1H NMR Quantification of DHA and EPA in Fish Oil
LVJinxiao WANGCong2) 3) 4) ZHANGXiuli2) 3) 4) LV Zhihua2) 3) 4) *andYUMingming2) 3) 4) *
1H NMR Quantification of DHA and EPA in Fish Oil
LVJinxiao1),#, WANGCong1), 2),3), 4),#, ZHANGXiuli1), 2),3), 4), LV Zhihua1), 2),3), 4), *,andYUMingming1), 2),3), 4), *
1)School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, China 2) Laboratory for Marine Drugs and Bioproducts of Qingdao National Laboratory for Marine Science and Technology, Qingdao 266003, China 3) Key Laboratory of Glycoscience & Glycotechnology of Shandong Province, Qingdao 266003, China 4) Key Laboratory of Marine Drugs, Ministry of Education of China, Qingdao 266003, China
Fish oil is a popular nutritional product consumed in China. The beneficial effects of fish oil have been attributed to docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Hence, it is crucial to develop a rapid and precise method to determine the contents of EPA and DHA in fish oil. In this study, a rapid and accurate proton nuclear magnetic resonancemethod for the quantification of DHA and EPA was developed. Dimethyl terephthalate was selected as an internal standard, and the signals at 2.391ppm for DHA and at 1.697ppm for EPA were chosen for the quantification. Validation of the method was performed in terms of specificity, precision, and stability. The results indicated that the method was precise and in line with the China Food and Drug Administration guidance. The method has been successfully applied to characterize fish oil capsules obtained from four pharmaceutical companies. This study indicated that the rapid1H NMR method is suitable for the quality control of fish oil.
1H NMR; EPA; DHA; fish oil;quality control
1 Introduction
Fish oil is the oil derived from marine pelagic fish species, such as tuna, salmon, swordfish, and mackerel (Jacobs, 2002; Smith and Sahyoun, 2005). Fish oil exerts some beneficial effects against several diseases such as autoimmune disease (Harbige, 2003), cardiovascular diseases (Kris-Etherton, 2002), depression (Nemets, 2006), and neurological disorders (Lukiw and Bazan, 2008). The beneficial effects of fish oil have been attributed to docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) (Fig.1) which have been associated with these health benefits. As a result, there are numerous fish oil products containing EPA and DHA in the markets. These fish oil products are derived from different sources, and the prices of these products are significantly different. While these products are labeled with the same ingredient levels, namely DHA (120mgg−1) and EPA (180mgg−1) (Yi, 2014). The quantitative methods of DHA and EPA in fish oil products have been developed by gas chromatography (GC) (Srigley and Rader, 2014), gas chromatography-mass spectrometry (GC-MS) (Yi., 2014), and high-performance liquid chromatography (HPLC) (Teng and Gowda, 1993). However, the acquisition time of these methods was over 1h, making them not suitable for the routine and rapid quantitative analysis of a large number of fish oil samples. Hence, it is necessary to develop a rapid analytical method that can simultaneously determine EPA and DHA in fish oil for quality control.
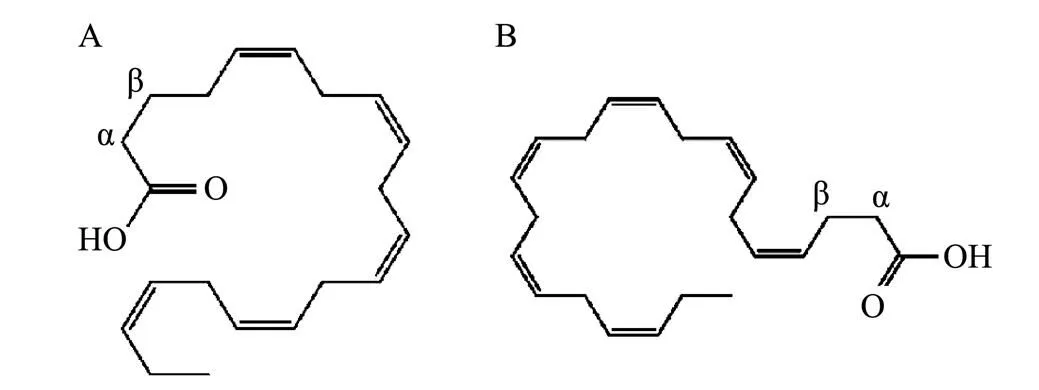
Fig.1 The structures of eicosapentaenoic acid (EPA, A) and docosahexaenoic acid (DHA, B).
Quantitative Nuclear Magnetic Resonance (qNMR) is a routine analytical technique, which is ‘green’, precise, and, most importantly, nondestructive (Wang, 2019). Besides, qNMR does not require the standard of compound (Pauli, 2005;Chauthe, 2012; Tada, 2013). The qNMR method has been widely applied for the determination of various compounds in the complex mixtures of foods (Hatzakis, 2008; Petrakis, 2008; Gouilleux, 2018), the natural products (Harvey, 2015), Chinese medicine (Fang and Tan, 2005) and plant extracts (Song, 2014; Jegou, 2015; Dong, 2018). Moreover, this method does not require sample prepurification or calibration curve standards (Tanaka, 2013). Therefore, it can be used for the quality control of DHA and EDA in fish oil products and serve as an effective alternative method to various chromatographic techniques.
In this study, we report a rapid and precise1H NMR method that can quantify DHA and EPA in fish oil products simultaneously. Also, the application of this method for the quality control of fish oil products is presented.
2 Materials and Methods
2.1 Chemicals and Reagents
DHA and EPA standards and dimethyl terephthalate (DMT, internal standard) were obtained from Sigma-Aldrich Corporation (Missouri, USA). Deuterated chloroform (CDCl3) was obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). Four kinds of fish oil softgels were obtained from 4common health food companies.
2.2 Sample Preparation
The working solution of the internal standard (4.14mgmL−1) was prepared in CDCl3with stock solutions of DHA (9.29mgmL−1) and EPA (10.45mgmL−1). Calibration cur- ves for DHA and EPA were constructed using seven concentrations over a range of 0.048-3.10mgmL−1(3.10, 1.55, 0.78, 0.39, 0.19, 0.10 and 0.048mgmL−1) and 0.038-2.45mgmL−1(2.45, 1.23, 0.61, 0.31, 0.15, 0.077 and 0.038mgmL−1), respectively. All calibration standard samples were freshly prepared each day. To quantify DHA and EPAin fish oil, 10 softgels were weighed precisely and the contents were taken out. Subsequently, the empty softgel shells are also weighed to calculate the mass of the contents of each softgel. The fish oil (10μL) was dissolved in a CDCl3solution (600μL) containing the internal standard (0.138mgmL−1) and vortex-mixed for 1min. And the mix- ture was used for the1H NMR analysis.
2.3 1H NMR Spectrometric Parameters
All1H-NMR spectra were recorded on an Agilent DD2 500MHz spectrometer with a 5-mm One NMR probe (CA, United States). The spectra were recorded with 32 scans over a pulse angle of 45˚, a spectral width (SW) of 8012.8 Hz, and a relaxation delay (D1) of 10.0s at 298K. Free induction decays (FIDs) were processed with a line broadening (LB) of 0.3Hz before Fourier transformation. The phase and baseline spectra were corrected manually. All data acquisition and processing were done with Mest- ReNova 11.0 (MestrelabResearch, S.L., Santiago de Com- postela, Spain).
2.4 Identification of Contents
The signals of DHA at 2.391ppm (α, β) and EPA at 1.697ppm (β) were used for the quantitative analysis. The amount of DHA and EPA in tablets or capsules was calculated by the following equation (Bharti and Roy, 2012):
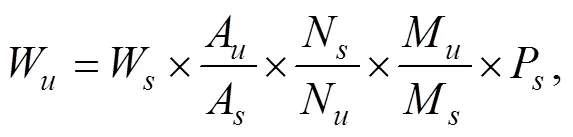
where,, andare the gravimetric weight, integral area, and the number of nuclei of DHA or EPA () and internal standard (), respectively.Mis the molecular mass ofDHA or EPA, andMis the molecular mass of the internal standard.Pstands for purity of the internal standard (99.99%). Based on the labeled contents data and linearity of the method, 100μL fish oil per sample was used for their quantification in the softgels.
2.5 Quantitative 1H NMR Validation Method
Validation was carried out according to the CFDA guidelines. The limit of detection (LOD) and limit of quantification (LOQ) were separately evaluated using the S/N values of 3 and 10, respectively (Zhang, 2016). The linearity, precision, repeatability, stability, and recovery were determined according to the CFDA guidelines (=6). The system suitability test was performed with six replicate injections of the standard solution. The stability of DHA and EPA at room temperature was determined by analyzing the samples at 0 and 120h. The recovery was calculated by Eq. 2). The amount of DHA and EPA was calculated using Eq. 1).
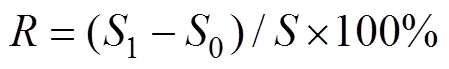
whereis the recovery;0and1are the measurements before and after the addition of standards;is the amount of added compounds DHA and EPA.
2.6 Sample Characterization
Four batches of fish oil from 4 commercial sources were analyzed1H NMR. The sample preparation was described above.
3 Results and Discussion
3.1 Development of 1H NMR Method
CDCl3was selected as a solvent in this study due to its superior solubilizing ability towards both DHA and EPA. The1H NMR spectra of DHA, EPA, and their mixture are shown in Fig.2. It is intuitively clear that the signals of both DHA and EPA appeared in the region with a small chemical shift. Therefore, DMT, with the signal in aromatic region (8.10 ppm), is a suitable internal standard for the quantification of DHA and EPA, because it does not overlap with the signals of DHA and EPA. Besides, it can be found from the comparison of Fig.2B and Fig.2C with Fig.2A respectively, the signals at 2.391ppm for DHA and at 1.697ppm for EPA are suitable to be chosen to quantify DHA and EPA in this study since these two peaks are separated from the other signal. Fortunately, there is no obvious overlap between the two signals and the signals of other substances in fish oil samples, so they can be applied to the quantification of EPA and DHA in fish oil samples. The1H NMR spectra of the mixed standard of DHA and EPA, and a fish oil sample are shown in Fig.3.
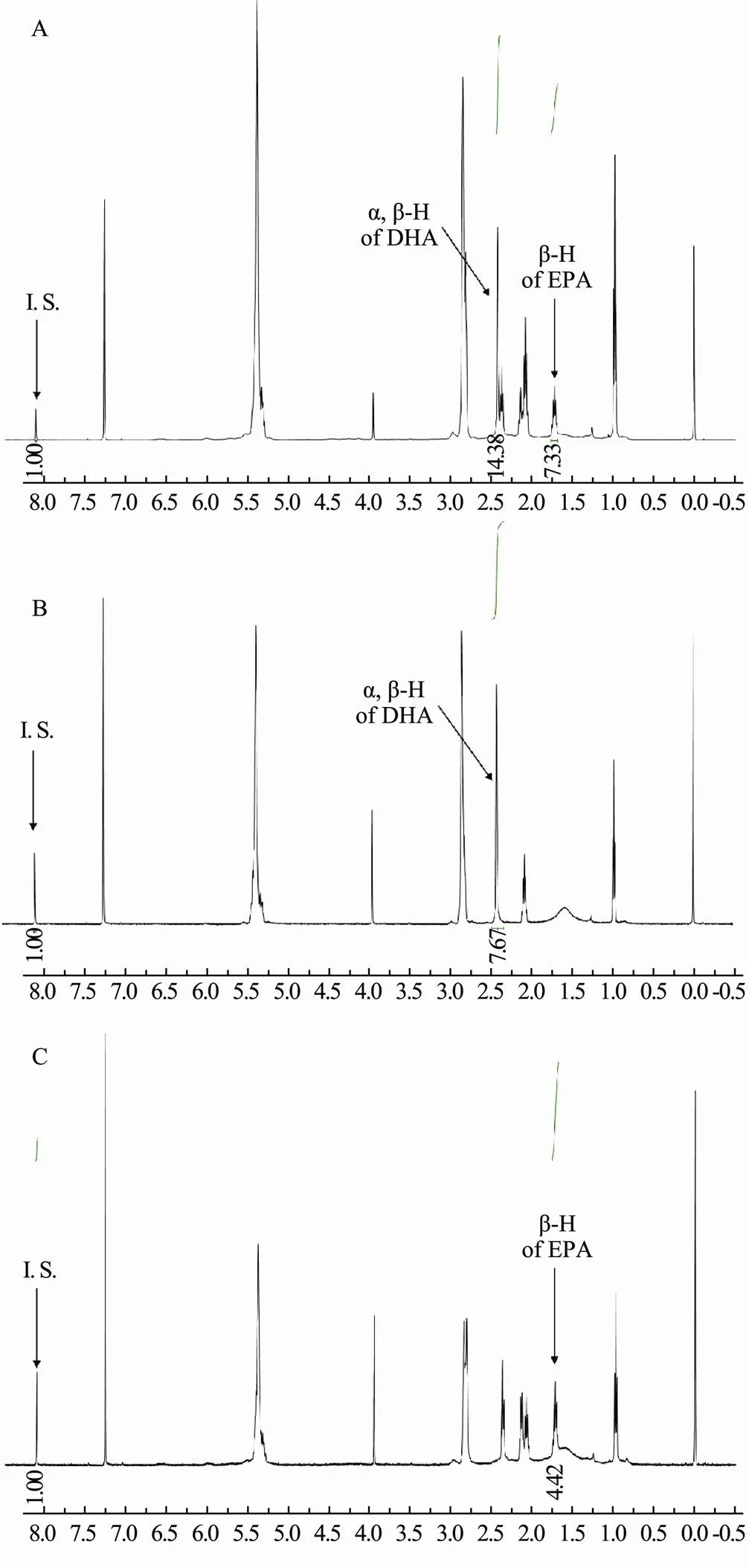
Fig.2 1H NMR spectra of mixed standards of DHA and EPA (A), DHA standard (B) and EPA standard (C).
In a quantitative experiment, the relaxation delay () and acquisition time () are very important parameters that influence the accuracy of the analysis. Therefore, these parameters were optimized to achieve good resolution and sensitivity for both DHA and EPA. To determine the robustness of this method, the(1, 5, 10, 15, 20, 25, and 30s) was evaluated, and theof 10s was selected for this study. Theof 2, 3, and 4s was evaluated, and the results showed that theof 2s was sufficient for the complete attenuation of FID. Hence, theof 10s and theof 2s were selected for this study. The1H NMR acquisition time was only 9min, which is much shorter than that of the long GC and HPLC elution times.
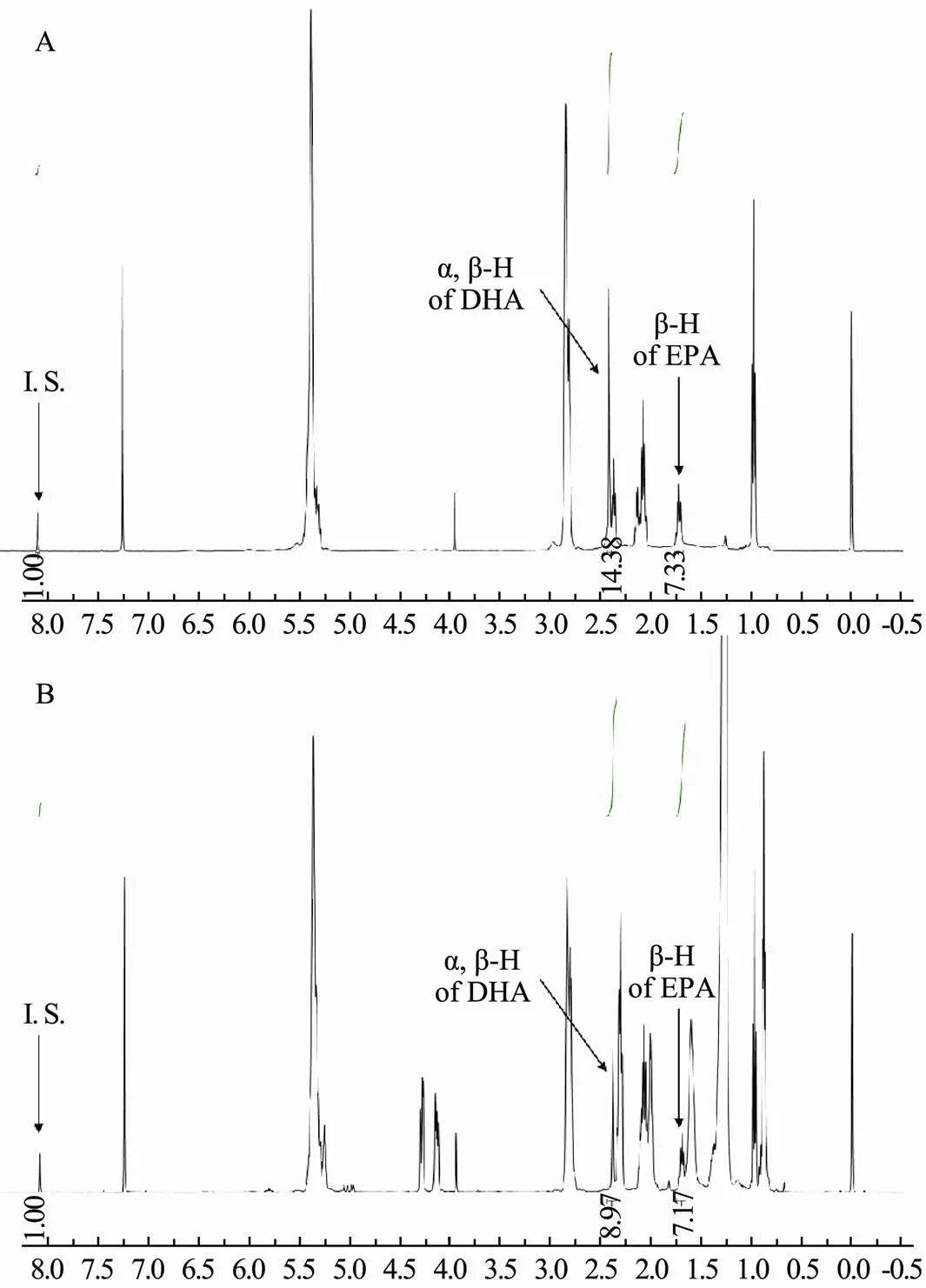
Fig.3 1H NMR spectra of DHA and EPA. A, mixed standards; B, fish oil softgels sample.
3.2 Validation of 1H NMR Methods
The linearity of the1H NMR method evaluated over the concentration range of 0.0485-3.10mgmL−1for DHA, and 0.0230-1.47mgmL−1for EPA, was excellent. Theandwere 0.015 and 0.05mgmL−1for DHA and 0.006 and 0.02mgmL−1for EPA (Table 1). The linear equations with good regression coefficients were shown in Fig.4. The precision of the method for DHA and EPA was 0.53% and 0.72% (Table 2), respectively, and the inter-day precision was 0.81% and 1.09%, respectively. The recovery rate for DHA and EPA was determined to be 102.9% and 106.8%, respectively. DHA and EPA in the samples were found to be stable for 120h according to the stability and 106.8%, respectively. DHA and EPA in the samples were then found to be stable for 120h according to the stability test. Validation of the1H NMR method indicated that the method was precise and in line with the CFDA guidance.

Table 1 Analysis results of linear correlation parameters, LOD and LOQ for DHA and EPA
Notes:, limit of detection;, limit of quantitation.= 6.

Table 2 The results of recoveries and precisions
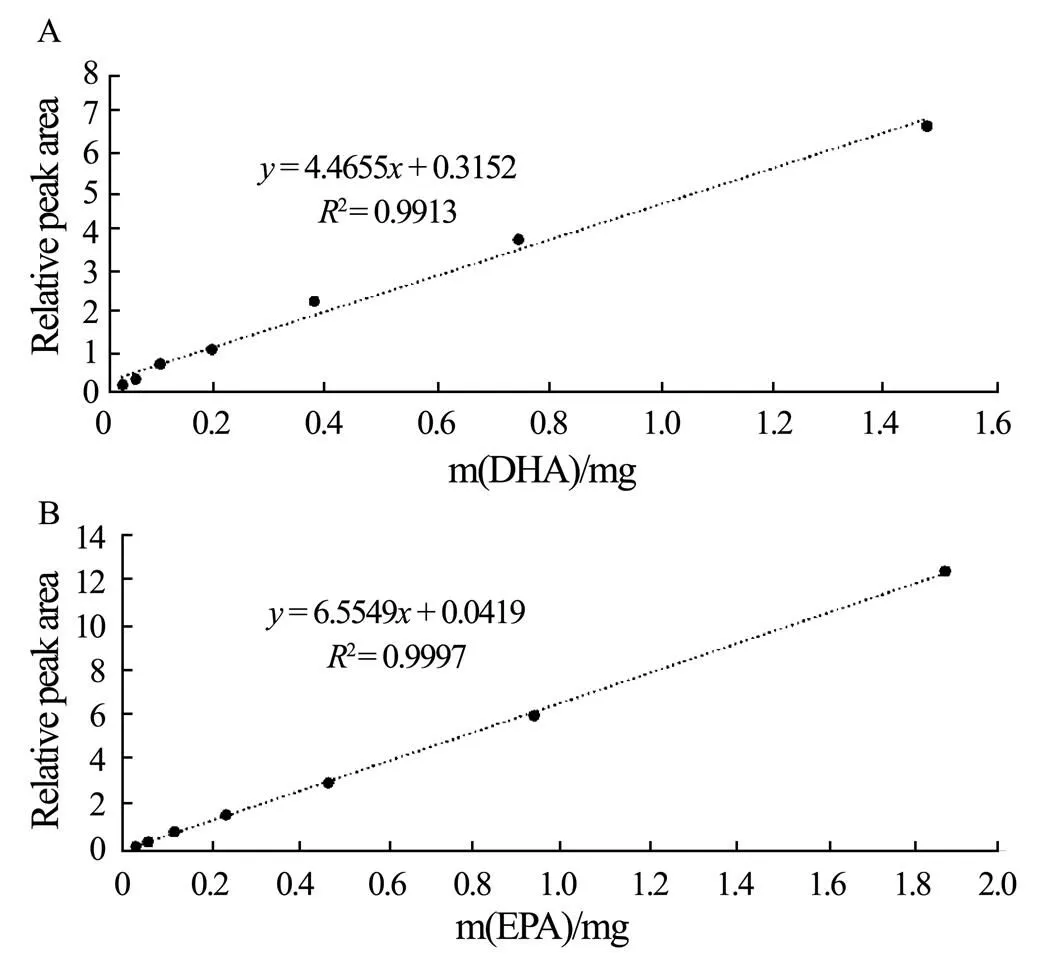
Fig.4 Calibration curves of DHA (A) and EPA (B).
3.3 Quantification of DHA and EPA in Fish Oil
The1H NMR method was successfully used to determine the concentration of DHA and EPA in fish oil softgels from 4 companies. The concentration of DHA was 132.55 ±0.22, 83.05±0.34, 122.09±0.24, and 156.30±0.31mgg−1, respectively (Table 3). The concentration of EPAwas 211.29±0.29, 207.99±0.46, 237.81±0.44, and 304.54±0.57mgg−1, respectively (Table 2). The results indicated the significant variation in the contents of EPA and DHA in the fish oils from different sources, and there is no relationship between the price and contents of EPA and DHA. Although the quality standards of fish oil in different countries and regions have many contents, the content of DHA and EPA, which are the most important active components, is an important part of it. Hence, the strict supervision of the labeling of the fish oil capsules is urgently required.
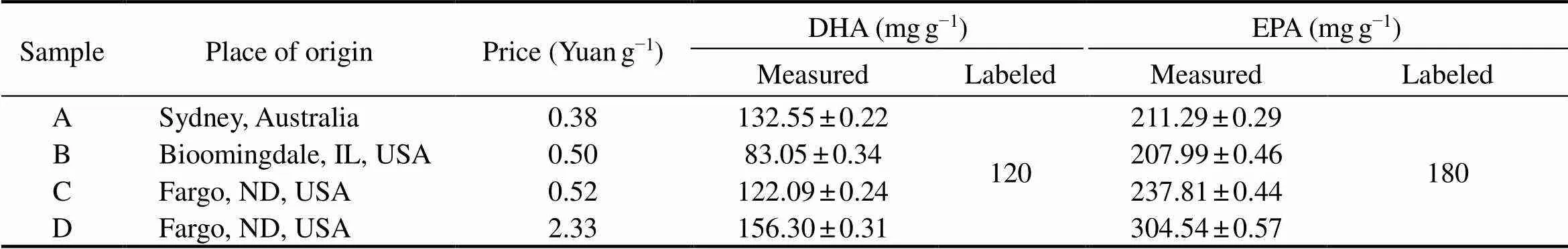
Table 3 The concentration (mgg−1) of DHA and EPA in fish oil softgels(N=3)
4 Conclusions
This paper describes a rapid, accurate, and validated1H NMR method for the quantification of DHA and EPA in fish oil capsules. In the past, the quantitative analysis of DHA and EPA in fish oil was mainly realized by chromatography. Although chromatographic methods, especially when combined with mass spectrometry, are highly sensitive. However, they also involved more complex pretreatment of samples. And fish oil contains many polyunsaturated fatty acids with a very similar structure. Therefore, the samples must be separated sufficiently on the column to accurately quantify EPA and DHA, which will take a lot of time. The accuracy and sensitivity of the NMR method have reached the level required in the production quality control and inspection process. Also, the acquisition time of the1H NMR method is much shorter than that of the GC and HPLC methods. Thus, this study indicates that qNMR is more suitable than GC and HPLC for the quality control of fish oil capsules. However, due to the limitation of test conditions, NMR is not available in every lab. However, it is reasonable to believe that with the development of science and technology, the instruments of NMR will be more and more popular, and the NMR method will be more widely accepted because of its advantages of simplicity and speed. Based on the results of the contents of DHA and EPA in samples, the strict supervision of the labeling of the fish oil products is urgently required.
Acknowledgements
This work was supported by the Open Research Fund Program of Shandong Provincial Key Laboratory of Glycoscience & Glycotechnology (Ocean University of China), and the Fundamental Research Funds for the Central Universities (Nos. 201912008, 201964019, and 201851025).
Bharti, S. K., and Roy, R., 2012. Quantitative1H NMR spectroscopy.,35: 5-26.
CFDA, 2015..
Chauthe, S. K., Sharma, R. J., Aqil, F., Gupta, R. C., and Singh, I. P., 2012. Quantitative NMR: An applicable method for quan- titative analysis of medicinal plant extracts and herbal products.,23: 689-696.
Dong, J. W., Li, X. J., Cai, L., Shi, J. Y., Li, Y. F., Yang, C., and Li, Z. J., 2018. Simultaneous determination of alkaloids di- centrine and sinomenine in Stephania epigeae by1H NMR spectroscopy.,160: 330-335.
Fang, X. S., and Tan, X. M., 2005. The qualitative analysis and quantitative analysis of purification of salvianolic acids by macroreticular resin.,30: 1331- 1334.
Gouilleux, B., Marchand, J., Charrier, B., Remaud, G. S., and Giraudeau, P., 2018. High-throughput authentication of edible oils with benchtop ultrafast 2D NMR., 244: 153-158.
Harbige, L. S., 2003. Fatty acids, the immune response, and autoimmunity: A question of n-6 essentiality and the balance between n-6 and n-3.,38: 323-341.
Harvey, A. L., Edrada-Ebel, R., and Quinn, R. J., 2015. The re-emergence of natural products for drug discovery in the genomics era.,14: 111-129.
Hatzakis, E., Koidis, A., Boskou, D., and Dais, P., 2008. Determination of phospholipids in olive oil by31P NMR spectroscopy.,56: 6232- 6240.
Jacobs, M. N., Covaci, A., and Schepens, P., 2002. Investigation of selected persistent organic pollutants in farmed Atlantic salmon (), salmon aquaculture feed, and fish oil components of the feed., 36: 2797-2805.
Jegou, C., Kervarec, N., Cerantola, S., Bihannic, I., and Stiger-Pouvreau, V., 2015. NMR use to quantify phlorotannins: The case of, a phloroglucinol-pro- ducing brown macroalga in Brittany (France).,135: 1-6.
Kris-Etherton, P. M., Hecker, K. D., Bonanome, A., Coval, S. M., Binkoski, A. E., Hilpert, K. F., Griel, A. E., and Etherton, T. D., 2002. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer.,113Suppl 9B: 71S-88S.
Lukiw, W. J., and Bazan, N. G., 2008. Docosahexaenoic acid and the aging brain.,138: 2510-2514.
Nemets, H., Nemets, B., Apter, A., Bracha, Z., and Belmaker, R. H., 2006. Omega-3 treatment of childhood depression: A controlled, double-blind pilot study.,163: 1098-1100.
Pauli, G. F., Jaki, B. U., and Lankin, D. C., 2005. Quantitative1H NMR: Development and potential of a method for natural products analysis., 68: 133-149.
Petrakis, P. V., Agiomyrgianaki, A., Christophoridou, S., Spyros, A., and Dais, P., 2008. Geographical characterization of Greek virgin olive oils (cv. Koroneiki) using1H and31P NMR fingerprinting with canonical discriminant analysis and classification binary trees.,56: 3200-3207.
Smith, K. M., and Sahyoun, N. R., 2005. Fish consumption: Recommendationsadvisories, can they be reconciled?, 63: 39-46.
Song, Y. L., Jing, W. H., Chen, Y. G., Yuan, Y. F., Yan, R., and Wang, Y. T., 2014.1H nuclear magnetic resonance based-metabolomic characterization of Peucedani Radix and simultaneous determination of praeruptorin A and praeruptorin B.,93: 86- 94.
Srigley, C. T., and Rader, J. I., 2014. Content and composition of fatty acids in marine oil omega-3 supplements.,62: 7268-7278.
Tada, A., Takahashi, K., Ishizuki, K., Sugimoto, N., Suematsu, T., Arifuku, K., Tahara, M., Akiyama, T., Ito, Y., Yamazaki, T., Akiyama, H., and Kawamura, Y., 2013. Absolute quantitation of stevioside and rebaudioside A in commercial standards by quantitative NMR.,61: 33-38.
Tanaka, R., Yamazaki, M., Hasada, K., and Nagatsu, A., 2013. Application of quantitative1H-NMR method to determination of paeoniflorin in., 67: 657-661.
Teng, J. I., and Gowda, N. M. M., 1993. Analysis of n-3 fatty acids in fish oils by high-performance liquid chromatography., 35: 627-630.
Wang, C., Zhang, X., and Yu, M., 2019. Rapid determination of acarbose in tablets by1H NMR spectroscopy., 15.
Yi, T., Li, S. M., Fan, J. Y., Fan, L. L., Zhang, Z. F., Luo, P., Zhang, X. J., Wang, J. G., Zhu, L., Zhao, Z. Z., and Chen, H. B., 2014. Comparative analysis of EPA and DHA in fish oil nutritional capsules by GC-MS.,13: 190.
Zhang, X. L., Wang, C., Chen, Z., Zhang, P. Y., and Liu, H. B., 2016. Development and validation of quantitative1H NMR spectroscopy for the determination of total phytosterols in the marine seaweed.,64: 6228-6232.
# The two authors contributed equally to the work.
E-mail: lvzhihua@ouc.edu.cn
E-mail:yumingming@ouc.edu.cn
December 2, 2019;
April 28, 2020;
May 3, 2020
(Edited by Ji Dechun)
 Journal of Ocean University of China2020年5期
Journal of Ocean University of China2020年5期
- Journal of Ocean University of China的其它文章
- Interannual Variability of Shelf and Slope Circulations in the Northern South China Sea
- Estimation and Prediction of Typhoons and Wave Overtopping in Qingdao, China
- Estimating Design Loads with Environmental Contour Approach Using Copulas for an Offshore Jacket Platform
- Geochemistry and Petrogenesis of Volcanic Rocks from the Continent-Ocean Transition Zone in Northern South China Sea and Their Tectonic Implications
- Study on the Geo-Environmental Evolution of the Laolonggou Lagoon Under the Impacts of the Caofeidian Reclamation Project in Hebei Province
- Operational Laboratory Methods for GDGTs Groups Separation
