Mucosal-associated invariant T cells in hepatitis B virus-related liver failure
Hong Xue, Han Li, Lin-Ling Ju, Xu-Dong Han, Tiao-Chun Cheng, Xi Luo, Lin Chen, Jian-Guo Shao, Yong-Jun She, Zhao-Lian Bian
Abstract
Key words: Mucosal-associated invariant T cells; Chronic hepatitis B; Liver failure; Prognosis; Hepatitis B virus
INTRODUCTION
Liver failure is a life-threatening clinical syndrome caused by multiple factors, leading to severe impairment or decompensation of synthesis, detoxification, metabolism and biotransformation, mainly presenting a group of clinical manifestations such as jaundice, coagulopathy, hepatorenal syndrome, hepatic encephalopathy and ascites[1]. Liver failure is a devastating disease with high morbidity and mortality. In China, the main cause of liver failure is hepatitis B virus (HBV) infection[2]. It develops rapidly and has extremely poor prognosis[3], so judging the severity and predicting the prognosis of liver failure are important.
Although there are various indicators or scoring systems for assessing the severity and prognosis in patients with liver failure, such as alanine aminotransferase, aspartate transaminase, prothrombin activity (PTA), model for end-stage liver disease (MELD) score and MELD-sodium (MELD-Na) score, these conventional scoring systems do not include extrahepatic organ failure and systemic inflammation, which limits their prognostic accuracy in liver failure. Because of the absence of gold standard markers for prognosis of liver failure[4], it is important to find additional reliable markers for predicting prognosis, enabling patients with poor prognosis to receive liver transplantation earlier to improve survival.
Mucosal-associated invariant T (MAIT) cells, the most abundant T cell subset that responds to bacteria in humans, are characterized by the invariant T-cell receptor (TCR) chain, Vα7.2-Jα33, and show high expression of the natural killer cell marker CD161. MAIT cells are found throughout the body, especially in human blood and liver. MAIT cells mediate protective immunity against infection and are increasingly being recognized for their importance in microbial immunity. As one of the largest T cell populations in the human liver, MAIT cells act as a “firewall” and play an important role in the front line of defense in the liver. MAIT cells are involved in the development of various liver diseases[5]. They can be activated in an antigen- and cytokine-dependent manner in chronic inflammatory liver disease[6]. Recent studies have reported that MAIT cells in blood and liver in patients with chronic liver inflammatory disease, including alcoholic or nonalcoholic fatty liver disease, cirrhosis, autoimmune liver disease and chronic hepatitis C and D[7-9]are decreased dramatically, but the phenotype of MAIT cells is more activated or shows functional impairment in different types of liver disease. However, circulating MAIT cells are not reversed after direct-acting-antiviral-mediated clearance of hepatitis C virus (HCV)[10]. Further studies explored the mechanism of MAIT cell involvement in liver inflammatory disease, including promoting liver inflammation and fibrosis dependent on monocytederived macrophages, maintaining intestinal mucosal permeability[11]and reacting with biliary epithelium and liver B cells[12]. From previous studies, tumor-infiltrating MAIT cells are a prognostic factor for hepatocellular carcinoma[13]and colorectal cancer[14]. Circulating MAIT cells are also a biomarker for response to standard treatment in primary biliary cholangitis[15]and immune thrombocytopenia[16]and prognosis in cervical cancer[17].
There are various reports about the frequency of circulating MAIT cells in patients with HBV infection. Yong and colleagues found that frequency of MAIT cells was decreased, presenting dysfunction in chronic hepatitis B (CHB) infection[18]. Boeijen et al[12]reported that MAIT cells were more activated and could be reversed by antiviral therapy, but the frequency of circulating MAIT cells was not decreased in CHB infection. In acute liver failure, it was reported that hepatic MAIT cells accumulated in the parenchyma, but the circulating MAIT cells were not detected and the etiology of liver failure was not revealed[19]. In China, the hepatitis B infection is the major cause of liver failure and T-cell-mediated immune injury plays a critical role in the pathogenesis of HBV-related liver failure. In this paper, we evaluated the change of circulating MAIT cells and its prognostic value in patients with HBV-related liver failure and explored the possible mechanism.
MATERIALS AND METHODS
Patient data
A total of 173 participants were enrolled, including 40 healthy controls, 48 CHB patients and 85 liver failure patients. We excluded 30 non-HBV related liver failure patients and enrolled 55 HBV related liver failure patients (Figure 1). Liver failure was diagnosed and treated according to the Guidelines in China[20]. CHB patients (n= 48) were diagnosed according to the American Association for the Study of Liver Disease 2018 guidelines for hepatitis B[21]. HBV-related liver failure (n= 55) was defined as liver failure accompanied with HBV activity, which was considered as HBV-DNA positive, and without other causes of liver failure. None of the patients in the CHB and HBVrelated liver failure groups received any antiviral therapy before the study, or antiviral drugs were discontinued for > 6 mo before the study. The research process is shown in a flow chart (Figure 1), and the demographic and clinical characteristics of the participants are presented in Table 1. This study was approved by the Ethical Committee of Nantong Third People’s Hospital, Nantong University, and patients gave signed informed consent.
Peripheral blood mononuclear cells isolation
Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation with GE Healthcare Ficoll-Paque PLUS Media (Thermo Scientific, Rosemont, IL, United States) as our previous study described[22]. Five milliliters of peripheral blood was centrifuged for 10 min at 300 ×gat 20 °C. The buffy coat interphase layer wascollected and then placed in a new 1.5-mL centrifuge tube. The buffy coat was mixed with an equal volume of phosphate-buffered saline. Then, 3 mL Ficoll was placed in a 15-mL centrifuge tube; the diluted buffy coat was layered on top of it; and the tube was centrifuged for 30 min at 400 ×gwith no break at room temperature. The PBMC layer was transferred and washed by phosphate-buffered saline. PBMCs were collected after centrifugation for 10 min at 300 ×gand stored in fetal bovine serum (Cell Sciences, Canton, MA, United States) with 10% dimethyl sulfoxide (Beyotime Biotechnology, Shanghai, China) in liquid nitrogen for further analysis.
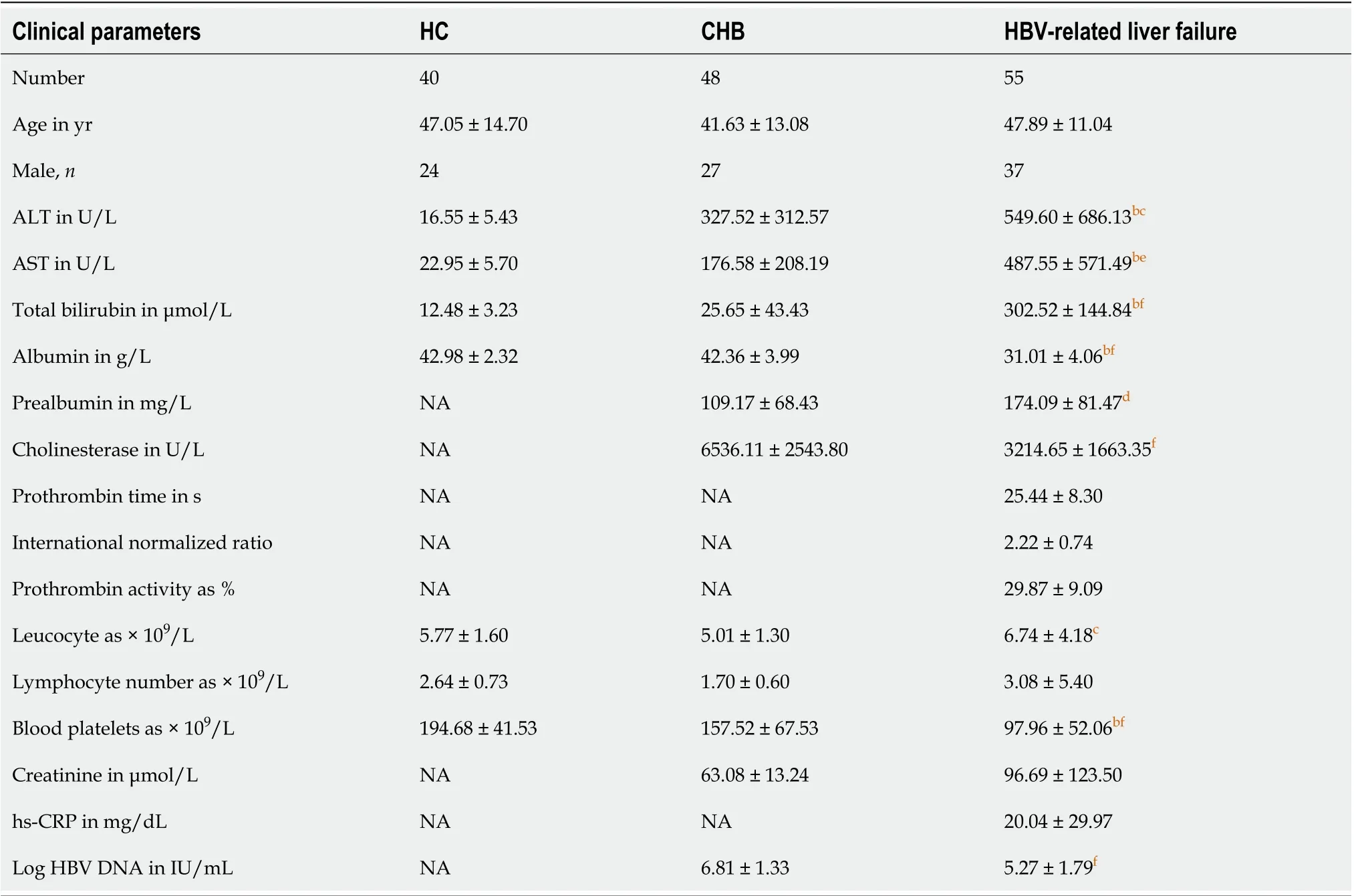
Table 1 Characteristics of healthy controls and chronic hepatitis B and hepatitis B virus-related liver failure patients
Flow cytometry
PBMCs were stained with combinations of the following antibodies: anti-TCRVα7.2-phycoerythrin (PE; BD Biosciences, Franklin Lakes, NJ, United States), anti-CD161-allophycocyanin (APC; BD Biosciences) and anti-CD3-fluorescein isothiocyanate (FITC; BD Biosciences). Flow cytometry was performed using FACS Calibur (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, San Carlos, CA, United States).
Plasma cytokine detection
Interleukin (IL)-7, IL-12p70, IL-18 and interferon (IFN)-α were measured using the Human Magnetic Luminex Assay Kit (R&D Systems, Minneapolis, MN, United States) in human plasma samples. The results were read and analyzed on Luminex MAGPIX Instrument System (Luminex, Austin, TX, United States).
Statistical analysis
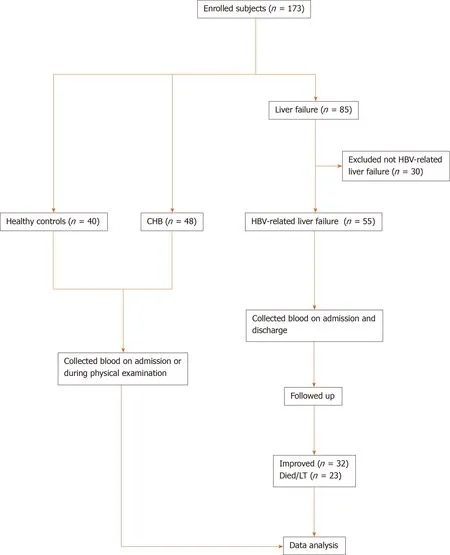
Figure 1 Flow chart for patient enrollment and analysis. Diagram showing the general process from enrolling patients to the final analysis. CHB: Chronic hepatitis B; HBV: Hepatitis B virus.
All data are presented as mean ± standard deviation. Analysis in patients before and after therapy was done by pairedttest. One-way analysis of variance was used to analyze differences between more than two groups. The comparison of two groups was carried out byttest. Statistical evaluations were conducted with GraphPad Prism software version 8.0 (La Jolla, CA, United States), andP< 0.05 was considered statistically significant.
RESULTS
Characteristics of healthy controls and patients with CHB and HBV-related liver failure
The detailed clinical characteristics of the participants, including healthy controls (HCs) (n= 40), patients with CHB (n= 48) and patients with HBV-related liver failure (n= 55), are presented in Table 1. There were no differences in age and gender between patients with CHB, patients with HBV-related liver failure and HCs. The levels of alanine aminotransferase (HBV-related liver failurevsHC: 549.60 ± 686.13vs16.55 ± 5.43,P< 0.0001; HBV-related liver failurevsCHB: 549.60 ± 686.13vs327.52 ± 312.57,P< 0.05), aspartate transaminase (HBV-related liver failurevsHC: 487.55 ± 571.49vs22.95 ± 5.70,P< 0.0001; HBV-related liver failurevsCHB: 487.55 ± 571.49vs176.58 ± 208.19,P< 0.001) and total bilirubin (HBV-related liver failurevsHC: 302.52 ± 144.84vs12.48 ± 3.23,P< 0.0001; HBV-related liver failurevsCHB: 302.52 ± 144.84vs25.65 ± 43.43,P< 0.0001) were dramatically increased in HBV-related liver failure group compared to HCs and CHB group. The levels of albumin (HBV-related liver failurevsHC: 31.01 ± 4.06vs42.98 ± 2.32,P< 0.0001; HBV-related liver failurevsCHB: 31.01 ± 4.06vs42.36 ± 3.99,P< 0.0001) and blood platelets (HBV-related liver failurevsHC: 97.96 ± 52.06vs194.68 ± 41.53,P< 0.0001; HBV-related liver failurevsCHB: 97.96 ± 52.06vs157.52 ± 67.53,P< 0.0001) were significantly decreased in HBV-related liver failure group compared to HCs and CHB group (Table 1).
Circulating MAIT cells were dramatically decreased in HBV-related liver failure
Circulating MAIT cells were defined as CD3+CD161+TCR Vα7.2+. The strategy for flow cytometry of MAIT cells is illustrated in Figure 2A. We counted the percentage of circulating MAIT cells in CD3+T cells and the number of circulating MAIT cells in 106PBMCs in patients with HBV-related liver failure, patients with CHB and HCs. The percentage and number of circulating MAIT cells in HBV-related liver failure patients (percentage: 2.00 ± 1.22vs5.19 ± 1.27%,P< 0.0001; number: 5.47 ± 4.93vs84.43 ± 19.59,P< 0.0001) and CHB (percentage: 3.59 ± 0.87vs5.19 ± 1.27%,P< 0.0001; number: 48.26 ± 15.45vs84.43 ± 19.59,P< 0.0001) were significantly lower than in HCs. Circulating MAIT cells exhibited a significant decrease in HBV-related liver failure patients compared with CHB patients (percentage: 2.00 ± 1.22vs3.59 ± 0.87%,P< 0.0001; number: 5.47 ± 4.93vs48.26 ± 15.45,P< 0.0001) (Figure 2B and C).
Quantity of circulating MAIT cells was associated with severity in HBV-related liver failure patients
Patients with HBV-related liver failure were divided into two groups according to the level of PTA: Early stage (n= 28) and middle/late stage (n= 27). The percentage and number of circulating MAIT cells were analyzed. A significant reduction in percentage (Figure 3A) and number (Figure 3B) of MAIT cells was found in patients with middle/late-stage disease compared with early stage. We analyzed the correlation between MAIT cells and parameters in Table 1, and data showed that PTA was not associated with percentage of MAIT cells (Figure 3C) but correlated with MAIT cell counts (P= 0.0321) (Figure 3D). Other parameters were not associated with the percentage or number of circulating MAIT cells in HBV-related liver failure (data not shown).
Partial recovery of circulating MAIT cells in surviving HBV-related liver failure patients
We collected PBMCs from 18 patients before and after therapy, including 13 surviving patients and five patients who died or received liver transplantation. The indicated time for post-therapy was defined as the endpoint of conservative treatment in patients who were cured, died or received liver transplantation. We analyzed the variation in percentage and number of circulating MAIT cells in these patients. In the survival group (n= 13), circulating MAIT cells were partially recovered after disease improvement not only in percentage (4.01 ± 1.21vs2.04 ± 0.95%,P< 0.0001) (Figure 4A), but also in cell count (17.24 ± 8.56vs7.41 ± 4.99,P< 0.0001) (Figure 4B) and they were still lower than in HCs, especially MAIT cell count (Figure 4A and B). However, the level of circulating MAIT cells showed no difference in before and after conservative treatment among those who died and received liver transplantation (n= 5) (Figure 4C and D).
Lower circulating MAIT cells indicated poor prognosis in HBV-related liver failure
Based on the outcome, we divided the HBV-related liver failure patients into the survival group (n= 32) and died/liver transplanted group (n= 23). Comparison of MAIT cells between them showed that the proportion (2.29 ± 1.01vs1.58 ± 1.38%,P< 0.05) (Figure 5A) and number of circulating MAIT cells (7.30 ± 5.70vs2.94 ± 1.47,P< 0.001) (Figure 5B) were significantly higher in the survival group than the died/LT group. We followed up the 55 HBV-related liver failure patients for 2-90 d, then the patients were divided into two groups, low expression (n= 27) and high expression (n= 28) of MAIT cells based on median proportion or cell counts of circulating MAIT cells. The Kaplan–Meier curve showed that lower expression of circulating MAIT cells (both percentage and cell count) predicted poor overall survival in HBV-related liver failure patients (P< 0.01) (Figure 5C and D). We compared the prognostic value of MELD score with MAIT cells (proportion and counts) and showed using receiver operating characteristic curve data that the proportion of MAIT cells can better predict the prognosis of HBV-related liver failure patients than MELD score and MAIT cell counts (Figure 6).
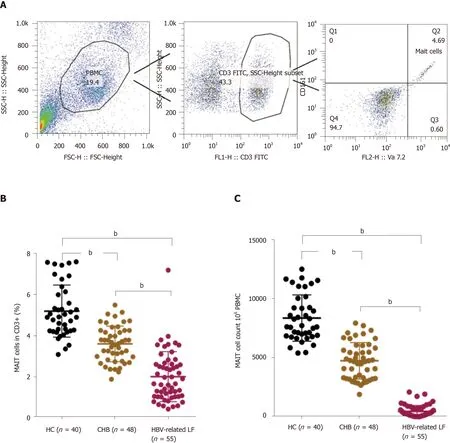
Figure 2 Circulating mucosal-associated invariant T cells were dramatically decreased in hepatitis B virus-related liver failure. A: The strategy for flow cytometry of mucosal-associated invariant T (MAIT) cells; B: The percentage of circulating MAIT cells in healthy controls (HC, n = 40), chronic hepatitis B (CHB, n = 48) patients and hepatitis B virus (HBV)-related liver failure (LF) patients (n = 55); C: Circulating MAIT cell count in 106 peripheral blood mononuclear cells in HCs and CHB and HBV-related liver failure patients. bP < 0.0001.
Plasma levels of IL-12 and IL-18 were increased in patients with HBV-related liver failure
It is well recognized that MAIT cells can be activated and exhausted by inflammatory cytokines, including IL-12, IL-18, IL-7 and IFN-α[23,24]. To explore the possible mechanism of decreased circulating MAIT cells in HBV-related liver failure, we detected these inflammatory cytokines in the plasma of HBV-related liver failure patients (n= 46) and HCs (n= 40). The level of IL-12 (20.26 ± 5.42 pg/mLvs17.76 ± 2.79 pg/mL,P= 0.01) and IL-18 (1470.05 ± 1525.38 pg/mLvs362.99 ± 109.64 pg/mL,P< 0.0001) were increased in HBV-related liver failure patients compared to HCs (Figure 7A and B). However, there was no difference in IL-7 and IFN-α between the two groups (Figure 7C and D). These data indicated that IL-12 and IL-18 contributed to the depletion of circulating MAIT cells in HBV-related liver failure patients.
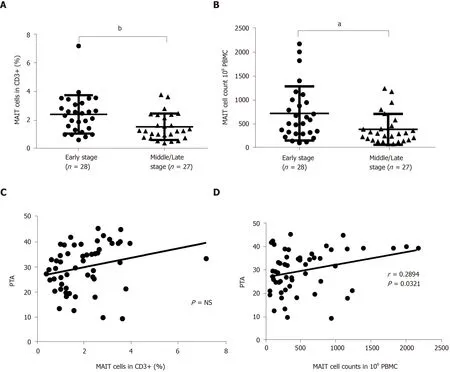
Figure 3 Quantity of circulating mucosal-associated invariant T cells was associated with severity of hepatitis B virus-related liver failure. A, B: The percentage (A) and number (B) of circulating mucosal-associated invariant T (MAIT) cells in early- (n = 28) and middle/late-stage (n = 27) hepatitis B virus (HBV)-related liver failure; C, D: Correlation between prothrombin activity (PTA) and percentage (C) or number (D) of circulating MAIT cells in patients with HBV-related liver failure. aP < 0.05, bP < 0.01. PBMC: Peripheral blood mononuclear cell. NS: No significance.
DISCUSSION
In previous studies, MAIT cells were reported in multiple tissues, including liver, lung, pancreas, female genital mucosa, spleen and lymph nodes[25]. They represent about 6% of all CD3+T cells in human blood and up to 20% in the lungs. More importantly, MAIT cells account for up to 40% of T cells in the liver[26], which indicates that they play a critical role in liver disease. MAIT cells are involved in many types of immune disorders, including viral[27]and bacterial[28]infections. From further study, we know that MAIT cells recognize microbial metabolites and rapidly respond to pathogens, release cytokines, lyse target cells and promote inflammatory response, including in infectious and noninfectious inflammatory diseases, by secreting Th1 and Th17 cytokines. MAIT cells also play a critical role in liver diseases by promoting hepatitis and fibrosis, maintaining intestinal permeability and responding to biliary epithelium cells and liver B cells. MAIT cells were reported to be activated in patients with cirrhosis and displayed a proinflammatory profile. The profibrogenic functions of MAIT cells suggested that targeting MAIT cells may constitute an attractive antifibrogenic strategy during chronic liver injury. In addition, MAIT cells have antibacterial potency and play a key role as biliary firewall protecting the epithelial lining from translocated bacteria. In patients with alcoholic liver disease, the antibacterial potency of MAIT cells are impaired as a consequence of contact with microbial products and microbiota, which results in susceptibility to infection.

Figure 4 Partial recovery of circulating mucosal-associated invariant T cells in survived hepatitis B virus-related liver failure patients. A, B: The percentage (A) and count (B) of circulating mucosal-associated invariant T (MAIT) cells before and after treatment of surviving patients (n = 13) with hepatitis B virus (HBV)-related liver failure, and in healthy controls (HC) (n = 40); C, D: The percentage (C) and number (D) of MAIT cells in patients (n = 5) who died or underwent liver transplantation in HBV-related liver failure before and after conservative treatment. bP < 0.01, dP < 0.0001. NS: No significance; PBMC: Peripheral blood mononuclear cell.
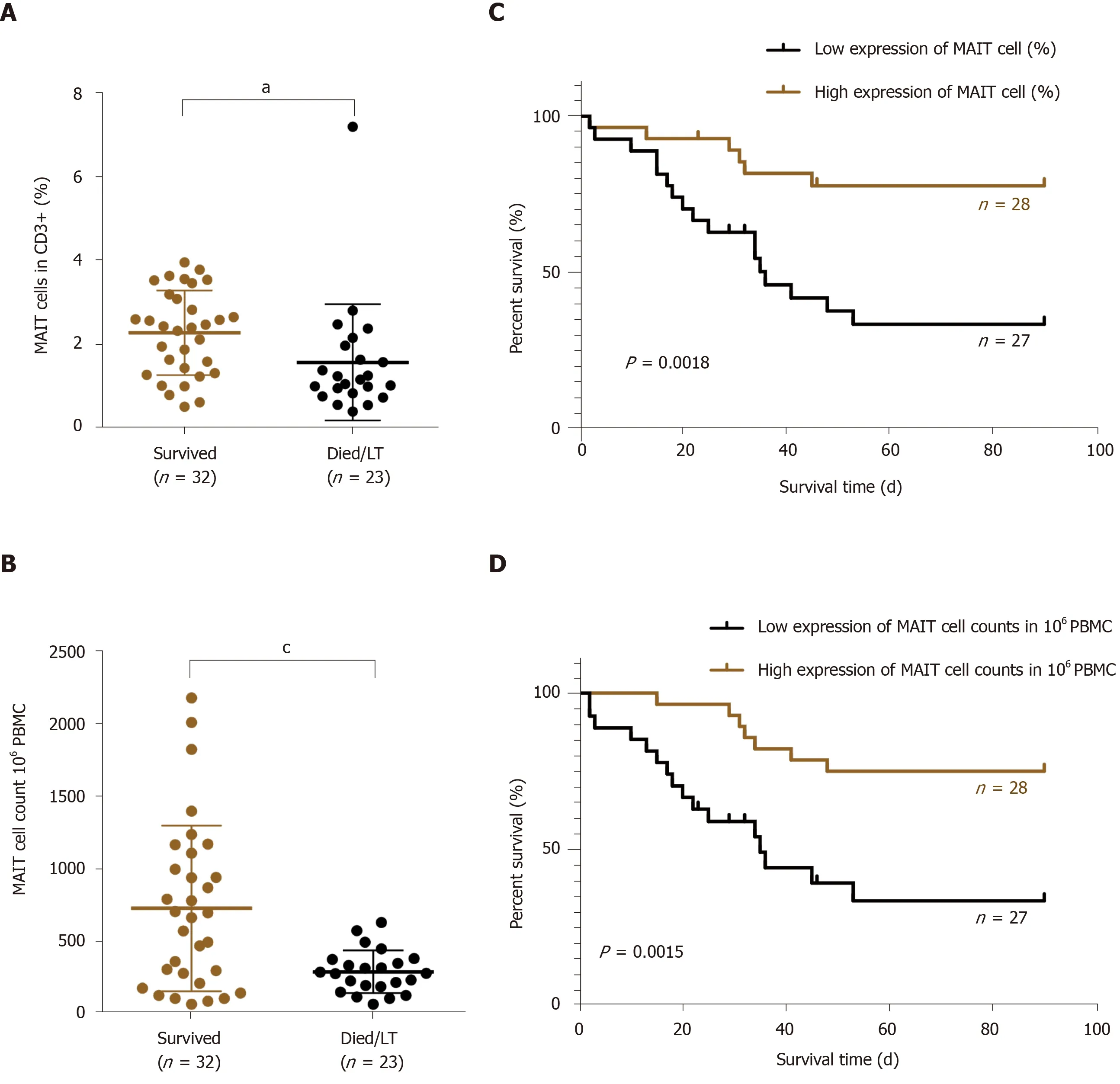
Figure 5 Lower count of circulating mucosal-associated invariant T cells indicated poor prognosis in hepatitis B virus-related liver failure. A, B: The proportion (A) and number (B) of circulating mucosal-associated invariant T (MAIT) cells in surviving (n = 32) and deceased/liver transplanted (LT) (n = 23) patients with hepatitis B virus (HBV)-related liver failure; C, D: The Kaplan–Meier curve of HBV-related liver failure patients with low (n = 27) and high (n = 28) expression of MAIT cells in percentage (C) and count (D). aP < 0.05, cP < 0.001. PBMC: Peripheral blood mononuclear cell.
The liver is an important immune organ in humans, with unique immune cells. MAIT cells are located around the bile ducts in the portal tracts and can exert antibacterial and antiviral effects. Hegdeet al[7]found that circulating MAIT cells were reduced in patients with alcoholic or nonalcoholic fatty liver disease-related cirrhosis. Yonget al[29]demonstrated that peripheral MAIT cells were significantly decreased in chronic HBV-infected patients. In line with the results above, we discovered that circulating MAIT cells were significantly reduced in patients with CHB. Additionally, we found that circulating MAIT cells were dramatically decreased in HBV-related liver failure compared with CHB. These findings may indicate that HBV-related liver failure may represent a more advanced and aggressive state of inflammatory cascade that may be incriminated in the more depressive effect on the status of circulating MAIT cells. However, Boeijenet al[12]suggested that MAIT cells were not depleted in chronic HBV-infected patients but more activated. The reason for this difference may include the number of patients and the different stages of the disease. In recent research, Niehauset al[30]demonstrated enrichment of MAIT cells in the peritoneal cavity, but circulating MAIT cells were dramatically decreased in patients with cirrhosis. Also, they reported that MAIT cells in ascites were almost intact and highly activated. This was because ascites in patients with decompensated cirrhosis represents a special immune environment, which is different from peripheral blood, resulting in different observed cell phenotypes and functions.
Immune responses play a crucial role in the pathogenesis of liver failure, and T-cellmediated immune responses play a critical role in the pathogenesis of HBV-related acute-on-chronic liver failure (ACLF). The enrichment of MAIT cells in the liver and its anatomical location close to the biliary tract suggest that MAIT cells play an important role in liver immune defense and possibly HBV-related liver failure. A recent study[31]suggested that T-cell-mediated immune response plays a vital role in acute HBV infection and the pathogenesis of HBV-related ACLF, which suggests that T-cell responses play a crucial role in virus control and immune-mediated liver damage. Another recent study[32]showed that chronic HBV and HCV infections always lead to T-cell depletion or dysfunction, which results in immune tolerance and viral persistence. The pathogenesis of ACLF is also a dysfunctional immune response caused by systemic inflammation and increased immune cell paralysis.
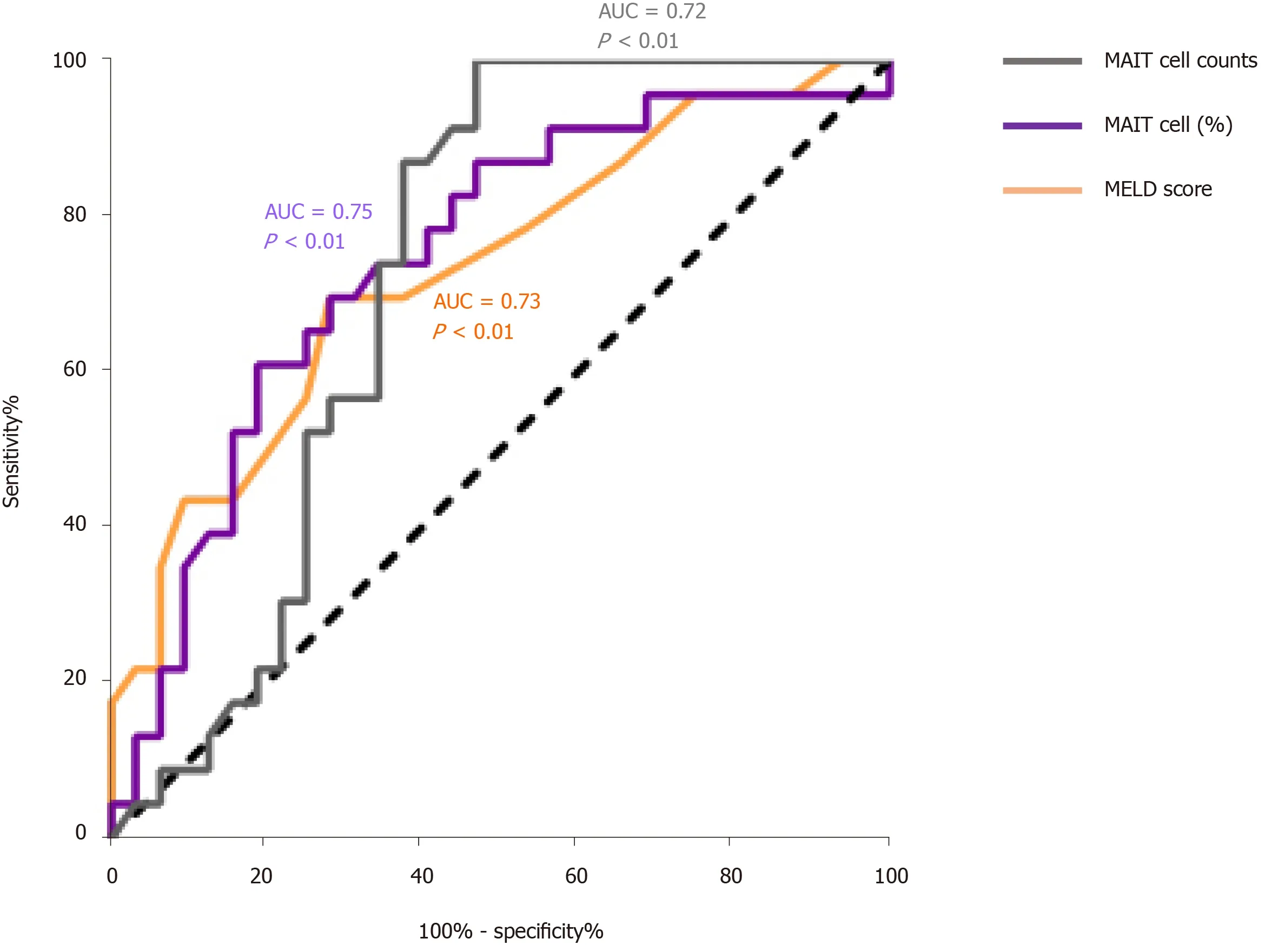
Figure 6 The prognostic value of model for end-stage liver disease score, mucosal-associated invariant T cell counts and percentage of mucosal-associated invariant T cells in patients with hepatitis B virus-related liver failure. The receiver characteristic operator curve of model for end-stage liver disease (MELD) score and mucosal-associated invariant T (MALT) cells counts and percentage (%). AUC: Area under the curve.
Böttcheret al[9]showed that MAIT cells were decreased in peripheral blood and liver tissue in autoimmune immune liver disease and found that intrahepatic MAIT cells tended to decrease with increasing fibrosis stage. They considered that the decrease was related to long-term exposure to cytokines and bacterial antigens, resulting in cell exhaustion. Together with their findings, our data showed a more significant decrease of circulating MAIT cells when the patients’ condition worsened. During the middle/late stage of liver failure, perhaps due to the long-term chronic course, circulating MAIT cells are increasingly depleted and the body mounts a dysfunctional immune response and enters an immunosuppressive state, resulting in viral persistence and aggravated conditions. Given that patients with end-stage chronic liver disease in most cases have at least some degree of malnutrition and decreased protein synthesis, maybe MAIT cells’ decrease is also associated with malnutrition, decreased protein synthesis and the reduction of lymphocytes.
Previous research has suggested that successful antiviral therapy does not restore the frequency of circulating MAIT cells, such as in patients with CHB or HCV or human immunodeficiency virus infection. Bolte and Rehermann[6]once mentioned that HCV infection leaves a lasting imprint on the MAIT cell population. We discovered that after treatment, the frequency and number of circulating MAIT cells partially recovered when HBV-related liver failure improved. We believe that in different immune environments of diseases, the circulating MAIT cells may undergo different immune processes (including immune exhaustion and chronic immune activation), resulting in different function and behavior of MAIT cells in the systemic circulation. This was an important discovery, and it is worthy of further study to explore the mechanism in patients with HBV-related liver failure.
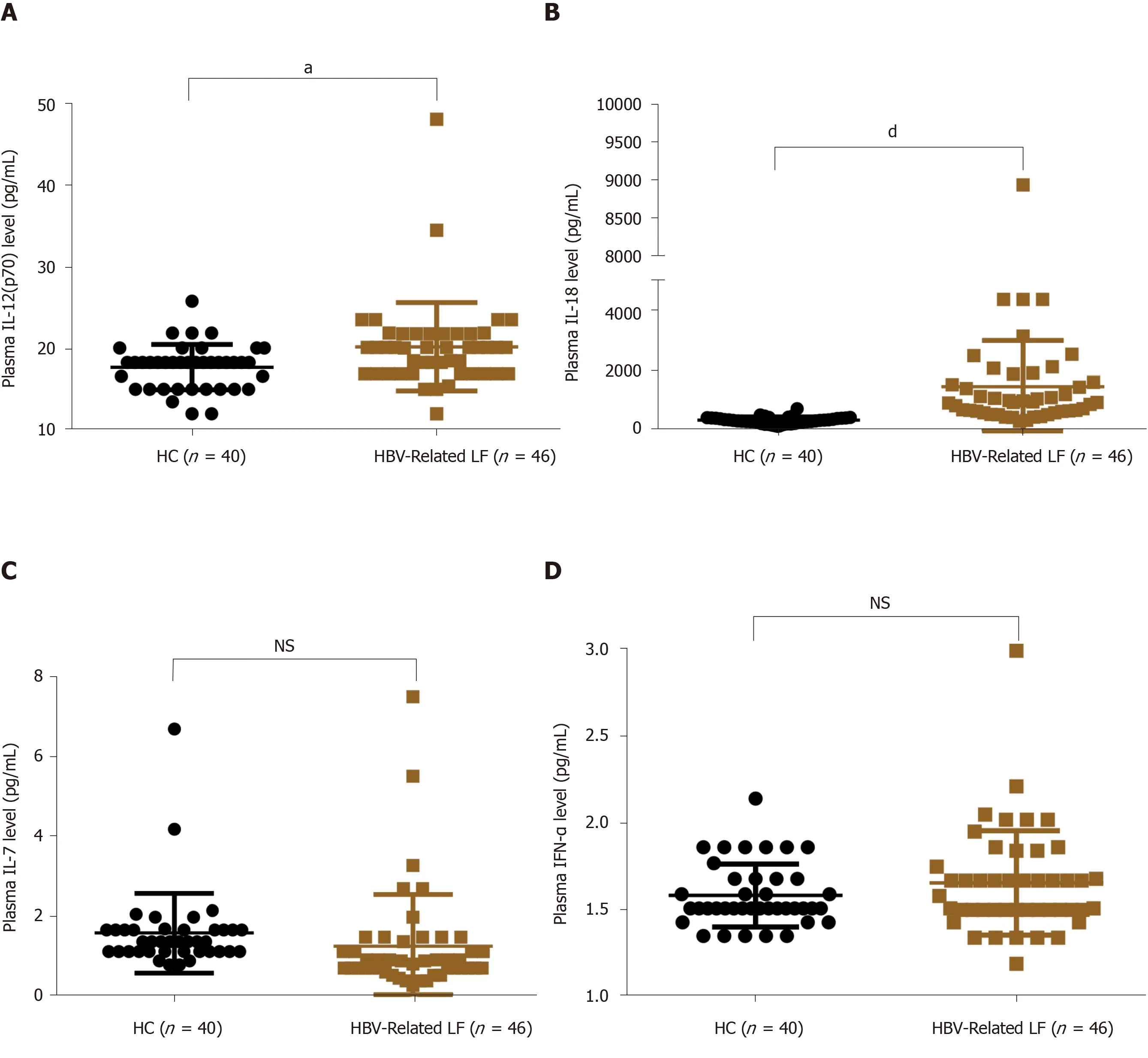
Figure 7 Plasma levels of interleukin-12 and interleukin-18 were increased in patients with hepatitis B virus-related liver failure. A-D: Plasma level of interleukin (IL)-12p70 (A), IL-18 (B), IL-7 (C) and interferon-α (D) in healthy controls (HC) (n = 40) and patients with hepatitis B virus (HBV)-related liver failure (n = 46). aP < 0.05; dP < 0.0001. NS: No significance.
Shenet al[31]have mentioned the lack of predictive prognostic biomarkers for hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF). Currently, the predictive scoring systems (including MELD and MELD-Na) for assessing the prognosis of patients with liver failure have defects, such as too complex, low sensitivity and limited application value. The prognosis of liver failure patients is poor. To find an objective, accurate and convenient prognostic marker for clinical application is still urgently needed. Given that the immune response plays a crucial role in the pathogenesis of liver failure, some immune-related prognostic markers appear, such as tumor necrosis factor-α-induced protein-8 like-2[33]. The latter is reported to have a potential role in predicting prognosis in patients with HBV-ACLF by negative regulation of cell-mediated immunity. Also, there were reports that peripheral myeloid-derived suppressor cells[34]and CD8 T cells acted as biomarkers for prognosis in HBV-ACLF. Our data indicate that circulating MAIT cells are an important marker for predicting the prognosis of HBV-related liver failure by showing obvious reduction of MAIT cells in deceased compared to surviving patients and a higher survival rate accompanied by higher expression of MAIT cells. It may be that depletion of MAIT cells leads to immunosuppression, increasing the risk of opportunistic infections, with severe consequences. Therefore, MAIT cells are vital for assessing the prognosis of patients with HBV-related liver failure, and early assessment can help physicians determine earlier which patients should receive liver transplantation and thus improve their prognosis.
Previous studies have shown that the exhaustion of blood MAIT cells may be caused by inflammatory cytokines, including IL-12, IL-18, IFN-α and IL-7. To explore further the possible mechanism of MAIT cell reduction in HBV-related liver failure, we quantified the plasma level of IL-7, IL-12p70, IL-18 and IFN-α. The results showed that plasma levels of IL-12 and IL-18 were increased obviously in HBV-related liver failure. Zhanget al[35]concluded that, in parallel to the reduction of MAIT cells, the increase in IL-12 and IL-18 may contribute to MAIT cell activation, loss or death. We inferred that depletion of circulating MAIT cells might be caused by stimulation by IL-12 and IL-18 in HBV-related liver failure.
In conclusion, circulating MAIT cells are decreased in HBV-related liver failure and associated with disease severity. Most importantly, circulating MAIT cells have a potential role in predicting prognosis in HBV-related liver failure patients. The mechanism of MAIT cell depletion needs to be further studied.
ARTICLE HIGHLIGHTS
Research background
Immune responses play a crucial role in the pathogenesis of liver failure. Mucosalassociated invariant T (MAIT) cells are a novel population of innate-like lymphocytes involved in inflammatory liver disease, and their potential role in liver failure remains unclear.
Research motivation
To determine the role of circulating MAIT cells in patients with hepatitis B virus (HBV)-related liver failure and better understand the pathophysiological mechanism of liver failure, which is helpful for clinical decision-making.
Research objectives
This study aimed to explore the relationship between the alteration of circulating MAIT cells and prognosis of HBV-related liver failure.
Research methods
Fifty-five patients with HBV-related liver failure were enrolled to assess the effect of circulating MAIT cells. Peripheral blood mononuclear cells were isolated and the expression of circulating MAIT cells was detected by flow cytometry.
Research results
The level of circulating MAIT cells was reduced significantly in HBV-related liver failure patients. There was also a more significant reduction of MAIT cells in patients with HBV-related liver failure compared with chronic hepatitis B patients. The alteration of circulating MAIT cells is associated with the severity and prognosis of HBV-related liver failure.
Research conclusions
We conclude that the circulating MAIT cells may be an important prognostic marker for HBV-related liver failure, and the lower expression of circulating MAIT cells may indicate a poor prognosis.
Research perspectives
The circulating MAIT cells are vital for assessing the severity and prognosis of patients with HBV-related liver failure, and the mechanism of MAIT cell depletion needs to be further studied.
ACKNOWLEDGEMENTS
The authors would like to thank Nantong Third People’s Hospital Affiliated to Nantong University for their technical support.
 World Journal of Gastroenterology2020年31期
World Journal of Gastroenterology2020年31期
- World Journal of Gastroenterology的其它文章
- COVID-19 pandemic: Pathophysiology and manifestations from the gastrointestinal tract
- Risk of malignancy in Caroli disease and syndrome: A systematic review
- Clinical characteristics and risk factors for liver injury in COVID-19 patients in Wuhan
- Prognostic value of pretreatment contrast-enhanced computed tomography in esophageal neuroendocrine carcinoma: A multicenter follow-up study
- Impact of interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer patients
- Feasibility and efficacy evaluation of metallic biliary stents eluting gemcitabine and cisplatin for extrahepatic cholangiocarcinoma
