Operational Laboratory Methods for GDGTs Groups Separation
LIU Zhan, LI Li, HE Juan, CHEN Lingdi, WANG Junjian, and JIA Guodong
Operational Laboratory Methods for GDGTs Groups Separation
LIU Zhan, LI Li*, HE Juan, CHEN Lingdi, WANG Junjian, and JIA Guodong
,,200092,
Glycerol dialkyl glycerol tetraethers (GDGTs), specific membrane lipids synthesized mainly by bacteria and archaea, can be divided into isoprenoids and methyl branched alkyl GDGTs (iGDGTs and brGDGTs, respectively). Three GDGTs groups (iGDGTs, brGDGTs, and other membrane lipids) in a peat sample were separated and collected in this study using semi-preparative high-performance liquid chromatography (HPLC) and silica gel column chromatography. The obtained recoveries for the whole analytical procedure were 85%–55% using semi-preparative HPLC and 70%–20% using gel column chromatography. In addition, in each method, the recoveries of brGDGTs and iGDGTs were similar, regardless of the huge difference in their contents. High purity was found in the fractionated groups, determined based on ether cleavage and reduction. Moreover, the semi-preparative HPLC method could realize a better separation efficiency than the silica gel method, but it was time-consuming and required expensive equipment, while the silica gel chromatography method featured merits of time saving and convenient operation at the cost of a slight reduction in separation efficiency. The advantages of the silica gel method make it an operational laboratory method for batch experiments and isotopic studies.
GDGTs separation; silica gel column chromatography; semi-preparative HPLC
1 Introduction
Glycerol dialkyl glycerol tetraethers (GDGTs), which were initially believed to be synthesized mainly by extre- mophilic archaea, have their carbon skeletons connected with glycerol by four ether bonds. The monolayer structure of the ether bond is highly stable compared with traditional ester bonds (Sinninghe Damsté., 2007). Other studies have demonstrated that these ether membrane lipids are widespread in various environments and are not only in harsh environments (Schouten., 2013). Two main kinds of GDGTs groups have been distinguished according to the difference in carbon skeleton structure: iGDGTs and branched GDGTs. The former is composed of isoprenoid carbon skeleton (biphytane with 0–4 cyclo- pentane moieties), while the latter contains two-alkyl skeletons containing up to four methyl branches and one cyclopentyl moiety substitute (Hoefs., 1997; Sinninghe Damsté., 2000; De Jonge., 2014). Generally, iGDGTs are believed to be synthesized by archaea, while brGDGTs are produced from soil bacteria, although there is some debate regarding possible additional sources (DeLong., 1998; Macalady., 2004; Schouten., 2007). In addition to their differences in carbon skeleton, the two GDGTs also feature differences in the spatial configuration of the head glycerol: 2, 3-di-O-alkyl-sn-glycerol has been found in iGDGTs, while 1, 2-di-O- alkyl-sn-glycerol in brGDGTs (Becker., 2013). The differences in the structure indicate both the biological diversity and complexity of the GDGTs.
Studies have shown the GDGTs composition varies sys- tematically in response to changes in environmental conditions. Based on these, many climate proxies have been established, for example, seawater temperature index–TEX86(Schouten., 2002), land source input index–BIT (Hopmans., 2004), methylation index of branched tetraethers–MBT (Weijers., 2007), cyclization ratio of branched tetraethers–CBT (Weijers., 2007), and methane indictor–MI (Zhang., 2011). Many studies have shown that brGDGTs are mainly produced by anaerobic soil bacteria and that a good correlation exists between MBT/CBT and mean annual air temperature (MAAT), as well as between MBT/CBT and soil pH (Weijers., 2007). These indices play increasingly important roles in terrestrial paleoenvironment reconstruction and contribute to GDGTs being the most promising biomarker.
However, further studies have shown that the GDGTs based indices, especially brGDGTs, have a particular feature: investigators found a clearly different distribution feature of brGDGTs in aquatic sediment and catchment soil, such as in the Norwegian strait (Peterse., 2009) and the lower Yangtze River–East China shelf area (Zhu., 2011). These phenomena have also been found in some lakes and coastal sediments (Tierney and Russell, 2009; Blaga., 2010; Tierney., 2010, 2012; Loomis., 2011; Wang., 2012; Buckles., 2014; Zell., 2014; De Jonge., 2015; Weber., 2015). Moreover, in the past 180kyr years, the rebuilt MAAT was significantly lower than the actual observed value in a Scotland lake, and the MAAT in the glacial period was significantly higher than that in the interglacial period in the South China Sea; these imply the constraints of the proxy (Tyler., 2010; Dong., 2015).
Therefore, the hypothesis that brGDGTs are derived from anaquatic column or sediment has been proposed. An isotopic study will be a possible solution to confirm this hypothesis (Schouten., 2013). Owing to the high molecular weight and strong polarity of the GDGTs, their isotopic composition cannot be detected directly by gas chromatography-isotope ratio mass spectrometry (GC-IRMS). The chemical degradation of the larger GDGTs molecules into GC-amenable detectable mo- lecules followed by GC-IRMS, and offline isolation by preparative liquid chromatography followed by elemental analysis-isotope ratio mass spectrometry (EA-IRMS) are the traditional methods for the isotope study of GDGTs. Thus far, most studies on stable carbon isotope of GDGTs employed the chemical degradation methods to detect the isotope; for example, Weijers. (2010) used semi-pre- parative high-performance liquid chromatography (HPLC) to obtain purified GDGTs; then, released the alkyl moieties by HI/LiAlH4treatment. In a previous study, the stable carbon isotope relationship between total organic matter and brGDGTs was attributed to a heterotrophic mecha- nism (Weijers., 2010). Research by Lu. (2018) on the Lantian paleosol also showed similar results by BBr3/LiEt3BH treatment. In addition to the chemical de- gradation method, high-performance liquid chromatography-isotope ratio mass spectrometry (HPLC-IRMS), developed in the past years, can directly detect the stable carbon isotope of individual GDGTs (Pearson., 2016; Colcord., 2017). Compared with the HPLC-IRMS method, GC-IRMS is equipped with well-established te- chnology. Besides, most published studies on GDGTs stable carbon isotope are based on the traditional method. The development of a similar method has made results com- parison feasible, so that the chemical degradation method is still relevant.
At present, continuous and long-timescale paleoenvironment research requires the processing of numerous samples. In addition, the mixture compounds in the che- mical degradation and the large volume material required for the EA-IRMS limit related research. Regarding the batch analysis of samples, semi-preparative HPLC is inappropriate as its mechanism allows the analysis of only one sample at a time. Thus, developing an operational laboratory manipulation that would allow the separation of many samples at the same time is necessary.
In this study, a new method based on silica column chromatography was built to isolate the GDGTs into three groups (iGDGTs, brGDGTs, and other membrane lipids). Ether cleavage was further applied to estimate the purification of the separation. Meanwhile, the traditional semi- preparative HPLC was also employed for comparison.
2 Materials and Methods
The sample used in this research was peat soil collected from the surface to the 5cm below surface in Xinjiang Pro- vince, China. The samples were stored in dry ice in the field and in a refrigerator at −40℃ in the laboratory.
2.1 Lipid Extraction
The sample was freeze-dried, homogenized, and then ex- tracted ultrasonically according to the procedure modified from Li. (2013). Each sample (about 5g) was first spiked with the internal standard of C46tetraether (Huguet., 2006) and then extracted in sequence using methanol (MeOH), dichloromethane (DCM)/MeOH (1:1), and DCM, twice for each extraction solvent. The supernatant liquid was collected after centrifugation for 15min. The extracted concentrated product was dissolved in 6% KOH in MeOH under a water bath of 50℃ for 12h for saponification. Then, neutral components were recovered by- hexane. The neutral lipids were separated into three subfractions for the following steps: GDGTs analysis, in which samples must be filtered through a 0.45mm-pore-size, 4mm-diameter PTFE filter, and then HPLC analysis.
2.2 GDGTs Fractionated by Silica Gel Column Chromatography
The silica gel (100–200mesh) was successively washed with DCM and-hexane for three times. Then, it was dried at 120℃ for 12h, deactivated under room temperature for 12h, and soaked in-hexane solution for storage. About 2g silica gel was loaded on a glass column with a diameter of 4mm and length of 8cm. The top subfraction of the total neutral lipids was dried under N2stream and dissolved in a small aliquot of-hexane; it was then applied on the top of the silica gel column. Five fractions were successively collected using 20mL of the following solvent systems:-hexane (aliphatics), DCM (ketones, al- kanols, and sterols),-hexane/isopropyl alcohol (IPA) (96: 3) (iGDGTs),-hexane/IPA (96:4) (brGDGTs), and-he- xane/IPA (9:1) (other ether membrane lipids). The first two fractions were aliphatics, alkanols, and sterols. The last three fractions were dried and dissolved with-he- xane/EtOAc (84:16) for HPLC in conjunction with atmo- spheric pressure chemical ionization mass spectrometry (HPLC-APCI-MS).
2.3 GDGTs Fractionated by Semi-Preparative HPLC
One neutral lipids subfraction was separated into three fractions by 20mL-hexane (F1, aliphatics), 20mL DCM (F2, ketones, alkanols, and sterols), and 20mL MeOH (F3, total GDGTs) by silica gel chromatography. The third part was collected for further processing. After blow-drying under N2, F3 was dissolved in 50μL-hexane/EtOAc (84: 16) for the semi-preparative HPLC-APCI-MS (Agilent 6130); the instrument was equipped with a semi-prepa- rative NH2column (Econosphere, 10mm×250mm×5μm; Alltech Prevail, USA) with 50μL injection volume. The column was eluted isocratically with 10% EtOAc in-he- xane for 90min, followed by a linear gradient to 50% EtOAc in 100min, and then back to 10% EtOAc in 120 min, with a flow rate of 1mLmin−1. The system was cleaned with 10% EtOAc and re-equilibrated to initial conditions (10min each). The target fraction was collected under the Agilent G1364C collector, depending on the time window detected by the mass spectrometer (Agilent 6130), which corresponded to 27–45min for iGDGTs, 50–80min for br-GDGTs, and 80–105min for other ether membrane lipids.
2.4 Ether Cleavage
The ether bond cleavage procedure of the GDGTs followed the methods modified from Weijers. (2010) and Lu. (2018). After these methods were carried out, aliquots of the separated GDGTs groups were subjected to excess 56% HI in H2O for 4h at 150℃ to cleave the ether bonds linking the alkyl skeleton and the glycerol side chain. The resulting alkyl iodides were extracted by-hexane for four times and then reduced to corresponding hydrocarbons by LiEt3BH for 3h at 75℃ in tetrahydrofuran and cooled to room temperature. Deionized water was used to quench the excess oxidizers and reductant in the solvent (five times). Finally, the products were extracted with-hexane and purified using a silica gel column, eluting with-hexane. The extract was dried under a gentle N2stream for gas chromatography-mass spectro- metry (GC-MS) analysis.
2.5 HPLC-APCI-MS for GDGT Analysis and Quantification
The GDGT analysis was conducted following a method described elsewhere (Hopmans., 2004; Dong., 2015). The analysis using HPLC-APCI-MS included the total GDGTs extracted from the peat, three separated fractions by silica column chromatography, and semi-pre- parative HPLC. Samples were dissolved in 500μL-he- xane/EtOAc (84:16) and 20μL of the sample was injected. Separation was achieved using an Agilent 1200 liquid chromatograph equipped with an automatic injector and a Prevail Cyano column (2.1mm×150mm×3μm; Alltech, Deerfield, IL) maintained at 40℃. The GDGTs were first eluted isocratically with-hexane and EtOAc as follows: 90%-hexane and 10% EtOAc for 90min, followed by a linear gradient to 50% EtOAc in 100min, and back to 10% EtOAc in 100min (1mLmin−1) till 120min. The system was cleaned with 10% EtOAc and re-equilibrated to initial conditions (10min each). The flow rate was 0.2mLmin−1. After each analysis, the column was cleaned by back-flushing with-hexane/EtOAc (84:16) at 0.2mLmin−1for 10min. Detection was performed on an Agilent 6460 triple-quadrupole mass spectrometer with an atomspheric pressure chemical ionization (APCI) ion source. The conditions for APCI mass spectroscopy were as follows: ne- bulizer pressure, 60psi; vaporizer temperature, 400℃; drying gas (N2) flow of 5Lmin−1; temperature, 200℃; capillary voltage, −3.5kV; corona, 5μA (3.2kV). The single- ion monitoring mode was set to detect the five isoprenoid GDGTs and nine branched GDGTs (1302, 1300, 1298, 1296, 1292, 1050, 1048, 1046, 1032, 1034, 1036, 1018, 1020, 1022) and the C46GDGT internal standard (744), with a dwell time of 237ms per ion. The GDGTs were quantified by the integration of peak areas of both target GDGTs and a known concentration of internal standard. Here, the liquid chromatography–mass spectroscopy detection limit for the C46GDGT was 30pg with a signal to noise ratio of >10.
2.6 GC-MS Detection
The alkyl moieties released from the GDGTs were detected on the GC-MS instrument (TSQ8000 Thermo Quest) to confirm the structural features. The capillary column (CP Sil-5CB, 30m×0.32mm×0.25μm, J&W) was equip- ped in a Trace GC system. Helium was the carrier gas with a flow rate of 1mLmin−1. For the GC procedure, the temperature was first 90℃, then increased by 15℃min−1to 170℃ (2min), increased by 10℃min−1to 250℃, maintained at this temperature for 2min, increased by 5℃min−1to 300℃, and then maintained at this temperature for 25 min. The TSQ 8000 Evo MS operated with an electron ionization ion source at 70 ev and a scan range of50–600 with a 0.24s cycle. The temperatures of transfer tube and ion source were both 280℃.
3 Results and Discussion
3.1 Separation Effect of GDGTs by Silica Column Chromatography and Semi-Preparative HPLC
Before separation, GDGTs compositions in the studied peat sample were detected by HPLC-APCI-MS. Five isoprenoid GDGTs (Fig.1, iGDGTs 0–4 and relevant composed alkyl moieties e–h), nine branched GDGTs (Fig.1, Ia, Ib, Ic, IIa, IIb, IIc, IIIa, IIIb, IIIc and relevant composed alkyl moieties a–d), and other ether membrane lipids (possible degradation products from GDGTs,., gly- cerol monobiphytanyl glycerol diether, glycerol monobiphytanyl monoether, glycerol dibiphytanol diether (GDD) in Fig.1; Liu., 2012, 2018; Becker., 2013) are shown in Fig.2. The obtained GDGTs are divided into three kingdoms: iGDGTs, brGDGTs, and other ether membrane lipids. The brGDGTs exhibited a higher proportion in the peat, containing about 80% of the total GDGTs, a finding similar to that of a previous study on peat sediments (Wei- jers., 2011). Two methods were applied for the purification of GDGTs in this study: silica gel column chroma- tography fractionation and semi-preparative HPLC fractionation. The separation effects of these two methods on the peat sample are demonstrated in Fig.2.Generally, both approaches could achieve the fundamental purpose of iso- lating the total GDGTs into three groups. The semi-pre- parative HPLC based on the retention time exhibited a good separation of the three kinds GDGTs owing to the high sensitivity of HPLC.
Moreover, silica gel column chromatography illustrated a similar isolation effect. The elution of the silica gel co- lumn with solvents of increasing polarity allowed the se- quenced withdrawal of iGDGTs, brGDGTs, and other mem- brane lipids. The results of the elution behavior of the ex- tracted lipids under different solvent mixtures of-hexane and IPA were tested. The ratios of 96:3 for iGDGTs, 96:4 for brGDGTs, and 9:1 for other ether membrane lipids were the appropriate proportions for the GDGTs separation (Fig.2). However, because of theextremely close polarities and chemical properties between the three kinds of GDGTs, avoiding crossover in the chromatography is quite difficult. Although the isolation fractionation of GDGTs was acceptable, little tiny brGDGTs (<3%) were still found in the iGDGTs fraction, and little tiny GDDs (<4%) in the brGDGTs fraction.
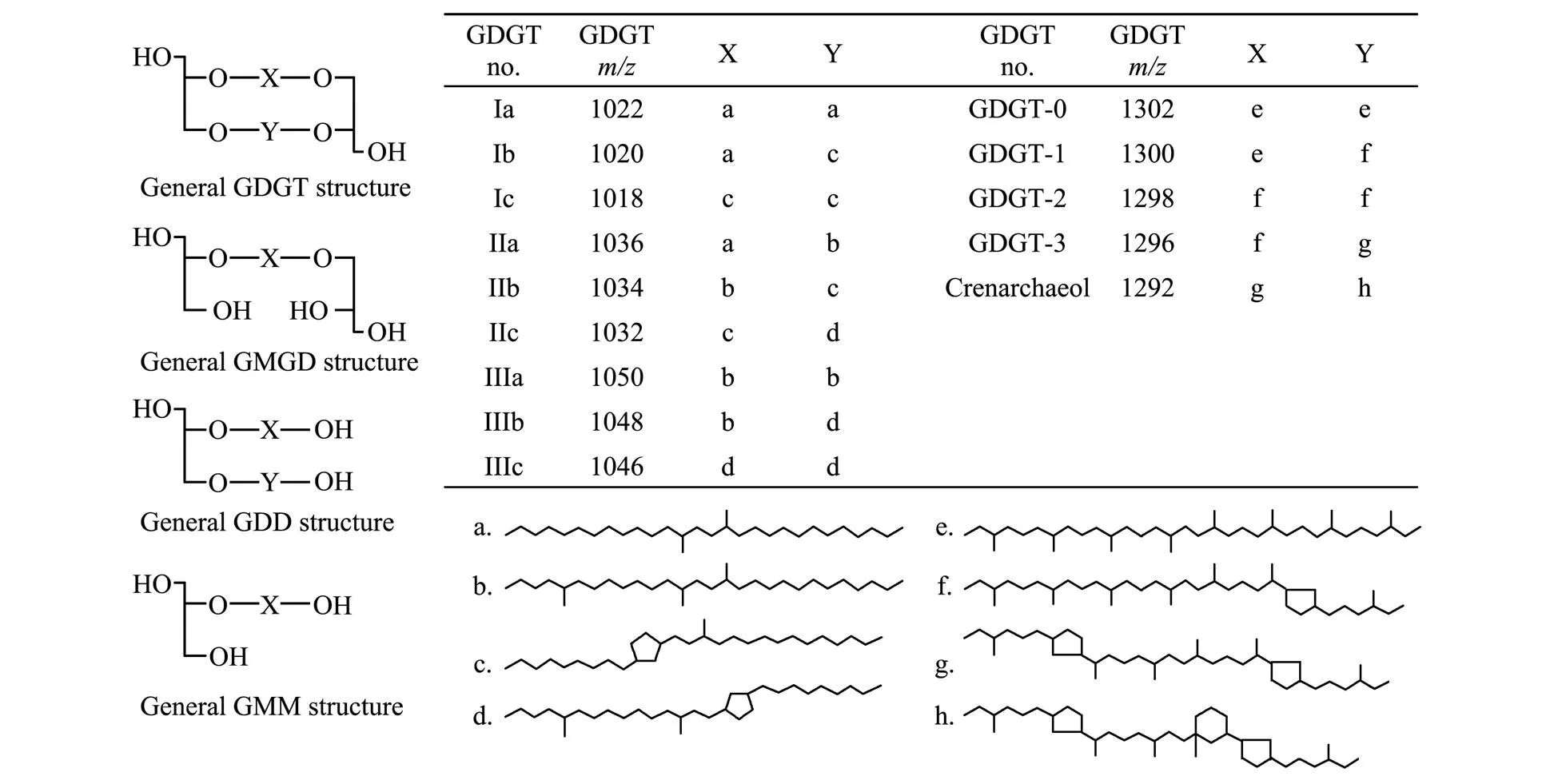
Fig.1 Basic structure of common GDGTs and possible degradation products of GDGTs.
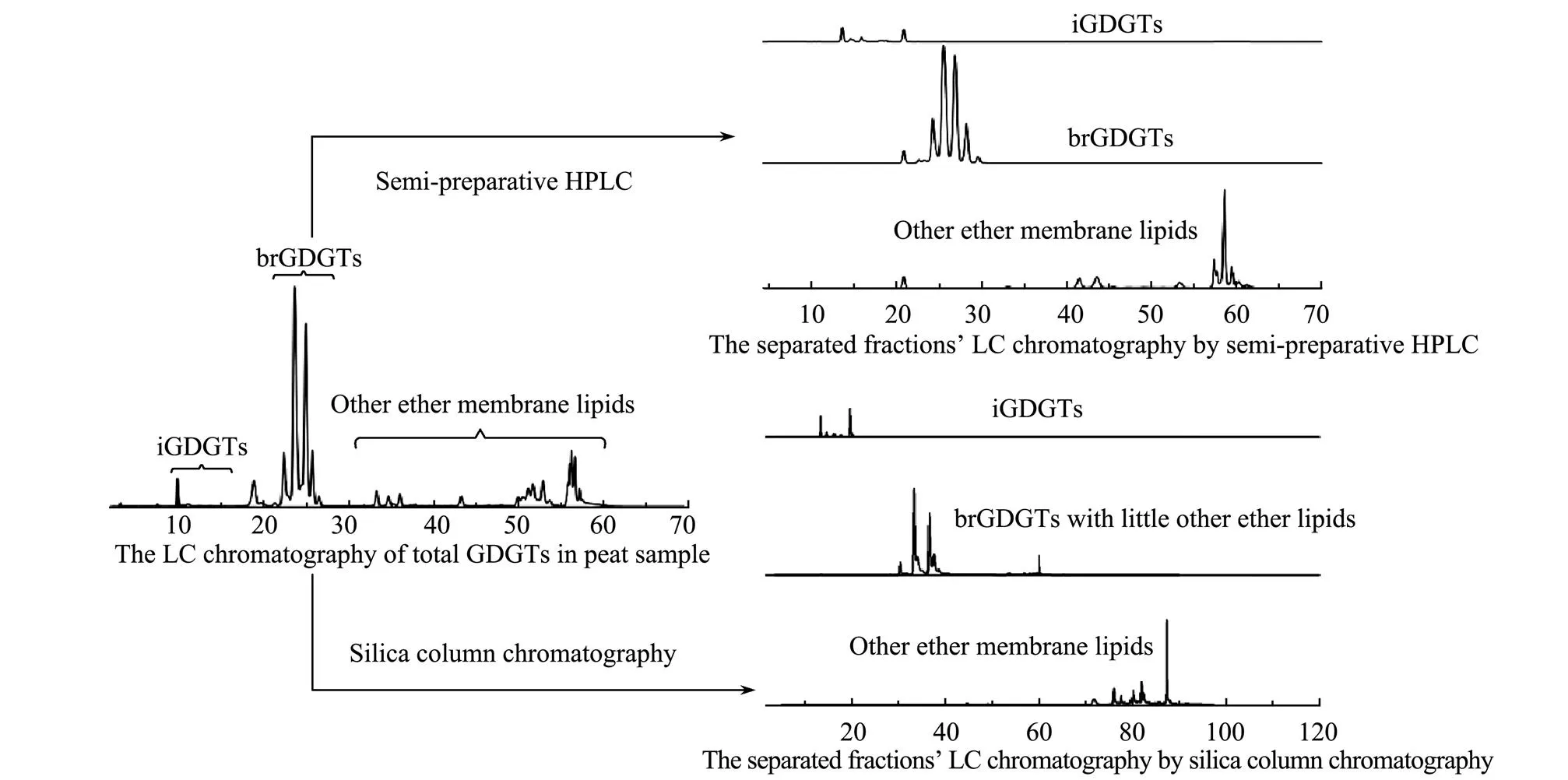
Fig.2 High-performance liquid chromatography of total GDGTs and separated fractions by semi-preparative liquid chromatography and silica gel column chromatography.
Furthermore, GDGTs with different structures would not perform similarly under the fixed mobile phase. While the solvent collection in the semi-preparative HPLC depended on the specific time window, the more sensitivity and accuracy of the HPLC system guarantees a possibility of obtaining a relatively stable and high yield.
3.2 Recovery Comparison
To evaluate the efficiency of the silica column chromatography, nine brGDGTs and GDGT-0 were used as representatives in the following calculation regarding the studied peat sample.
The brGDGTs concentrations in the peat were generally higher than those in the soil and ocean sediment, and the difference within different structural brGDGTs was huge. Taking the Xinjiang peat as an example, some concen- trations could be up to 15000ngg−1(IIa,1036) whilesome were only 3ngg−1(IIIc,1046). The brGDGTs con- centrations in the total GDGTs before fractionation were considered as the original content. The concentration results obtained from silica gel column chromatography and semi-preparative HPLC are illustrated in Fig.3. Comparing the collected brGDGTs with the total GDGTs, it is clear losses occurred in the collected brGDGTs by both methods.
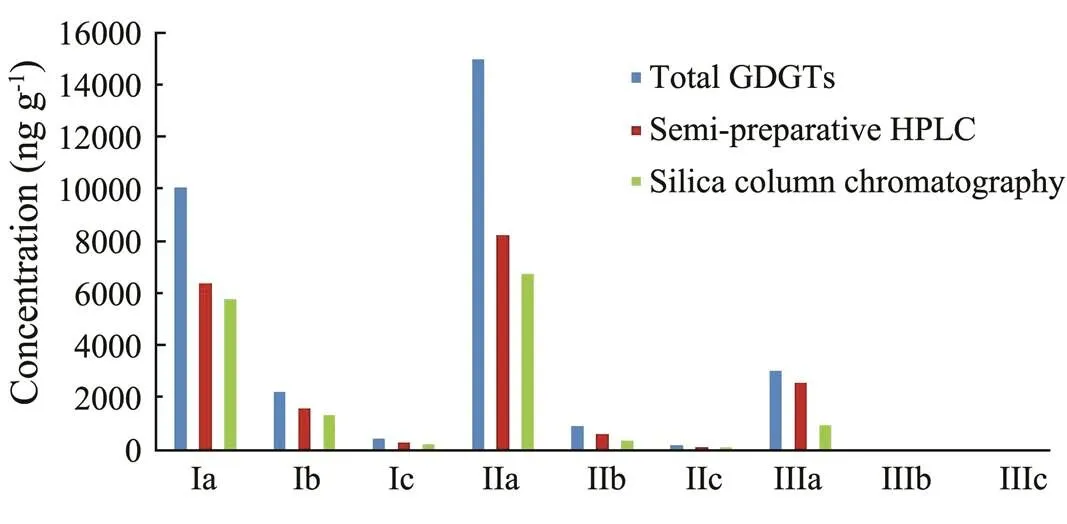
Fig.3 The brGDGTs concentrations of total GDGTs and separated fractions by semi-preparative HPLC and silica gel column chromatography.
The recoveries of each brGDGT are shown in Fig.4. Original compounds before separation were used for the efficiency assessment during the overall analytical protocol. The performances of nine brGDGTs in the semi-pre- parative HPLC were averaged at 70%, with the highest value of 85% for brGDGTs IIIa and the lowest (55%) for brGDGTs IIa. Moreover, the brGDGTs recovery from si- lica gel column chromatography was slightly lower than that from semi-preparative HPLC, with the highest yield of 70% for brGDGTs IIIc and the lowest of <20% for brGDGTs IIIb. The specific recoveries of each brGDGT are listed in the following Table 1.
Although the iGDGTs were less than the brGDGTs in the sample, we analyzed the performance of GDGT-0 as a representative. Its 69% recovery using the silica gel me- thod and 81% using the semi-preparative HPLC column were similar to the recoveries of brGDGTs using the corresponding methods.
Partial irreversible adsorption of GDGTs might exist in both the semi-preparative HPLC NH2column and silica gel column. The small injection volume and high sensiti- vity of the semi-preparative NH2column content in semi- preparative liquid chromatography were possibly the main causes of the relatively high recovery. Probably, the absorption characteristics of silica gel and the multiple trans- fer steps during the silica gel experiment would cause a high mass loss. This could account for the lower yield obtained using the silica gel method.
Although both methods could separate iGDGTs, br- GDGTs, and other membrane lipids from the total GDGTs, different recoveries were obtained for various brGDGTs. The recovery exhibited weak relationship with the concentration; similar recoveries were found in low concentrations of brGDGTs Ib, Ic, IIb, and IIc. However, explaining the special low recovery of brGDGTs IIIb in silica gel column chromatography is difficult.
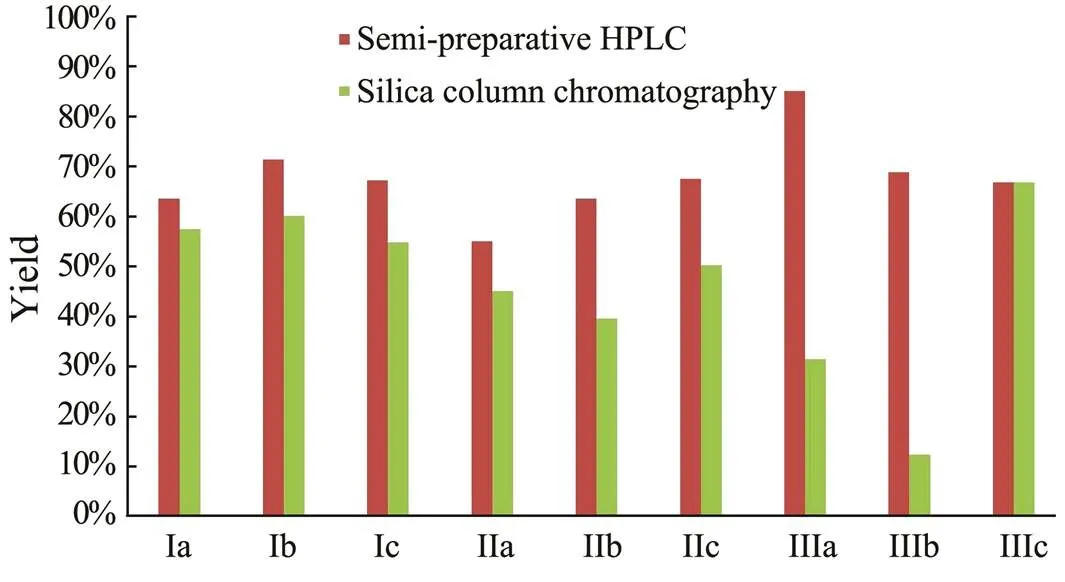
Fig.4 Recovery comparison of semi-preparative HPLC and silica gel column chromatography.
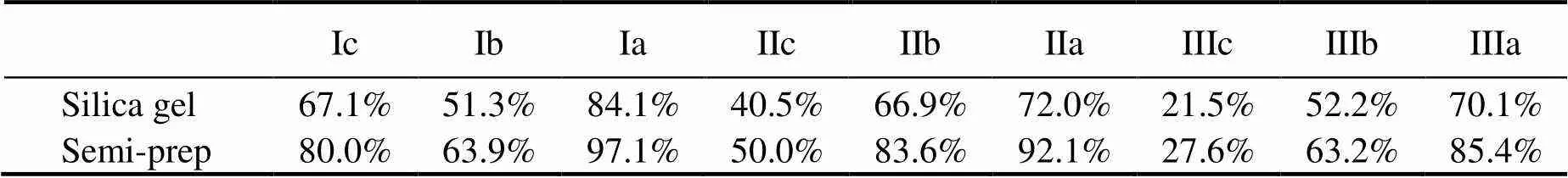
Table 1 Recoveries of different brGDGTs in the two methods
3.3 Purification Comparison
The released alkyl moieties from GDGTs after ether cleavage were detected to assess the purification of the fractionated GDGTs from silica column chromatography and semi-preparative HPLC. Theoretically, four kinds of iGDGTs-derived isoprenoid hydrocarbons and four kinds of brGDGTs-derived branched alkanes should be released. In addition, the GDGTs purification was essential to the isotopic detection.
Three isolated GDGTs fractionations from the silica gel column chromatography and the semi-preparative HPLC, as well as the corresponding GC chromatographs of hydro- carbons released by ether cleavage, are shown in Figs.5 and 6, respectively. In terms of products derived from iGDGTs collected by semi-preparative HPLC, only isopre-noid hydrocarbon ‘e’ derived from iGDGT-0 and iGDGT-1 was detected. The mass spectra of previous published data confirmed the structure of the compound (Fig.7) (Sin- ninghe Damsté., 2000; Weijers., 2010; De Jonge., 2014). Other cleavage products derived from iGDGTs,., ‘f’, ‘g’, and ‘h’ were not detected for their low contents. Additionally, four extra peaks existed in the spectra of the ether cleavage products. These materials were not compositionally related to GDGTs based on the GC-MS analysis. They may co-elute during the HPLC separation.
Coincidentally or not, indistinguishable peak mixtures also existed when the silica gel method was used. These mixtures may be caused by the insufficiency of the silica column chromatography, whose separation mainly depend- ed on the polarity differences among organic matters. After thehexane and DCM,-hexane/IPA 96:3 eluted not only iGDGTs but also many other compounds, and with similar polarities; these were all exhibited on the gas chro- matogram and thus made the chromatogram complex. The absence of ‘f’ in the semi-preparative HPLC could be accounted for the instability of ether cleavage reaction, which happened accidentally.
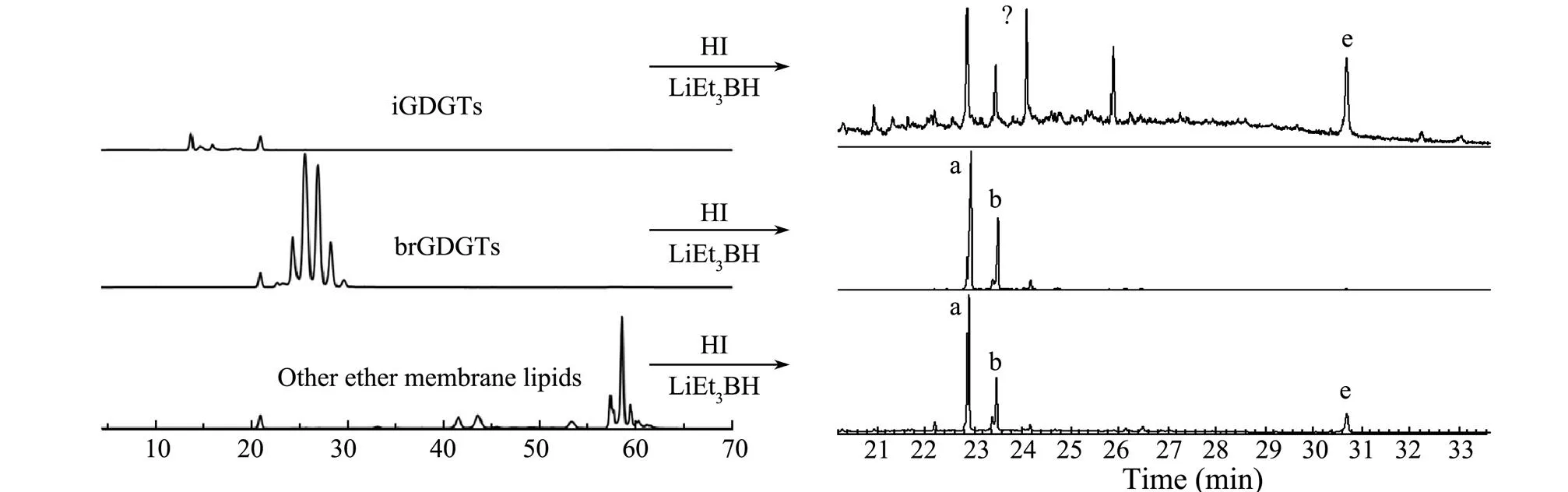
Fig.5 High-performance liquid chromatography and gas chromatography of separated GDGTs and their hydrocarbon products released after ether cleavage in semi-preparative HPLC method.
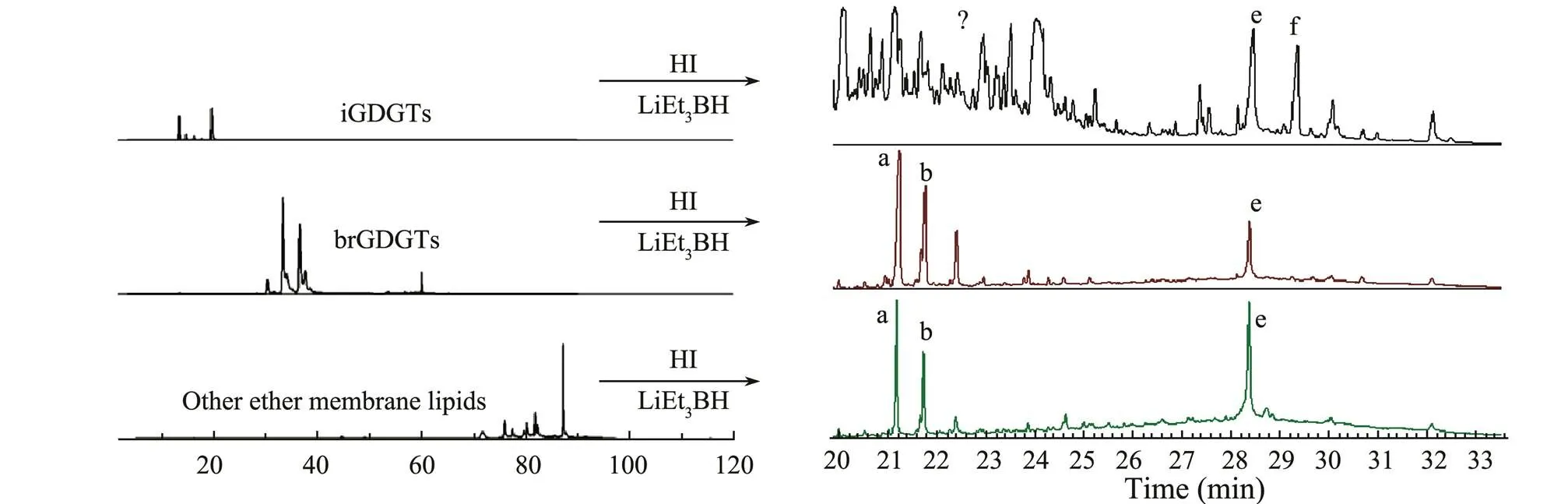
Fig.6 High-performance liquid chromatography and gas chromatography of separated GDGTs and their hydrocarbon products released after ether cleavage in silica gel method.

Fig.7 Mass spectra of compounds ‘a’, ‘b’, ‘e’, and ‘f’.
For the ether cleavage products of the second groups, the gas chromatograms were relatively pure (Figs.5 and 6). Besides the expected alkyl moieties ‘a’ and ‘b’ from br- GDGTs, an extra hydrocarbon ‘e’ was also present in ether cleavage production in the silica column collection. This was considered as a released product from the iGDDs during the silica column collection, as shown in Fig.2. Compared with the iGDGTs and their released alkyl moieties, the brGDGTs were much more concise, with little impurity, and the hydrocarbon was consistent with their parent compounds shown in the liquid chromatography.
Typical alkyl moieties ‘a’ and ‘b’ were present in the third fraction after HI/LiEt3BH treatment (Figs.5 and 6). Generally, the hydrocarbons ‘a’ and ‘b’ were believed to be from brGDGTs. Their presence in the third fraction im- plies that this fraction mainly comprised the natural degradation products of iGDGTs and brGDGTs, such as gly- cerol monobiphytanyl glycerol diether, GDD, and glycerol monobiphytanol monoether (structures shown in Fig.1). Same carbon skeleton as that in the GDGTs produced the same hydrocarbon ‘a’, and ‘b’ after ether cleavage from these GDDs or other structural ether lipids. Hydrocarbon ‘e’ was also detected in the third fraction of the silica gel column. Although iGDGTs were less than the brGDGTs in the peat sample, iGDDs might still have existed in the third groups. In addition, the yields of the ether cleavage reactions of the iGDGTs and brGDGTs were different: on average, 70% for iGDGTs and 40% for brGDGTs. These could account for the high abundance of hydrocarbon ‘e’ in the third fraction.
The ether cleavage result indicates the GDGTs group separation was necessary, especially for the isotope study of GDGTs, because the same cleavage products were found in the third groups, especially the products ‘a’ and ‘b’. Both iGDGTs fractionation and GDD fractionation could produce these hydrocarbons. Without GDGTs group sepa- ration, the isotopes of iGDGTs and brGDGTs would be mixture information. Remarkably, co-elution happened even in the HPLC separation, especially in the iGDGTs fractionation.
Although the semi-preparative HPLC exhibited a slightly better separation performance, its special equipment requirement and time consumption makes it impractical for the large-number batch experiments in paleoclimatic change studies. Considering the merits of rapidness, easy laboratory operation, no special equipment requirement, and acceptable separation results, silica column chromatography for the isolation of different groups of GDGTs is practicable. Although the semi-preparative HPLC featured automatic operation, repetitive transfer processes would take up too much time. In addition, the collection process was slow due to the limitation by the mobile phase. Therefore, the semi-preparative HPLC is more appropriate in high- precision analysis,., specific compound study, while the silica column chromatography is more suitable for batch samples,., paleoclimate and paleoenvironment studies.
4 Conclusions
In this study, GDGTs separation effect by silica column chromatography was compared with that by the semi-pre- parative HPLC method using a peat sample.
Using the silica column chromatography, 60% was obtained, a little lower than that obtained by the semi-pre- parative HPLC (70%). The alkyl moieties released after ether cleavage from the fractionated GDGTs indicated the separation efficiency. Some compositions unrelated to GDGTs were co-eluted in the iGDGTs collected by silica column chromatography. Meanwhile, the finding of same branched alkanes cleavage products (., ‘a’, ‘b’) in the third groups imply caution should be taken in the isotopic study of the GDGTs in the sedimentary records. Overall, the HI/LiEt3BH treatment results indicated similar purification effects between the silica column chromatography method and the semi-preparative HPLC approach. The merits of time saving, easy operation, and low cost make the silica column chromatography method a better choice for batch experiments in paleoclimate reconstruction stu- dies.
Despite the good performance of the new method, further research covering various samples is needed to expand the application scope and test the accuracy under different circumstances.
Acknowledgements
We thank Dr. Liang Dong for providing the peat sample from Xinjiang. This research was supported by the National Natural Science Foundation of China (Nos. 416730 42, 41876042, and 41776049).
Becker, K. W., Lipp, J. S., Zhu, C., Liu, X. L., and Hinrichs, K. U., 2013. An improved method for the analysis of archaeal and bacterial ether core lipids., 61: 34- 44.
Blaga, C. I., Reichart, G. J., Schouten, S., Lotter, A. F., Werne, J. P., Kosten, S., Mazzeo, N., Lacerot, G., and Sinninghe Damsté, J. S., 2010. Branched glycerol dialkyl glycerol tetraethers in lake sediments: Can they be used as temperature and pH proxies?, 41: 1225-1234.
Buckles, L. K., Weijers, J. W. H., Tran, X. M., Waldron, S., and Sinninghe Damsté, J. S., 2014. Provenance of tetraether mem-brane lipids in a large temperate lake (Loch Lomond, UK): Im- plications for glycerol dialkyl glycerol tetraether (GDGT)- based palaeo thermometry., 11: 5539-5563.
Colcord, D. E., Pearson, A., and Brassell, S. C., 2017. Carbon isotopic composition of intact branched GDGT core lipids in Greenland lake sediments and soils., 110:25-32.
De Jonge, C., Hopmans, E. C., Zell, C. I., Schouten, S., and Sinninghe Damsté, J. S., 2014. Occurrence and abundance of 6-methyl branched glycerol dialkyl glycerol tetraethers in soils: Implications for palaeoclimate reconstruction., 141: 97-112.
De Jonge, C., Stadnitskaia, A., Hopmans, E. C., Cherkashov, G., Fedotov, A., Streletskaya, I. D., Vasiliev, A. A., and Sinninghe Damsté, J. S., 2015. Drastic changes in the distribution of branched tetraether lipids in suspended matter and sediments from the Yenisei River and Kara Sea (Siberia): Implications for the use of brGDGT-based proxies in coastal marine sediments., 165: 200-225.
DeLong, E. F., King, L. L., Massana, R., Cittone, H., Murray, A., Schleper, C., and Wakeham, S. G., 1998. Dibiphytanyl etherli- pids in nonthermophilic Crenarchaeotes., 64: 1133-1138.
Dong, L., Li, Q. Y., Li, L., and Zhang, C. L., 2015. Glacial- interglacial contrast in MBT/CBT proxies in the South China Sea: Implications for marine production of branched GDGTs and continental teleconnection., 79: 74- 82.
Hoefs, M. E. L., Schouten, S., King, L. L., Wakeham, S. G., De Leeuw, J. W., and Sinninghe Damsté, J. S., 1997. Ether lipids of planktonic archaea in the marine water column., 63: 3090-3095.
Hopmans, E. C., Weijers, J. W. H., Schefuss, E., Herfort, L., Sin- ninghe Damsté, J. S., and Schouten, S., 2004. A novel proxy for terrestrial organic matter in sediments based on branched and isoprenoid tetraether lipids., 24: 107-116.
Li, L., Li, Q. Y., Tian, J., Wang, H., and Wang, P. X., 2013. Low latitude hydro-climatic changes during the Plio–Pleistocene: Evidence from high resolution alkane records in the South China Sea., 78: 209-224.
Liu, X. L., Lipp, J. S., Birgel, D., Summons, R. E., and Hinrichs, K. U., 2018. Predominance of parallel glycerol arrangement in archaeal tetraethers 375 from marine sediments: Structural features revealed from degradation products., 115: 12-23.
Liu, X. L., Lipp, J. S., Schröder, J. M., Summons, R. E., and Hin- richs, K. U., 2012. Isoprenoid glycerol dialkanol diethers: A series of novel archaeal lipids in marine sediments., 43: 50-55.
Loomis, S., Russell, J. M., and Sinninghe Damsté, J. S., 2011. Distributions of branched GDGTs in soils from western Ugan- da and implications for a lacustrine paleothermometer., 42: 739-751.
Lu, H., Liu, W., and Sheng, W., 2018. Carbon isotopic composition of branched tetraether membrane lipids in a loess-paleosol sequence and its geochemical significance., 504: 150-155.
Macalady, J. L., Vestling, M. M., Baumler, D., Boekelheide, N., Kaspar, C. W., and Banfield, J. F., 2004. Tetraether-linked mem- brane monolayers inspp.: A key to survival in acid., 8: 411-419.
Pearson, A., Hurley, S. J., Walter, S. R. S., Kusch, S., Lichtin, S., and Zhang, Y. G., 2016. Stable carbon isotope ratios of intact GDGTs indicate heterogeneous sources to marine sediments., 181: 18-35.
Peterse, F., Kim, J. H., Schouten, S., Klitgaard Kristensen, D., Koç, N., and Sinninghe Damsté, J. S., 2009. Constraints on the application of the MBT/CBT paleother mometer in high latitude environments (Svalbard, Norway)., 40: 692-699.
Schouten, S., Hopmans, E. C., Schefuß, E., and Sinninghe Damsté, J. S., 2002. Distributional variations in marine crenarchaeotal membrane lipids: A new tool for reconstructing ancient sea water temperature?,204: 265- 274.
Schouten, S., Sinninghe Damsté, J. S., and Hopmans, E. C., 2013. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review., 54: 19-61.
Schouten, S., Van Der Meer, M. T. J., Hopmans, E. C., Rijpstra, W. I. C., Reysenbach, A. L., Ward, D. M., and Sinninghe Damsté, J. S., 2007. Archaeal and bacterial glycerol dialkyl gly- cerol tetraether lipids in hot springs of Yellowstone National Park (USA)., 73: 6181- 6191.
Sinninghe Damsté, J. S., Hopmans, E. C., Pancost, R. D., Schou- ten, S., and Geenevasen, J. A. J., 2000. Newly discovered non- isoprenoid dialkyl diglycerol tetraether lipids in sediments., 17: 1683-1684.
Sinninghe Damsté, J. S., Rijpstra, W. I. C., Hopmans, E. C., Schou- ten, S., Balk, M., and Stams, A. J. M., 2007. Structural cha- racterization of diabolic acid-based tetraester, tetraether and mixed ether/ester, membrane-spanning lipids of bacteria from the order Thermotogales., 188: 629- 641.
Tierney, J. E., and Russell, J. M., 2009. Distributions of bran- ched GDGTs in a tropical lake system: Implications for lacus- trine application of the MBT/CBT paleoproxy., 40: 1032-1036.
Tierney, J. E., Russell, J. M., Eggemont, H., Hopmans, E. C., Verschuren, D., and Sinninghe Damsté, J. S., 2010. Environmental controls on branched tetraether lipid distributions in tropical East African lake sediments: A new lacustrine paleothermometer?, 74: 4902- 4918.
Tierney, J. E., Schouten, S., Pitcher, A., Hopmans, E. C., and Sinninghe Damsté, J. S., 2012. Core and intact polar glycerol dialkyl glycerol tetraethers (GDGTs) in Sand Pond, Warwick, Rhode Island (USA): Insights into the origin of lacustrine GDGTs., 77: 561-581.
Tyler, J. J., Nederbragt, A. J., Jones, V. J., and Thurow, J. W., 2010. Assessing past temperature and soil pH estimated from bacterial tetraether membrane lipids: Evidence from the recent lake sediments of Lochnar, Scotland., 115: G01015.
Wang, H., Liu, W., Zhang, C. L., Wang, Z., Wang, J., Liu, Z., and Dong, H., 2012. Distribution of glycerol dialkyl glycerol tetraethers in surface sediments of Lake Qinghai and surroun- ding soil., 47: 78-87.
Weber, Y., De Jonge, C., Rijpstra, W. I. C., Hopmans, E. C., Stad- nitskaia, A., Schubert, C. J., Lehmann, M. F., Sinninghe Da- msté, J. S., and Niemann, H., 2015. Identification and carbon isotope composition of a novel branched GDGT isomer in lake sediments: Evidence for lacustrine branched GDGT pro- duction., 154: 118-129.
Weijers, J. W. H., Schouten, S., Den Donker, J. C., Hopmans, E. C., and Sinninghe Damsté, J. S., 2007. Environmental controls on bacterial tetraether membrane lipid distribution in soils., 71: 703-713.
Weijers, J. W. H., Steinmann, P. H., Hopmans, E. C., Schouten, S., and Sinninghe Damsté, J. S., 2011. Bacterial tetraether mem-brane lipids in peat and coal: Testing the potential of the MBT–CBT temperature proxy for climate reconstruction., 42: 477-486.
Weijers, J. W. H., Wiesenberg, G. L. B., Bol, R., Hopmans, E. C., and Pancost, R. D., 2010. Carbon isotopic composition of branched tetraether membrane lipids in soils suggest a rapid turnover and a heterotrophic life style of their source orga- nism(s).,7: 2959-2973.
Zell, C., Kim, J. H., Hollander, D., Lorenzoni, L., Baker, P., Silva, C. G., Nittrouer, C., and Sinninghe Damsté, J. S., 2014. Sources and distributions of branched and isoprenoid tetraether lipids on the Amazon shelf and fan: Implications for the use of GDGT-based proxies in marine sediments., 139: 293-312.
Zhang, Y. G., Zhang, C. L., Liu, X. L., Li, L., Hinrichs, K. U., and Noakes, J. E., 2011. Methane index: A tetraether archaeal li- pid biomarker indicator for detecting the instability of marine gas hydrates., 307: 525- 534.
Zhu, C., Weijers, J. W. H., Wagner, T., Pan, J. M., Chen, J. F., and Pancost, R. D., 2011. Sources and distributions of tetraether lipids in surface sediments across a large river-dominated continental margin., 42: 376-386.
. E-mail: lilitju@tongji.edu.cn
September 20, 2019;
February 17, 2020;
March 4, 2020
(Edited by Chen Wenwen)
 Journal of Ocean University of China2020年5期
Journal of Ocean University of China2020年5期
- Journal of Ocean University of China的其它文章
- Phaeocystis globosa Bloom Monitoring: Based on P. globosa Induced Seawater Viscosity Modification Adjacent to a Nuclear Power Plant in Qinzhou Bay, China
- Effect of pH, Temperature, and CO2 Concentration on Growth and Lipid Accumulation of Nannochloropsis sp. MASCC 11
- Fuzzy Sliding Mode Active Disturbance Rejection Control of an Autonomous Underwater Vehicle-Manipulator System
- The 9–11 November 2013 Explosive Cyclone over the Japan Sea- Okhotsk Sea: Observations and WRF Modeling Analyses
- Real-Time Position and Attitude Estimation for Homing and Docking of an Autonomous Underwater Vehicle Based on Bionic Polarized Optical Guidance
- Characterization of the Complete Mitochondrial Genome of Arius dispar (Siluriformes: Ariidae) and Phylogenetic Analysis Among Sea Catfishes
