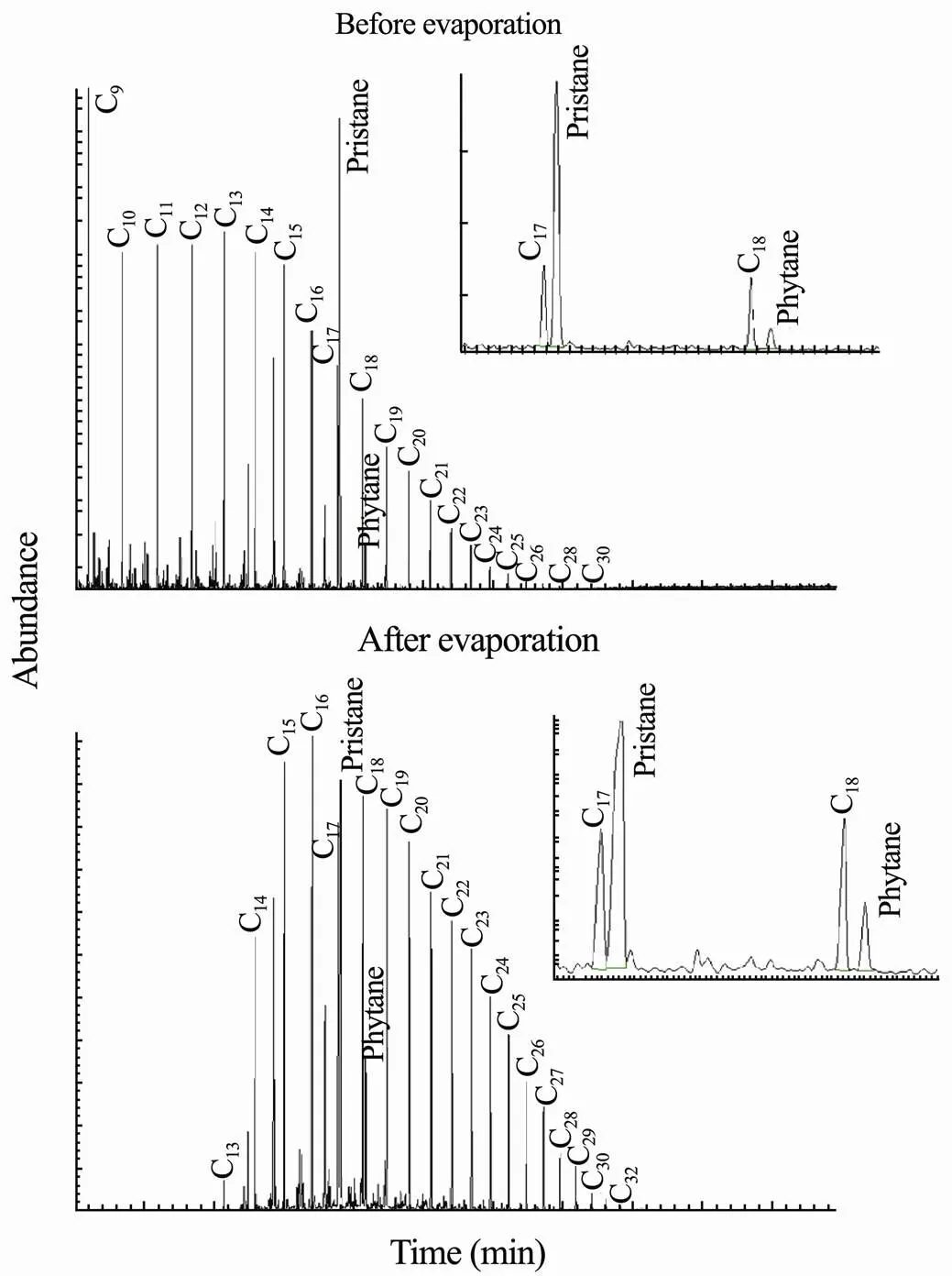
Fig.7 Comparison of extracted ion chromatograms of n- alkanes (m/z of 57) for condensate oil 1 before and after evaporation.

Fig.8 Comparison of extracted ion chromatograms of n- alkanes (m/z of 57) for condensate oil 2 before and after evaporation.
Fig.8 describes the extracted ion chromatographic results of n-alkanes in condensate oil 2 before and after evaporation process. The chromatogram indicates the presence of n-alkanes in oil 2 ranging from C9to C30before evaporation, then reduces to the range of C12to C30after evaporation. Based on the peak responses, the ratios of pristane/phytane, C17/pristane and C18/phytane are: 7.522, 0.765 and 5.098 for non-evaporated oil samples, and 6.598, 0.475 and 2.759 for evaporated oil samples. With the respect of oil fingerprinting, these ratios are indicative of the differences in chemical characteristics of two original condensate oils but not suitable for the evaporated oils.
3.3.3 Light polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons are one of the most important groups of hazardous environmental pollutants presented in crude oils. The high molecular weight of PAHs (4 to 6 rings) are recalcitrant to degradation and the low molecular weight ones (2 and 3 rings) can be evapo- rated more easier (Yin., 2015b). With this respect, the 2 rings of naphthalene with its C1alkylated homologs and 3 rings of phenanthrene with its C1alkylated homologs in the two condensate oils were analyzed. The extracted ion chromatographic results of naphthalenes and phenanthrenes in condensate oil 1 with and without evaporation are shown in Fig.9. The parent naphthalene and C1-naphthalene homologs are clearly presented in the source condensate oils, while most of the naphthalene compounds have been removed after 30d evaporation, indicating their significant volatility. Opposite to naphthalenes, although the peak responses of parent phena- threne and C1-phenathrenes are low before evaporation, they seem to be concentrated after oil evaporation. The apparent chromatographic peaks of phenanthrenes in the evaporated oil 1 indicate the low volatility of the 3 rings PAHs.
Fig.10 describes the extracted ion chromatograms of parent nanphthalene, C1-naphthalene, parent phenanthrene and C1-phenanthrenes in condensate oil 2 before and after evaporation. The peak abundances of naphthalenes in oil 2 almost disappear after 30d evaporation, indicating their high evaporation conditions. A certainamount of phenanthrenes are presented in condensate oil 2 and the peak patterns of C1alkylated phenanthrenes can be differentiated from condensate oil 1, indicating the potential for the forensic analysis of the evaporative con- densate oils. Based on the observations of the chromatograms of phenanthrenes in oil 2, significant peak responses are still found after about a month of evaporation process, proving their poor volatility (Pantsyrnaya., 2011).

Fig.9 The extracted ion chromatograms of naphthalenes (m/z 128 and 142) and phenanthrenes (m/z 178 and 192) in condensate oil 1 before and after evaporation process.

Fig.10 The extracted ion chromatograms of naphthalenes (m/z 128 and 142) and phenanthrenes (m/z 178 and 192) in condensate oil 2 before and after evaporation process.
Based on all of the experiments, evaporation can be clearly considered as the major weathering process for condensate oils, which contributes about 90% of the con- densate oil mass degradation. It seems that the environ- mental impacts of condensate oil spills can be insignifi- cant. However, we must mention that the researches that focused on the highly degradation-resistant oil components, proved that the oil compositions such as heavy parent and alkylated PAHs (Snyder., 2015; Yin., 2015b; John., 2016; Tidwell., 2016; Clement., 2017) and their environmental derivatives (.., oxyhydrocarbons (Aeppli., 2012; Tidwell., 2015, 2016) are certainly required more attentions, because of the worse ecological risks caused by these slow weathered oil components would be posed for a long-term period. In this study, the phenanthrenes were also clearly found more resistant to volatilization. In consideration of the aquatic solubility (Pantsyrnaya., 2011; Gong., 2014) and toxicity of phenanthrenes (Bado-Nilles., 2008; Bellas., 2008; Turcotte., 2011; Fallahtafti., 2012; Incardona., 2014), these residual toxic chemicals in condensate oils should always be emphasized.
4 Conclusions
For gathering more hints for better estimating the consequences of condensate oil spill accident, this work has preliminarily investigated varied evaporation conditions of two condensate oils in the laboratory. A series of experiments were designed for understanding the evaporative effects of different environmental factors include turbulence, salinity, temperature, time, oil film area, and thickness on condensate oils. Also, the chemical changes of light normal alkanes and low molecular weight PAHs in condensate oils were analyzed based on their chromatographic peak responses. According to the experimental data, the condensate oil evaporation has been found not highly effected by the studied turbulence, salinity and temperature. Since large oil area and thin thickness of condensate oil film can be easily formed in the ocean, high evaporative result of the condensate oils can be presumed. The time has been confirmed as the major control factor for the evaporation of condensate oils. The results show that the first ten minutes is extremely important for the oil evaporation of two condensates. Within one minute, the total oil evaporation can be 47% to 70% and rise to 64% to 78% in ten minutes. The rate of evaporation has declined considerably after about 10min. After 1h of evaporation, about 75% to 80% of the oil mass has been removed from the system, then eight days later the total oil loss can be more than 90%. The full-scanned chromatographic data have also proved the high portion of volatile oil hydrocarbons in condensate oils. The amount changes of the light normal alkanes with C9to C13carbon numbers are significant through the evaporation process. The diagnostic ratios of pristane/phytane, C17/ pristane and C18/phytane are shown as: 9.167, 0.378 and 2.805 in condensate oil 1 and 7.522, 0.765 and 5.098 in condensate oil 2, which are distinguishable from the two sources of condensate oils. The 2 rings PAHs such as naphthalene and C1alkylated naphthalenes are able to be completely removed by the evaporation weathering, as opposed to the phenanthrene and C1alkylated phenanthrenes. However, the remaining semi-volatile phenanthrenes in condensate oil residues have been proved more recalcitrant and should be carefully concerned.
According to this study, although the most of the spilled condensate oil mass are presumed to be volatilized into the local atmosphere within an hour, the ecological risks of the about 10% of remained oil components in the seawater still need to be carefully concerned, especially when the total volume of oil split is large, such as the Sanchi accident. The 10% of the Sanchi condensate oil is capable to generate 11130 tons of residues, it is already larger than the total quantity of oil spilt about 10900 tons of the latest Hebei Spirit oil spill in 2006 (Yim., 2012). As a matter of fact, the eight days of oil burning might exhaust the most of the condensate oil in the tanker and minimize the spilled volume of the residues in some way. However, it should always emphasize that the dissolved, dispersed or deposited fractions of the remained Sanchi oil (the condensate oil and the heavy fuel oil) would potentially cause negative ecological risks to the aquatic organisms, and undoubtedly the fate and transport of the evaporated toxic oil chemicals and the by-products of oil combustion also need to be properly monitored.
Acknowledgements
This research is partly supported by the National Natural Science Foundation of China (No. 41807341), the Young Orient Scholars Program of Shanghai (No. QD2017038), the Doctoral Scientific Research Starting Foundation of Shanghai Ocean University, and Shanghai Special Research Fund for Training College’s Young Teachers (No. ZZSHOU18025). The authors wish to thank the colleagues from State Oceanic Administration involved in the environmental monitoring program of Sanchi oil spill for their assistance in executing field observations. The technicians in the chemistry laboratory of East China Sea Environmental Monitoring Center are acknowledged for their help on the massive sample preparations and analyses.
Aeppli, C., Carmichael, C. A., Nelson, R. K., Lemkau, K. L., Graham, W. M., and Redmond, M. C., 2012. Oil weathering after the deepwater horizon disaster led to the formation of oxygenated residues., 46: 8799-8807.
Bado-Nilles, A., Gagnaire, B., Thomas-Guyon, H., Le Floch, S., and Renault, T., 2008. Effects of 16 pure hydrocarbons and two oils on haemocyte and haemolymphatic parameters in the Pacific oyster,(Thunberg)., 22: 1610-1617.
Bellas, J., Saco-Alvarez, L., Nieto, O., and Beiras, R., 2008. Ecoto-xicological evaluation of polycyclic aromatic hydro- carbons using marine invertebrate embryo-larval bioassays., 57: 493-502.
Carswell, C., 2018. Unique oil spill in East China Sea frustrates scientists., DOI: 10.1038/d41586-018-00976-9.
Clement, T. P., John, G. F., and Yin, F., 2017. Chapter 16-Assessing the increase in background oil-contamination levels along alabama’s beaches resulting from the deepwater Horizon Oil Spill A2-Fingas, Mervin. In:. 2nd Edition, Gulf Professional Publishing, Boston, 851-888.
Dzou, L. I. P., and Hughes, W. B., 1993. Geochemistry of oils and condensates, K Field, offshore Taiwan: A case study in migration fractionation., 20: 437-462.
Fallahtafti, S., Rantanen, T., Brown, R. S., Snieckus, V., and Hodson, P. V., 2012. Toxicity of hydroxylated alkylphenanthrenes to the early life stages of Japanese medaka ()., 106: 56-64.
Fingas, M., 1996. The evaporation of oil spills: Prediction of equations using distillation data., 3: 191-192.
Fingas, M. F., 1998. Studies on the evaporation of crude oil and petroleum products - II. Boundary layer regulation., 57: 41-58.
Fingas, M. F., 1999. The evaporation of oil spills: Development and implementation of new prediction methodology., 281-287.
Fingas, M. F., 2004. Modeling evaporation using models that are not boundary-layer regulated., 107: 27-36.
Fingas, M. F., 2012. Studies on the evaporation regulation me- chanisms of crude oil and petroleum products., 2: 246.
Gong, Y., Zhao, X., O’Reilly, S. E., Qian, T., and Zhao, D., 2014. Effects of oil dispersant and oil on sorption and desorption of phenanthrene with Gulf Coast marine sediments., 185: 240-249.
Hollebone, B., 2017. Chapter 3-Oil physical properties: Measurement and correlation. In:. 2nd Edition, Fingas, M., ed., Gulf Professional Publishing, Boston, 185-207.
Incardona, J. P., Gardner, L. D., Linbo, T. L., Brown, T. L., Esbaugh, A. J., and Mager, E. M., 2014. Deepwater horizon crude oil impacts the developing hearts of large predatory pelagic fish., 111: E1510-E1518.
John, G. F., Han, Y. L., and Clement, T. P., 2016. Weathering patterns of polycyclic aromatic hydrocarbons contained in submerged deepwater horizon oil spill residues when re-exposed to sunlight., 573: 189-202.
Liu, Z. F., Liu, J. Q., Zhu, Q. Z., and Wu, W., 2012. The weathering of oil after the deepwater horizon oil spill: Insights from the chemical composition of the oil from the sea surface, salt marshes and sediments., 7pp.
National Research Council, 2003.. Washington D C, 1-20.
Pantsyrnaya, T., Blanchard, F., Delaunay, S., Goergen, J. L., Guédon, E., and Guseva, E., 2011. Effect of surfactants, dispersion and temperature on solubility and biodegradation of phenanthrene in aqueous media., 83: 29-33.
Snyder, S. M., Pulster, E. L., Wetzel, D. L., and Murawski, S. A., 2015. PAH exposure in Gulf of Mexico demersal fishes, post-deepwater horizon., 49: 8786-8795.
Speight, J. G., 2015. Chapter 1-Occurrence and formation of crude oil and natural gas.. Gulf Professional Publishing, Boston, 1-43.
Thompson, K. F. M., 1987. Fractionated aromatic petroleums and the generation of gas-condensates., 11: 573-590.
Tidwell, L. G., Allan, S. E., O’Connell, S. G., Hobbie, K. A., Smith, B. W., and Anderson, K. A., 2015. Polycyclic aroma- tic hydrocarbon (PAH) and oxygenated PAH (OPAH) air- water exchange during the deepwater horizon oil spill., 49: 141-149.
Tidwell, L. G., Allan, S. E., O’Connell, S. G., Hobbie, K. A., Smith, B. W., and Anderson, K. A., 2016. PAH and OPAH flux during the deepwater horizon incident., 50: 7489-7497.
Turcotte, D., Akhtar, P., Bowerman, M., Kiparissis, Y., Brown, R. S., and Hodson, P. V., 2011. Measuring the toxicity of alkyl-phenanthrenes to early life stages of medaka () using partition-controlled delivery., 30: 487-495.
Wang, Y., Zhu, C., Sun, B., Shi, Z., Yu, S., and Hao, E., 1995. Simulant experiments of crude oil purification in shallow sea area -The evaporation and degradation process of dispersed oil., 26: 389-396.
Yim, U. H., Kim, M., Ha, S. Y., Kim, S., and Shim, W. J., 2012. Oil spill environmental forensics: The Hebei spirit oil spill case., 46: 6431-6437.
Yin, F., Hayworth, J. S., and Clement, T. P., 2015a. A tale of two recent spills-comparison of 2014 Galveston Bay and 2010 deepwater horizon oil spill residues., 10pp.
Yin, F., John, G. F., and Hayworth, J. S., and Clement, T. P., 2015b. Long-term monitoring data to describe the fate of polycyclic aromatic hydrocarbons in deepwater horizon oil submerged off Alabama’s beaches., 508: 46-56.
Yin, L., Zhang, M., Zhang, Y., and Qiao, F., 2018. The long- term prediction of the oil-contaminated water from the Sanchi collision in the East China Sea., 37: 69-72.
. E-mail: jb_zhang@shou.edu.cn
E-mail: fangyin@shmtu.edu.cn
February 1, 2019;
August 27, 2019;
October 11, 2019
(Edited by Ji Dechun)
 Journal of Ocean University of China2020年3期
Journal of Ocean University of China2020年3期
- Journal of Ocean University of China的其它文章
- Estimation of the Reflection of Internal Tides on a Slope
- The New Minimum of Sea Ice Concentration in the Central Arctic in 2016
- Investigation of the Heat Budget of the Tropical Indian Ocean During Indian Ocean Dipole Events Occurring After ENSO
- Image Dehazing by Incorporating Markov Random Field with Dark Channel Prior
- Biochemical Factors Affecting the Quality of Products and the Technology of Processing Deep-Sea Fish,the Giant Grenadier Albatrossia pectoralis
- Propulsion Performance of Spanwise Flexible Wing Using Unsteady Panel Method
