Is there a relationship between dopamine and rhegmatogenous retinal detachment?
Alessio Martucci , Massimo Cesareo , Maria Dolores Pinazo-Durán, Michela Di Pierro, Matteo Di Marino Carlo NucciMassimiliano Coletta, Raffaele Mancino
1 Ophthalmology Unit, Department of Experimental Medicine, University of Rome Tor Vergata, Rome, Italy 2 Ophthalmic Research Unit “Santiago Grisolia”/FISABIO and Cellular-Molecular Ophthalmobiology Group/University of Valencia, Valencia,Spain 3 Department of Clinical Sciences and Translational Medicine, University of Rome Tor Vergata, Rome, Italy
Abstract
Key Words: 3,4-dihydroxyphenylacetic acid; DOPAC; dopamine; dopaminergic amacrine cells; dopaminergic neurotoxicity; macular pucker; oxidative stress; photoreceptor degeneration; rhegmatogenous retinal detachment;vitreous hemorrhage
Introduction
In the late 1950s, Carlsson identified dopamine (DA) as a potential neurotransmitter in the brain. In the last decades,DA has gained much attention and the importance and the complexity of its multiple roles in the retinal function have been appreciated. The first studies revealed the importance of DA as chemical messenger for light adaptation. Subsequently, DA has been involved in more trophic functions of the retina such as growth, development, cell death and experimental myopia (Witkovsky, 2004). Therefore, DA seems to have a central role in development, visual signaling, and refractive changes of the eye (Zhou et al., 2017).
Amacrine cells situated in the inner nuclear layer are the most sparsely distributed neurons within the retina and a major source of retinal dopamine (Sankaran et al., 2018).
In the outer retina, DA has been shown to be related to a decrease in the cell to cell coupling among horizontal cells provided by the gap junction, which are specialized electrotonics sinapses (Witkovsky, 2004). Moreover, interactions between DA and retinal pigment epithelium (RPE) have been reported (Witkovsky, 2004). DA, in fact, affects both faces of the RPE.
Finally, DA has been shown to modulate the production of cyclic adenosine monophosphate (cAMP). Interestingly,cAMP signaling plays a pro-apoptotic role in certain cell type. This may imply a possible role of dopamine both in the induction of RRD and in the sequelae of the disease, such as photoreceptors damage (Ladilov and Appukuttan, 2014). DA is enzymatically metabolized into 3,4-dihydroxyphenylacetic acid (DOPAC) and the DA/DOPAC ratio in the retina is around 1:1 (Witkovsky, 2004). Synthesis or degradation of DA and its metabolites is not performed by the vitreous gel and Witkovsky et al. (1993) suggested that vitreal DA amounts could reflect retinal dopamine release. As high-performance liquid chromatography cannot correctly detect the low vitreal DA concentrations, DOPAC is currently used as surrogate measure of retinal DA (Cohen et al., 2012).
Basing on the important role of DA in the eye, the aim of this study was to compare vitreous concentration of DOPAC in vitreous samples of patients affected by RRD, macular pucker and vitreous hemorrhage, in order to investigate the possible differences in DA release in such retinal diseases.
Subjects and Methods
This pilot prospective monocentric study adhered to the tenets of Declaration of Helsinki and was approved by the Ethics Committee of Rome Tor Vergata University Hospital(R.S.92.10) on September 24, 2010. All subjects involved signed a written informed consent. Vitreous samples were collected from all participants on the day of surgery.
Clinical characteristics of enrolled patients
For this study, vitreous sample of 18 patients that underwent pars plana vitrectomy for RRD or other vitreoretinal diseases were collected. Patients were subdivided into a RRD group,consisting in 9 eyes with RRD, and a control group (CTRL group), consisting in 7 eyes of patients with macular pucker and 2 eyes of patients with vitreous hemorrhage. Subjects were recruited during routine ophthalmologic examinations in Ophthalmology Clinic Department of Tor Vergata University Hospital.
All the patients underwent a comprehensive ophthalmological examination that consisted in: best corrected visual acuity (BCVA) determination, intraocular pressure determination by Goldmann tonometry, anterior segment and, after pupil dilation, fundus oculi examination. All the patients also underwent ultrasound and optical coherence tomography examination.
To avoid possible bias, with exclusion of vitreous hemorrhage, that required six months of watch and wait approach,all the subject must have had a recent development of the disease (within 5 days). Patients must not be affected by other medical conditions or underwent previous ocular or systemic surgery and must not be using topical or systemic medications. All the patients were enrolled within 30 days from the beginning of the study and underwent surgery on the same day of the week, of the same month, almost at the same time. These strict inclusion criteria made the enrollment very complex.
3, 4-Dihydroxyphenylacetic acid assessment
Before infusion started, for the purpose of the study, we collected 300-400 μL vitreous humor from each patient. Vitreous was placed in cryo Eppendorf tubes, stored at -70°C,and assayed within 2 weeks of collection. Vitreous humor was deproteinized, by adding ice-cold 70% HClO4(20 μL),centrifuged at 20,690 × g for 10 minutes at 4°C, neutralized by adding 5 M K2CO3(20 μL) in ice, and filtered using a 0.45 μM Millipore-HV filter. Samples were therefore evaluated by High-Performance Liquid Chromatography (HPLC)(100 μL) to determinate DOPAC concentration without chemical manipulation of the sample, apart from perchloric acid deproteinization. This technique minimizes the risks of modifications of DOPAC induced by proteins as well as avoids any potential HPLC analytical column obstruction due to proteins. Concentration of DOPAC was determined on 100 μL of perchloric acid extract by an ion-pairing HPLC technique using a Kromasil 250 × 4.6 mm, 5-μm particle size column, with its own guard column (Eka Chemicals AB,Bohus, Sweden), and using tetrabutylammonium hydroxide as the pairing reagent (Di Pierro et al., 1995). The separation was obtained by forming a step gradient (adapted to the increase in column length compared to the original method)with the following buffers: buffer A, 10 mM tetrabutylammonium hydroxide, 10 mM KH2PO4, 0.25% methanol pH 7.00; buffer B, 2.8 mM tetrabutylammonium hydroxide, 100 mM KH2PO4, 30% methanol pH 5.50. The gradient was: 10 minutes 100% buffer A; 3 minutes 90% buffer A; 10 minutes 70% buffer A; 12 minutes 55% buffer A; 15 minutes 45%buffer A; 10 minutes 25% buffer A; 5 minutes 0% buffer A.By using water-jacketed glassware, the flow rate throughout the chromatographic runs was 1.2 mL/min and the column temperature was kept at a constant 23°C. A Surveyor LC Pump (ThermoFinnigan Italia, Rodano, Milan, Italy) connected to a Surveyor PDA Detector (ThermoFinnigan Italia)at 200-300 nm was used as the HPLC instrument. Data were collected and examined using the ChromQuest program(ThermoQuest, Milano, Italy). To identify and calculate the different metabolites concentration areas, retention times and absorbance spectra of the peak of sample chromatograms were compared with those of freshly prepared ultrapure standards (Di Pierro et al., 1995; Mancino et al., 2011;Nucci et al., 2013a).
Statistical analysis
All data were recorded into an EXCEL database (Microsoft,Redmond, WA, USA). Data were analyzed using SPSS 23.0 statistical software (IBM, Armonk, NY, USA). Kolgomorov-Smirnov test was performed to confirm Gaussian distribution of the parameters. The mean ± SD for parameter with Gaussian distributions was calculated. Independence among categorical variables was evaluated using a chi-square test.A Student’s t-test was used to evaluate differences among groups in terms of age and mean HPLC DOPAC. Pearson correlation coefficient was calculated between DOPAC and post-operative BCVA, and between DOPAC and the days elapsed between diagnosis and surgery. A P < 0.05 was considered statistically significant. To evaluate the statistical power of the study, a post-hoc power analysis was performed(Rosner, 2011).
Results
Descriptive statistics
Descriptive statistic is reported in Table 1.
A total of 18 patients were enrolled in the study, 9 for each group. RRD group consisted in 9 eyes of 9 patients, 6 males and 3 females, aged between 32 and 76 years (mean 56.7 ±15.0), while CTRL group consisted in 9 eyes of 9 patients, 8 males 1 female, aged between 43 and 76 years (mean 65.7 ±10.3). Groups were homogeneous in terms of age (P = 0.08)and gender (χ2= 1.29, P = 0.26). The post-hoc power analysis showed a 100% of power with α = 0.05.
DOPAC levels
The biochemical analysis showed an HPLC DOPAC mean value of 8.60 ± 1.94 ng/mL in the RDD group and of 0 ng/mL in the CTRL group (Figure 1). A Student’s t-test revealed that RRD group had significantly higher HPLC DOPAC values than CTRL group (P = 0.002).
Pearson correlation coefficient analysis showed a slightly significant positive correlation was found among DOPAC and post-operative BCVA (r = 0.470, P = 0.049). No cor-relation was found between DOPAC and the days elapsed between diagnosis and surgery (P = 0.317).

Table 1 Descriptive statistics of the examined groups
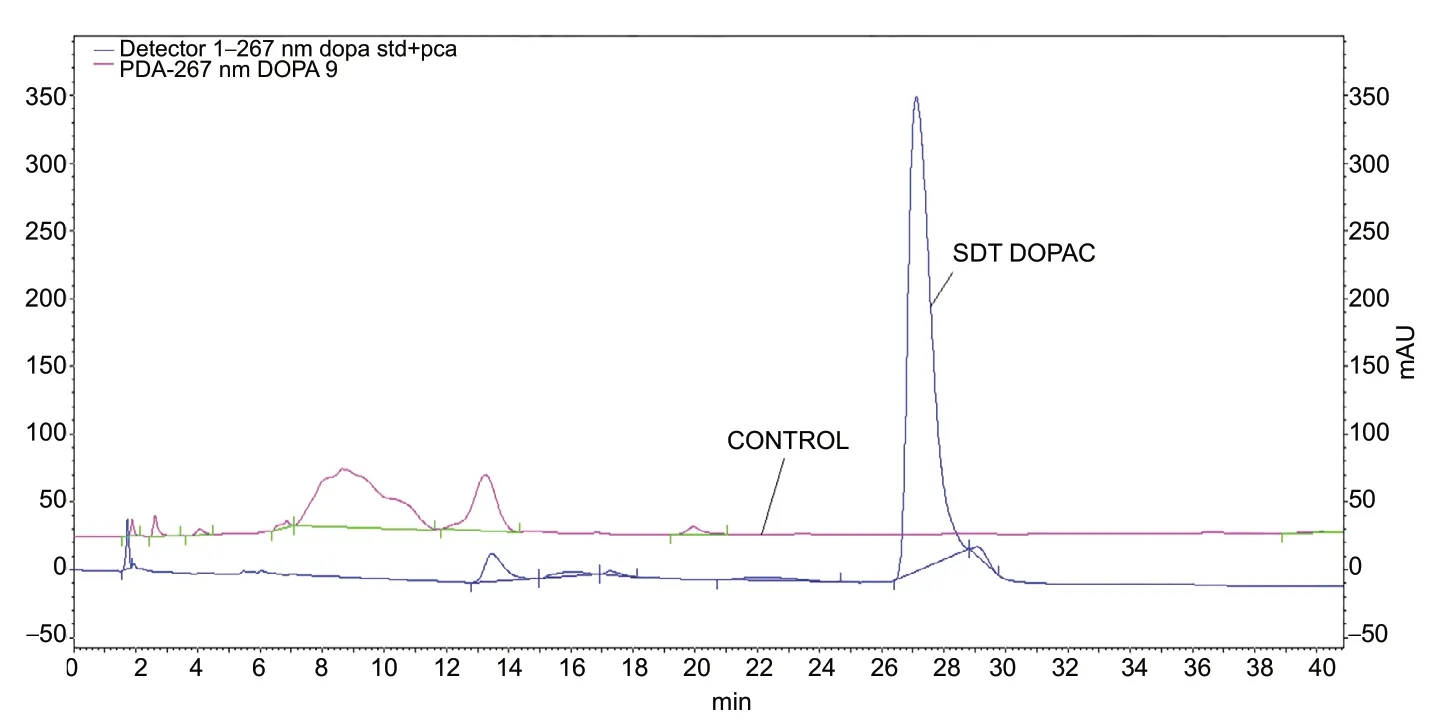
Figure 1 High-performance liquid chromatography.
Discussion
To our knowledge, this is the first study showing significantly higher levels of DOPAC, a surrogate index of retinal dopamine levels, in the vitreous sample of patients affected by RRD compared to those affected by vitreous hemorrhage and macular pucker. In particular, vitreous hemorrhage and macular pucker samples did not show any trace of the substance. Although we may not exclude that dopamine release is consequent to the mechanical trauma induced by RRD,that’s would explain the absence of DOPAC in the vitreous samples of vitreous hemorrhage and macular pucker, we may speculate that dopamine may have a potential, although still unknown, role in the development of the disease and/or in the photoreceptors loss after RRD development as previously shown in rd mouse model (Djamgoz et al., 1997; Firsov and Astakhova, 2016).
Interestingly, DA derived reactive oxygen species are toxic towards a wide range of cellular lines. For this reason, and to our knowledge for the first time, we investigated the possible role of DA in RRD.
Oxidative stress is considered one of the causes of cellular damage and plays a role in triggering programmed cell death(Li et al., 2013). It not only has a fundamental role in developing and accelerating neurodegenerative disorders (Li et al.,2013; Niedzielska et al., 2016), but also seems to play an important role in several eye disorders such as RRD (Nucci et al., 2011, 2013a, b, 2018; Suzuki et al., 2016; Mancino et al.,2018; Martucci and Nucci, 2019). An elevated vitreous level of oxidative stress biomarkers has been previously reported by Cederlund et al. (2013) in a study showing that oxidative stress was related to the RRD severity. Accelerated cellular damage, in fact, is mostly due to an imbalance between reactive oxygen species production and antioxidant capacity(Nucci et al., 2013b; Martucci et al., 2019).
The damage to dopaminergic neurons induces a spontaneous oxidization of cytosolic DA and produces O2-and reactive quinones (Miyazaki and Asanuma, 2008). Beside oxidative stress caused by reactive oxygen species, the pathogenicity of quinone has been recently considered a source of dopaminergic neuron-specific oxidative stress (Miyazaki and Asanuma, 2008). Generation of oxygen radicals by the quinone futile cycle has been proposed as a viable model of the damage of RPE cells in culture by dopa (Akeo et al., 1989).
The apical face of RPE express D5 receptors and dopamine action seem to involve alterations in cAMP and in anion conductance of the RPE membrane (Gallemore et al.,1990; Nao-i et al., 1990). Basing on the ability of dopamine to modulate the production of cAMP and the crucial role of cAMP in apoptosis of some cell types (Kolb et al., 1992),it can be hypothesized that apoptosis may be induced by contribution of this messenger through other pro-apoptotic factors (Kolb et al., 1992). It is still unknown whether alterations in dopamine pathways contribute to retinal degeneration, though they surely precede the photoreceptor death as revealed in both the Royal College of Surgeons rat and the rd mouse models (Djamgoz et al., 1997; Firsov and Astakhova,2016). Dopamine antagonists, in fact, as well as dopamine reduction, block photoreceptor alteration in the retinal degeneration mice retinal organ culture model, suggesting that dopamine antagonists may have a therapeutic role in retinal degenerative diseases (Feltgen and Walter, 2014).
Due to the technical difficulties to dose dopamine, measurements of vitreous DOPAC as surrogate index of retinal dopamine levels was previously validated (Megaw et al.,2001). Interestingly, Pearson correlation coefficient showed that there is no correlation between the content of DOPAC and the days elapsed between the diagnosis of the disease and surgery. More importantly, our data suggest that higher DOPAC values in the vitreous are associated to worse post operative visual acuity. Thus, supporting an important role of DOPAC, and eventually of dopamine, in the patient’s visual outcome.
Overall, our findings are suggestive of new possible scenarios in the pathogenesis and management of RRD. It is therefore clinically important to study the vitreous composition as it may provide support to newer targeted therapeutic protocols in RRD (Suzuki et al., 2016). Further prospective studies, on a larger cohort of RRD patients, may be useful to further delineate the possible role of dopamine in RRD.
Author contributions:RM conceived and planned the experiments. RM,MDM, and MC carried out the experiments. MDP, MDM, MC, and RM contributed to sample preparation. AM, MDPD, MC, MDM, MDP, CN,MC, and RM contributed to the interpretation of the results. AM and MC took the lead in writing the manuscript. All authors approved the final version of the manuscript.
Conflicts of interest:None declared.
Financial support:None.
Institutional review board statement:The study protocol was approved by the Ethics Committee of Rome Tor Vergata University Hospital(R.S.92.10) on September 24, 2010. This study was performed according to the statement of the Declaration of Helsinki, and informed consent of the study procedure was obtained from all participants.
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the forms the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement:This study followed the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals developed by the International Committee of Medical Journal Editors.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of the University Hospital Tor Vergata in Italy.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Adrian Smedowski, Medical University of Silesia School of Medicine in Katowice, Poland.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Ethanol extract from Gynostemma pentaphyllum ameliorates dopaminergic neuronal cell death in transgenic mice expressing mutant A53T human alpha-synuclein
- Peripheral nerve injury induced changes in the spinal cord and strategies to counteract/enhance the changes to promote nerve regeneration
- Genetic targeting of astrocytes to combat neurodegenerative disease
- Pathological significance of tRNA-derived small RNAs in neurological disorders
- Applications of advanced signal processing and machine learning in the neonatal hypoxic-ischemic electroencephalography
- Protective effect of hydrogen sulfide on oxidative stress-induced neurodegenerative diseases

