Impact of interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer patients
Shi-Wen Mei, Zheng Liu, Fang-Ze Wei, Jia-Nan Chen, Zhi-Jie Wang, Hai-Yu Shen, Juan Li, Fu-Qiang Zhao, Wei Pei, Zheng Wang, Xi-Shan Wang, Qian Liu
Abstract
Key words: Interval time; Advanced rectal cancer; Disease-free survival; Pathologic complete response; Neoadjuvant therapy; Surgery; Complication; Sphincter preservation
INTRODUCTION
According to the latest report, with the increase in morbidity and mortality, rectal cancer ranks as the third most common cancer in China and is a major public health problem[1]. In addition, according to the epidemiological characteristics of rectal cancer in China, locally advanced rectal cancer is most common. Advanced primary tumor stage negatively impacts prognosis with local recurrence and synchronous metastases[2]. Over the past few decades, neoadjuvant chemoradiotherapy has been shown to reduce the rate of local recurrence after surgery for advanced rectal cancer to some extent, but overall survival (OS) was not improved significantly in some large randomized trials[3,4]. In recent years, with the increasing incidence of rectal cancer in China and the higher proportion of advanced rectal cancer, neoadjuvant chemoradiotherapy has gradually become the standard treatment scheme for locally advanced rectal cancer in China. In several prospective cohort and retrospective studies[5,6], neoadjuvant chemoradiotherapy has been indicated to lead to longer disease-free survival (DFS) in patients with pathologic complete response (pCR) and tumor downstaging. Subsequently, related studies were carried out on how to improve the pCR and tumor downstaging rates. By studying different surgical procedure methods and preoperative chemoradiotherapy doses and protocols, researchers further improved the pCR rate and tumor downstaging rate[7-9]. In recent years, some centers have also begun to observe whether the pCR rate and DFS rate can be further improved by examining the time interval to surgery after preoperative chemoradiotherapy.

The timing of surgery after neoadjuvant therapy for rectal cancer is an unresolved subject. Therefore, the first prospective randomized controlled study on the issue was established as the Lyon Trial R90-01. Francoiset al[10]enrolled 201 patients between 1991 and 1995 and published the results of the study in 1999. In the trial, the registered patients were divided into two groups according to the time of surgery after neoadjuvant therapy: A 2-wk group and a 6-8 wk group. They concluded that the longer time interval group (6-8 wk) had a significant advantage in clinical tumor response and pathologic downstaging, while there were no significant differences between the groups in postoperative complications, local recurrence, sphincterpreservation, or short-term survival. The second phase of the trial was the 17-year follow-up of the two groups of patients. Although the conclusion was that preoperative neoadjuvant therapy can effectively eliminate residual tumor cells, which is a sign of a good prognosis, the OS rates were similar in the groups with long followup, and the time interval to surgery after neoadjuvant chemoradiation of 6-8 wk as the standard treatment protocol was widely accepted in clinical practice[11]. More recent studies have described that a prolongation of the time interval achieved higher rates of downstaging and pCR and an especially favorable DFS[12]. The guidelines of the National Comprehensive Cancer Network recommend that surgery should be performed 5–12 wk following neoadjuvant chemoradiation[13]. In the GRECCAR-6 randomized trial[14], which divided patients into interval times of 7 and 11 wk, there was no significant difference in the effect of the time intervals on the pCR rate, whereas the quality of specimens was poorer and the rate of complications was higher in patients with an interval of 11 wk. Overall, the optimal time interval for operation is still a topic to be researched through clinical trials.
This study aimed to evaluate the optimal time interval to perform surgery following neoadjuvant chemoradiotherapy for increasing downstaging and pCR rates and improving DFS.
MATERIALS AND METHODS
Patients
Our retrospective cohort included 231 patients with locally advanced rectal cancer who underwent neoadjuvant therapy followed by radical surgery at the Colorectal Surgery Unit of the National Cancer Center/National Sciences Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College between November 2014 and August 2017 and had clinical, neoadjuvant, pathologic, and follow-up information available. This research was authorized by the ethics committee of our institution, and informed consent to collect clinical data was obtained from each patient following the principles of the World Medical Association Declaration of Helsinki. The patients enrolled in this study needed to meet the following criteria: (1) All patients had endoscopy biopsy-proven adenocarcinoma within 12 cm of the anal verge through endoscopy; (2) Tumors were scanned by pelvic magnetic resonance imaging and thoracic and abdominal-pelvic enhanced computed tomography (CT) and classified as locally advanced rectal cancer without metastases (stage cT3/T4 or cT any cN1/2, cM0) at the time of diagnosis; and (3) Laparoscopic surgery was performed following the completion of neoadjuvant therapy. The exclusion criteria were as follows: (1) Clinical tumor-node-metastasis (TNM) stages I and IV; (2) Patients diagnosed with other carcinomas and lesions; (3) Obstruction, perforation, bleeding, and palliative resection; and (4) Patients who refused to accept chemoradiotherapy.
Neoadjuvant therapy
Based on clinical stages II and III, all enrolled patients received the following neoadjuvant therapy regime. Preoperative radiotherapy was performed at a dose of 45 Gy to all pelvic tissue, 25 fractions/5 wk in a long-course regime, and then the linear accelerator was used to inject 5.4 Gy/3 fractions/3 d into the primary tumor with an energy of 10 MV. Radiation therapy technique used was intensity modulated radiotherapy. The radiating field involved the upper margin 1.5 cm above the sacral region and the lateral margin 1.5 cm outside the pelvic bone, including pelvic lymph nodes. One of the three following chemotherapy regimens was administered concurrent with radiation: (1) XELOX regimen and capecitabine 825 mg/m2orally administered twice daily 5 d/wk and oxaliplatin 130 mg/m2IV on the first day every 3 wk as a cycle; (2) Oral capecitabine 825 mg/m2twice daily 5 d/wk alone; and (3) Other regimens of oxaliplatin combined with others.
Surgical procedure
Surgery was scheduled 6-8 wk after the completion of chemoradiotherapy, but due to clinical factors such as logistics and scheduling, the actual interval varied from 2 to 60 wk. All surgical procedures were performed by colorectal surgeons specialized in oncology using the standardized laparoscopic total mesorectal excision (TME) technique defined by Healdet al[15]and a pelvic autonomic nerve preservation technique. The proximal and distal colon was anastomosed end to end by using the double stapler technique. To prevent severe complications from being caused by postoperative anastomotic leakage, a protective stoma was used as a diversion method based on the anastomotic tension and colonic blood supply during the operation, and 51 (22.1%) patients underwent protective stoma with the proximal ileum. The other key surgical procedures were left colic artery preservation (6.9%), multivisceral resection (5.2%), and lateral lymph node dissection (9.1%). All specimens were of high quality and completely measured by expert pathologists.
Pathologic analysis
All postoperative pathological specimens were fixed in paraffin within 24 h. The prepared specimens were analyzed by two pathologists specializing in colorectal cancer, and group information was kept confidential to the pathologists. The resected specimens were reviewed according to TNM staging (ypTNM), and the tumor tissues were analyzed by microscopy by the pathologists. The Dworak classification was used to determine tumor regression grades, with grades 0 to 4. pCR was defined as no residual cancer cells in the primary tumor tissue and no metastasis of tumor cells in the lymph nodes of the dissected region. Meanwhile, according to the WHO classification of digestive system tumors[16], the differentiation degree of tumor tissue was determined.
Follow-up
The first follow-up occurred 3 mo after surgery, and follow-up visits were conducted every 3 mo for the first two years at our center. Clinical examination and enhanced CT examination of the chest, abdomen, and pelvic cavity were performed, and colonoscopy was performed every 6 mo during the first two years. Beyond two years, the patients were followed every 6 mo, and colonoscopy was performed once a year. DFS was defined as the time from the date of radical resection to the time when disease recurrence was determined by radiological examinations. OS was defined as the time from the date of radical resection to the time when death caused by cancer occurred. The last follow-up date of this study was March 22, 2020. Patients who did not reach the endpoint or who were lost to follow-up before that date were censored. The median follow-up was 41 mo.
Statistical analysis
For statistical analysis, the chi-squared test or Fisher’s exact test was used to compare binary and categorical variables. Continuous data were analyzed byt-test if they were normally distributed and are displayed as the mean ± SD. If continuous data were not normally distributed, they were compared using the Mann-WhitneyUtest and are displayed as medians (ranges). Comparisons of the effects of the following variables on the two groups were carried out: Clinical characteristics, neoadjuvant information, surgical procedure, postoperative complications, pCR status, and other factors on intervals. All variables that achievedP< 0.05 in univariate analysis were included in the multivariable analysis. Multivariable regression analysis was performed to further identify factors independently associated with the time interval to surgery. The Kaplan-Meier method and Cox proportional hazards regression analysis were used to extract independent factors, especially time interval, associated with DFS and OS. All statistical analyses were performed using the Statistical Package for the Social Sciences version 26.0 for Mac (IBM Corp., Armonk, NY, United States).
RESULTS
Clinical characteristics and outcomes
Overall, 231 patients with locally advanced rectal cancer received neoadjuvant therapy followed by laparoscopic TME surgery from November 2014 to August 2017 at the Department of Colorectal Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The enrolled patients had an average age of 58.7 ± 11.2 years at the time of surgery, and the population consisted of 160 (69.3%) males and 71 (30.7%) females. The average body mass index (BMI) was 24.1 ± 3.2 kg/m2. The time interval between neoadjuvant chemotherapy and surgery ranged from 2 to 60 wk, with a median of 9 wk. The patient and tumor characteristics of the 139 (60.2%) patients who underwent surgery ≤ 9 wk after chemoradiotherapy (group A) and the 92 (39.8%) patients who underwent surgery > 9 wk after chemoradiotherapy (group B) are presented in Table 1. The two groups were balanced by age, sex, American Society of Anesthesiologists (ASA) score, BMI, preoperative carcinoembryonic antigen level, clinical T stage, clinical N stage, and preoperative concurrent chemotherapy regimen. The surgery results and tumor outcomes are presented in Table 2. All surgical procedures were performed by laparoscopy, and the surgical procedures were not significantly different and were unaffected by the neoadjuvant surgery time in the groups (P= 0.241). More patients underwent sphincter preservation surgery in group A than in group B, although the difference was not statistically significant (group A, 52.5%vsgroup B, 43.5%;P= 0.179). In the comparison of complications between the two groups, there were no significant differences in the incidence or type of complications; the incidence of complications in group A was similar to that in group B (8.6%vs7.6%;P= 0.894). Blood loss and operation time were significantly different between the two groups. More blood loss and longer operative times were found in group B than in group A (B, 100.9 ± 133.9 mLvsA, 68.0 ± 92.1 mL;P= 0.007 and B, 222.2 ± 67.8 minvsA, 194.5 ± 65.2 min;P= 0.001). Pathologic T stage (ypT) and pathologic N stage (ypN) were significantly different between the two groups (ypT,P= 0.022; ypN,P= 0.014). The rate of pCR was obviously higher in group B than in group A (group B, 27.2%vsgroup A, 10.8%;P= 0.001). Additionally, patients in group A had more lymph nodes retrieved (group A, 19.0 ± 8.8vsgroup B, 15.7 ± 8.7;P= 0.001) and positive lymph nodes (group A, 1.6 ± 3.2vsgroup B, 0.8 ± 1.7;P= 0.045) than those in group B. The exhaust time, defecation time, micturition time, and degree of differentiation were not affected by the interval time.
Multivariate analysis
All factors that achieved aP< 0.05 in univariate analysis were selected for binary logistic regression analysis since those factors have a possible association with being affected by interval time, including pathologic T stage (ypT), pathologic N stage (ypN), intraoperative blood loss (mL), operation time, total lymph nodes, positive lymph nodes, and rate of pCR. The binary logistic regression analysis showed that a shorter time interval (≤ 9 wk) between neoadjuvant therapy and surgery had the effect of decreasing the rate of pCR [odds ratio (OR) = 2.668; 95%CI: 1.276-5.578;P= 0.009]. In addition, operative time (OR = 1.006; 95%CI: 1.001-1.01;P= 0.01) and the total number of lymph nodes retrieved (OR = 0.952; 95%CI: 0.918-0.986;P= 0.007) were independent factors affected by time interval. The results are displayed in Table 3.
Pathologic outcomes
Seventy-three (31.6%) patients in the cohort experienced downstaging of disease, which was defined as clinical TNM > pathologic TNM without pCR. Forty (17.3%) patients achieved a pCR. Unfortunately, 118 (51.1%) patients did not reach the point of downstaging. There was a significant difference in T downstaging (group A, 40.3%vsgroup B, 43.5%;P= 0.016). Patients who underwent surgery after a longer interval had a significantly higher rate of N downstaging than those who underwent surgery after a shorter interval following neoadjuvant therapy (group A, 28.1%vsgroup B, 44.6%;P= 0.010, Table 4).
Survival outcomes
The follow-up period ranged from 2 to 62 mo, and it was not different between the two groups. Twelve (8.7%) patients in group A and 8 (8.6%) patients in group B experienced local recurrence (P= 0.987). The total number of deaths in the cohort was 26 (18.7%) from group A and 12 (13%) from group B (P= 0.661). The patients in group A experienced more distant recurrence than those in group B (group A, 28%vsgroupB, 10.9%,P= 0.001). Table 5 presents the tumor outcomes between the interval groups. The impacts of potential prognostic factors on DFS and OS (age, sex, BMI, ASA score, clinical T stage, clinical N stage, surgical procedure, pathologic T stage, pathologic N stage postoperative complications, differentiation, and preoperative concurrent chemotherapy regimen) were examined with Kaplan-Meier curves. There were significant differences in the DFS curves between the two groups for pathologic T stage, pathologic N stage, and time interval (Figure 1). There was no significant difference between the two OS curves for pathologic N stage or time interval (Figure 1), whereas there was a significant difference in the two OS curves for postoperative complications, as shown in Table 6. According to Table 7, the time interval was found to be independently associated with DFS but not with OS (> 9 wkvs≤ 9 wk: OR: 0.570; 95%CI: 0.328-0.991;P= 0.046). Meanwhile, pathologic T stage was an independent factor for DFS.
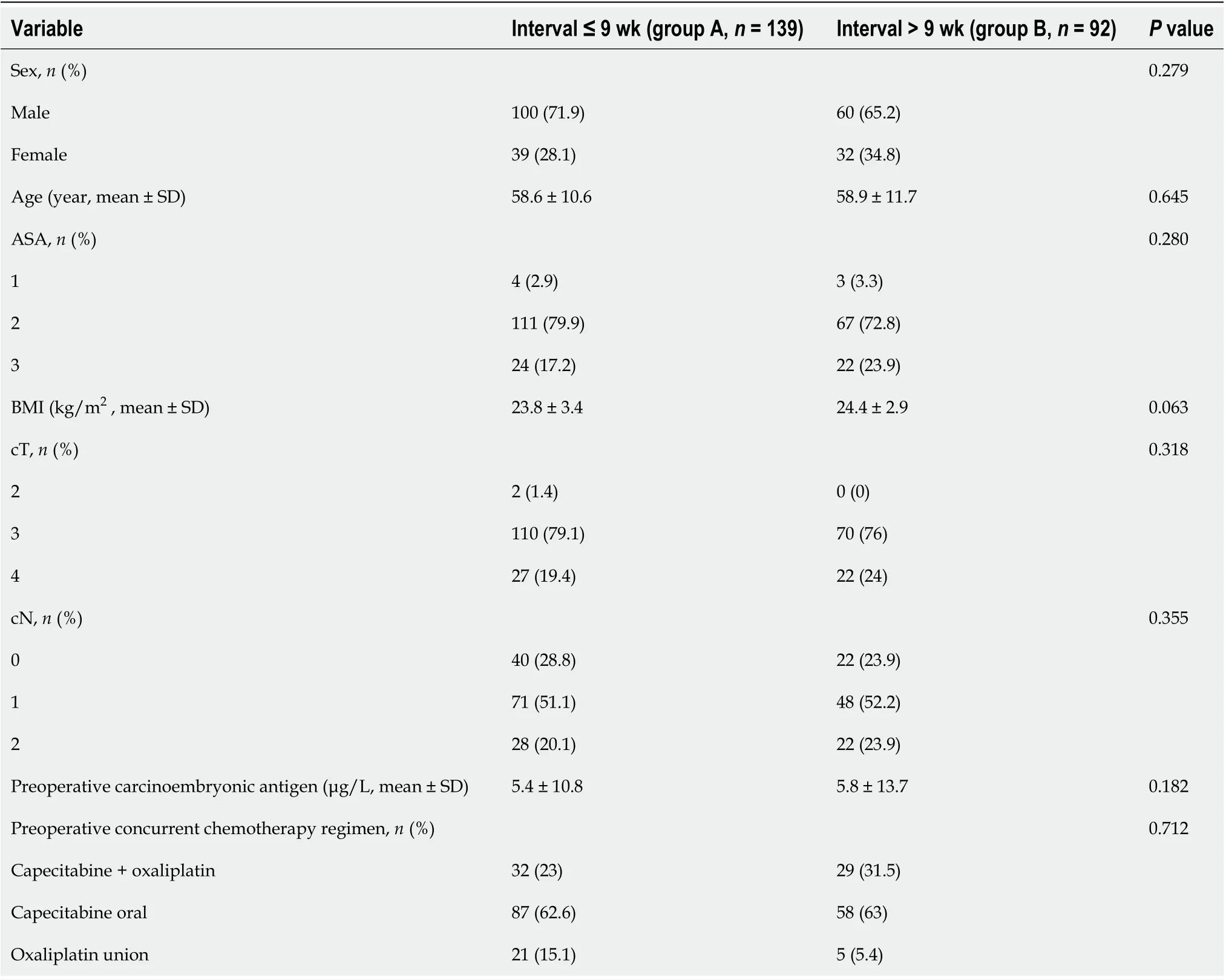
Table 1 Patient and tumor characteristics
DISCUSSION
The standard treatment regimen for clinical TNM stage II-III rectal cancers is neoadjuvant chemoradiotherapy followed by surgery with TME in China[17,18]. The treatment regimen can effectively decrease the rates of local recurrence and distant metastasis and may increase the rate of DFS[19]. Our results suggested that a longer time interval (> 9 wk) resulted in a better pathologic response and more favorable DFS. The results also demonstrated that a high pCR rate (P= 0.009; OR: 2.668; 95%CI: 1.276-5.578) and downstaging were favorable prognostic factors. Similarly, in the pathologic response study, although the TNM downstaging rate did not reachstatistical significance in the longer interval group, the data analysis presented a trend towards an increased TNM downstaging rate in patients who underwent surgery > 9 wk after chemoradiotherapy (group A, 29.5%vsgroup B, 34.8%,P= 0.129). Recently, an increasing number of studies have suggested that a longer time interval to surgery is associated with a high incidence of pCR. Our findings are consistent with this conclusion regarding pathologic response. Garcia-Aguilaret al[20]investigated the time interval to surgery in a 6-wk group and a longer time interval group. They compared the tumor response and surgical complications of the two groups. Finally, they concluded that a longer time interval to surgery may increase the pCR rate withoutincreasing complications. de Campos-Lobatoet al[21]described that perioperative complications were not affected by chemoradiotherapy or interval time. The pCR rate of patients with a longer time interval (≥ 8 wk) after chemoradiotherapy was significantly improved (P= 0.03), and the 3-year local recurrence rate decreased (P= 0.04). Probstet al[22]collected 17255 patients from a national database and stated that a longer time interval to surgery of 8 wk improved the pCR rate. Similarly, a systematic analysis[23]found that 4 of 7 studies reported that the rate of pCR was significantlyhigher with a longer interval time, and 3 of 8 studies indicated that prolonging the time interval to surgery increased the rate of tumor downstaging. In the first phase of the Lyon[10]trial, which was reviewed above, the conclusion was that a prolonged time interval to surgery can lead to better pathologic downstaging, but it does not contribute much to the control of local recurrence. We believe that the differences in the results of this study may be because the neoadjuvant radiotherapy dose was small (39 Gy) and that there was no concentrated radiation on the tumor. In contrast, in 2016, Sunet al[24]found that downstaging was increasing in less than 8 wk after radiotherapy. Steinet al[35]concluded that prolonging the time interval to surgery after neoadjuvant therapy did not lead to tumor downstaging. Our study described a favorable prognosis of DFS in patients who underwent surgery more than 9 wk after neoadjuvant chemoradiotherapy. However, there was a significant balance in OS between the two groups. To some extent, in the second phase of the Lyon trial[11], the DFS and OS rates were similar in the two groups at 17 years of follow-up. Regarding the pCR rate results, our study result was also in agreement with that of the randomized trial by Akgunet al[12]. They also concluded that prolonging the interval (> 8 wk) to surgery after chemoradiotherapy could increase the pCR rate, with the highest increase at 10-11 wk. However, there was no information on DFS or OS outcomes. In many studies, lower pathologic T stage or N stage was associated with improving DFS and decreasing recurrence and was even a vital predictor of survival[25,26]. Kimet al[27]reported that pathologic N downstaging was an important prognostic factor. Our study demonstrated that pathologic T downstaging and time interval delayed beyond 9 wk significantly improved the oncological outcomes of DFS and OS, and we found that there was a lower recurrence rate associated with delaying
surgery. Our conclusion coincided with that of Tulchinskyet al[28], who found that a longer time interval (> 7 wk) to surgery was associated with a higher rate of pCR and near pCR (17%vs35%,P= 0.03), decreased recurrence, and improved DFS (P= 0.05).

Table 2 Surgical results and tumor outcomes
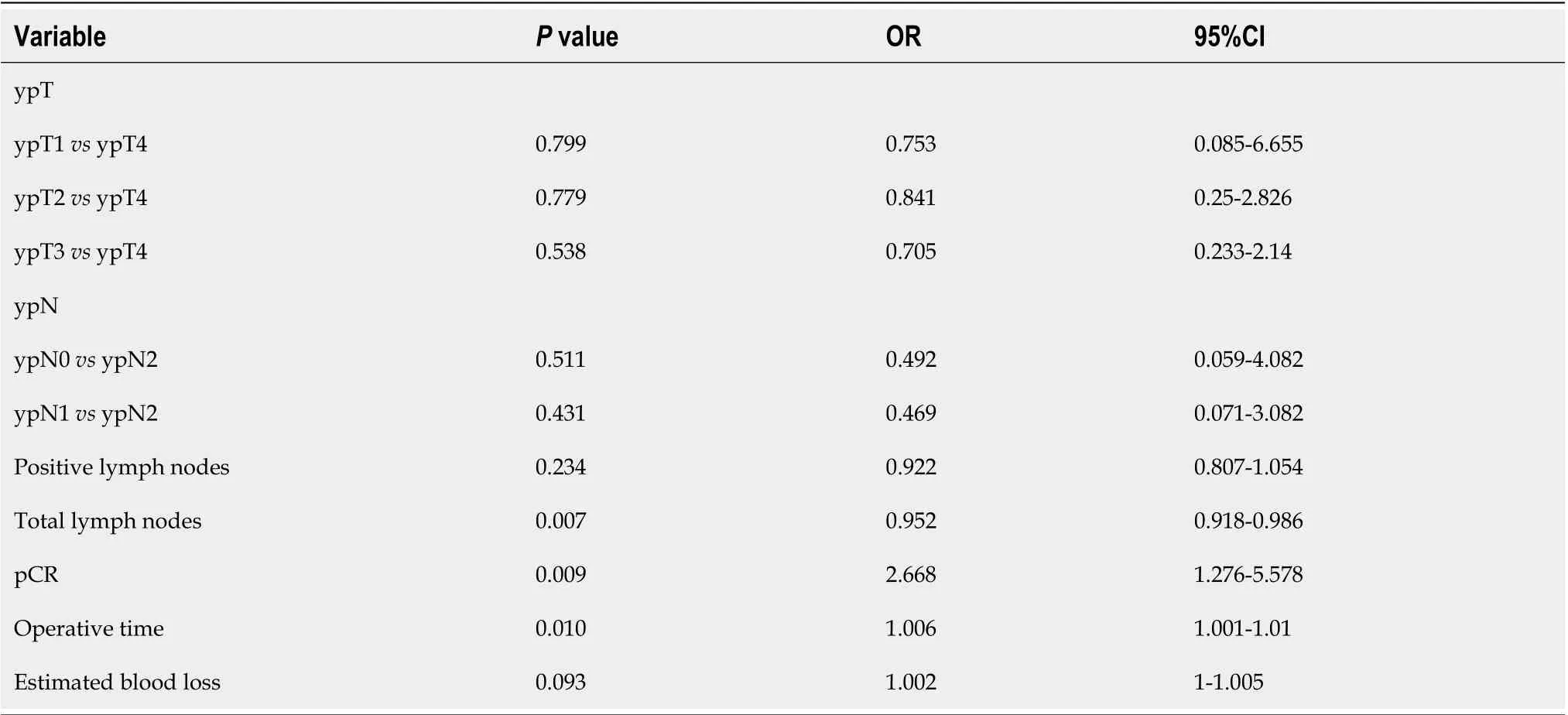
Table 3 Multivariate logistic regression analysis identifying independent factors affected by interval time
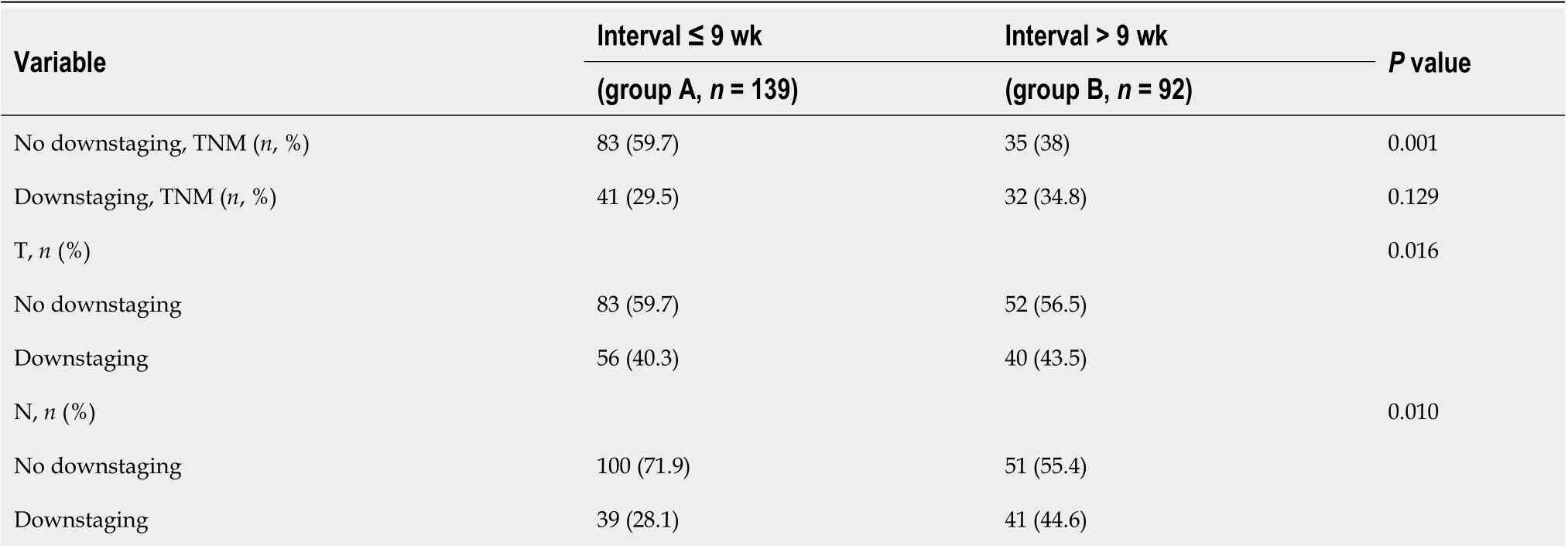
Table 4 Pathologic response
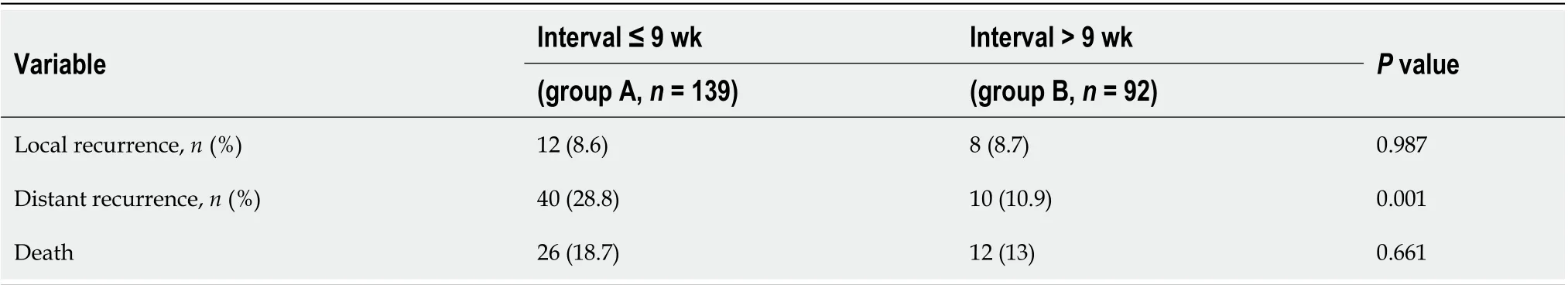
Table 5 Patterns of disease recurrence according to study group

Table 6 Kaplan-Meier survival estimates

BMI: Body mass index; ASA: American Society of Anesthesiologists; DFS: Disease-free survival; OS: Overall survival.

Table 7 Cox regression analysis
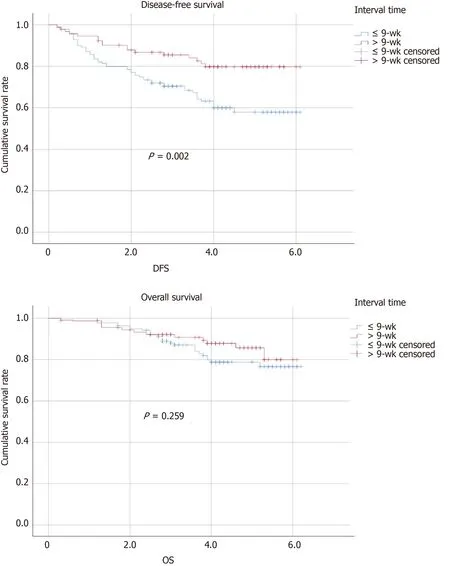
Figure 1 Kaplan-Meier curves of disease-free survival and overall survival in the ≤ 9-wk group and > 9-wk group with 5-year follow-up. DFS: Disease-free survival; OS: Overall survival.
Sphincter preservation was performed in more than half of the patients in our study (56.7%), and there was no significant difference between the two groups (P= 0.161). The longer interval of neoadjuvant surgery had no influence on the rate of sphinctersaving procedures, confirming the findings of other studies. A meta-analysis published in 2017 involving 13 studies that registered 19652 patients found that there were no significant differences (P= 0.743) in sphincter-preserving procedures[29]. Conversely, Campbellet al[30]reported that in the longer time interval group (8-16 wk), 74 (65%) patients underwent abdominoperineal resections. However, the most recently published studies show no beneficial effect of longer intervals on sphincter preservation[31,32].
The rate of postoperative complications was 12% in our study. According to the Clavien-Dindo classification[33], all complications were graded as grades 1 to 3. Anastomotic leakage occurred more often in the shorter interval (≤ 9 wk) group than in the longer interval group (3.6%vs2.2%). Three patients underwent reoperation in the interval > 9 wk group, and one patient underwent reoperation in the interval ≤ 9 wk group. The other types and rates of complications were similar between the groups in our analysis. However, many surgeons hold the view that further prolongation of the time interval to surgery will increase the risk of the operation because pelvic fibrosis caused by radiotherapy may lead to intraoperative or postoperative bleeding. According to our previous research, intraperitoneal chemotherapy can lead to postoperative anastomotic leakage, especially in patients undergoing preoperative neoadjuvant therapy[34]. In fact, Steinet al[35]reported that in the shorter interval group, the incidence of complications was higher than that in the longer time interval group. Three patients suffered from anastomotic leakage, and two patients healed by reoperation. In addition to the blood supply factors, leakage of the anastomotic site may be caused by injury to the intestinal canal at the anastomotic site caused by radiation irradiation. The Lyon R90-01 trial[10]compared the postoperative complications of the two groups and found that the incidence of complications was 18% in the 2-wk group and 17% in the 6-8 wk group, and there was no significant difference between the two groups. A total of 265 patients from 24 medical centers were randomly enrolled in the GRECCAR-6[36]trial, and the patients were divided into a 7-wk interval group and an 11-wk interval group. The study described that complications were significantly increased in the longer interval group, but there was no significant difference compared with the 7-wk interval group (P= 0.404); regarding the rate of anastomotic leaks, there was balance in the two groups (P= 0.611). The study concluded that a longer time interval (11 wk) after surgery may improve the risk of complications. We analyzed the data of Mooreet al[37]and found a high incidence of anastomotic complications in the longer time interval group undergoing surgery after chemoradiation (P= 0.05). In summary, most studies suggested that a prolonged time interval may lead to higher complications and lower sphincter preservation.
Our study has several limitations. The first limitation of this study is its retrospective nature. Due to the lack of data from large, prospective, randomized controlled studies, we believe that the results of this study are of value. The second may be bias in the information collected, but the homogeneity in the cohort was well compared. A future multicenter randomized control or cohort trial with a larger sample size may be needed to identify the optimal time interval for increasing the rate of pCR and improving oncologic outcomes. Third, the complication rate in our center is relatively low for neoadjuvant therapy in advanced rectal cancer.
In conclusion, a time interval to surgery of > 9 wk after neoadjuvant therapy increases the pCR rate and has an impact on DFS, but it does not have an impact on OS.
ARTICLE HIGHLIGHTS
Research background
Surgery with total mesorectal excision following neoadjuvant therapy is a standard regime for locally advanced rectal cancer. The optimal interval time between neoadjuvant therapy and surgery is still under debate.
Research motivation
There is a lack of consensus concerning the interval time between neoadjuvant therapy and surgery. Whether shorter or longer interval time is a controversial topic. And there are limited data regarding outcomes associated with different neoadjuvant therapy-surgery times.
Research objectives
The main aim of this study was to investigate whether different interval times affect the rate of pathologic complete response (pCR), preoperative outcomes, and survival status.
Research methods
We performed a retrospective cohort study and enrolled locally advanced rectal cancer patients with neoadjuvant therapy. Information regarding the clinicopathological features, clinical outcomes, and follow-up was collected and analyzed. Multivariate logistic regression analysis was performed to evaluate the possible factor affected by interval time.
Research results
The interval time between neoadjuvant therapy and surgery > 9 wk increased the incidence of pCR and had a better impact on disease-free survival (DFS).
Research conclusions
Prolonging the interval time between neoadjuvant therapy and surgery may be associated with improved rates of pCR, decreased disease recurrence, and improved DFS but has little impact on postoperative complications and sphincter preservation.
Research perspectives
Prospective randomized trials are required to evaluate the optimal time interval that is needed to achieve minimum morbidity, maximal tumor downstaging, and minimum disease recurrence.
 World Journal of Gastroenterology2020年31期
World Journal of Gastroenterology2020年31期
- World Journal of Gastroenterology的其它文章
- COVID-19 pandemic: Pathophysiology and manifestations from the gastrointestinal tract
- Risk of malignancy in Caroli disease and syndrome: A systematic review
- Mucosal-associated invariant T cells in hepatitis B virus-related liver failure
- Clinical characteristics and risk factors for liver injury in COVID-19 patients in Wuhan
- Prognostic value of pretreatment contrast-enhanced computed tomography in esophageal neuroendocrine carcinoma: A multicenter follow-up study
- Feasibility and efficacy evaluation of metallic biliary stents eluting gemcitabine and cisplatin for extrahepatic cholangiocarcinoma
