Differential expression of glial cell line-derived neurotrophic factor splice variants in the mouse brain
Xiao-He Gu , Heng Li , Lin Zhang , Tao He Xiang Chai He Wei, Dian-Shuai Gao
1 Department of Anatomy and Neurobiology, Xuzhou Medical University, Xuzhou, Jiangsu Province, China 2 Department of Neurosurgery, First Affiliated Hospital of Soochow University, Suzhou, Jiangsu Province, China
Abstract
Key Words: Δ78 locus; brain region; dopaminergic neurons; glial cell line-derived neurotrophic factor; mouse brain; precursor protein;α-pro-GDNF; β-pro-GDNF; sorting protein-related receptor with A-type repeats; splice variants
Introduction
Glial cell line-derived neurotrophic factor (GDNF), widely expressed in the central nervous system, protects and repairs dopaminergic neurons (Tomac et al., 1995; Liu et al., 2013;Ortmann et al., 2018). GDNF is, by far, the most effective neurotrophic factor for dopaminergic neurons (Lin et al.,1993). It has been reported that two GDNF splice variants are expressed in humans and rodents-full-length α-pro-GDNF and a shorter β-pro-GDNF lacking a 78-bp sequence (Suter-Crazzolara et al., 1994; Trupp et al., 1995; Matsushita et al.,1997; Grimm et al., 1998). α- and β-pro-GDNF are expressed in the same tissues, but in different proportions (Trupp et al.1995, Airavaara et al., 2011). β-GDNF mRNA is relatively highly expressed during brain development (Suter-Crazzolara et al. 1994). Furthermore, these two splice isoforms are translated into precursor proteins with different signal peptide sequences (β-pro-GDNF has a deletion of 26 amino acids)(Penttinen et al., 2018). However, the mRNA and protein expression levels of α- and β-pro-GDNF in dopaminergic neurons in the different brain regions are unknown.
α- and β-pro-GDNF have distinct secretory pathways.While α-pro-GDNF is secreted constitutively, β-pro-GDNF is secreted in an activity-dependent manner (Lonka-Nevalaita et al., 2010). Given the role of sorting protein-related receptor with A-type repeats (SorLA) in sorting GDNF (Chen et al., 2005; Oh-hashi et al., 2009; Glerup et al., 2013), it is tempting to speculate that SorLA might regulate the protein expression of GDNF splice variants. SorLA, a 250 kDa membrane glycoprotein, is mainly expressed in mammalian brain cells (Jacobsen et al., 1996, Yamazaki et al., 1996). SorLA is one of five members of the vacuolar protein sorting-related receptor family, which has a domain homologous to vacuolar protein sorting 10 (Willnow et al., 2008; Hermey 2009;Willnow et al., 2011). Vacuolar protein sorting 10 transports carboxypeptidase Y from the Golgi apparatus to secretory vacuoles in the yeast (Marcusson et al., 1994). Early studies show that SorLA plays important roles in transporting and processing amyloid precursor protein (Andersen et al., 2005;Nielsen et al., 2007; Schmidt et al., 2007).
Given that there are only a few studies on the expression of GDNF splice variants in the brain, we investigated the expression of α- and β-pro-GDNF at the mRNA and protein levels in seven mouse brain regions containing dopaminergic neurons.
Materials and Methods
Animals
Twenty-four specific-pathogen-free C57BL mice, weighing 20-25 g and 5 weeks of age, including 12 males and 12 females, were provided by the Experimental Animal Center of Xuzhou Medical University, China [license No. SYXK(Su) 2016-0028]. All mice were maintained as previously described (Wang et al., 2018), and efforts were made to reduce animal suffering. All experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996) and the Regulations of the People’s Republic of China on the administration of laboratory animals. This work was approved by the Animal Ethics Committee of Xuzhou Medical University, China on July 14, 2016.
Brain tissue preparation
After intraperitoneal injection of a mixture of ketamine (100 μg/g; Henry Schein Animal Health, Dublin, OH, USA) and xylazine (10 μg/g; Sigma-Aldrich, St. Louis, MO, USA) (Qi et al., 2019), brain tissues from seven regions containing dopaminergic neurons were obtained from each mouse under a stereomicroscope, including the olfactory bulb, amygdala,hypothalamus, hippocampus, corpus striatum, pons, and substantia nigra/ventral tegmental area. All dissected tissues were immediately stored at -80°C.
RNA and protein extraction
Nine mice were used to extract RNA, and nine RNA samples were collected for each brain region and the whole brain.Brain tissues (50-100 mg) were washed three times with icecold PBS to remove residual blood, followed by the addition of 1 mL TRIzol (Invitrogen, Life Technologies, Carlsbad, CA,USA). After homogenizing with an electric homogenizer,RNA was extracted according to the manufacturer’s instructions. Whole brain RNA was obtained by mixing the RNA samples of the seven brain regions.
A total of 15 mice were used for protein isolation. First,the same brain regions from five mice were mixed randomly to extract proteins, and then, three protein samples were obtained for each brain region and the whole brain. Next,the brain tissues were cleaned with ice-cold PBS, followed by the addition of RIPA lysis buffer (KeyGEN BioTECH, Co.,Ltd., Nanjing, China) containing phenylmethylsulfonyl fluoride (1:100). The mixture was homogenized, incubated for 30 minutes at 4°C, and then centrifuged at 13,800 × g for 30 minutes at 4°C. The supernatant was harvested, and protein concentration was measured using a bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology, Haimen,China). Whole brain protein was obtained by mixing the protein samples of the seven brain regions.
Design of specific primers
Because of the lack of sequences for mouse GDNF splice variants in the National Center for Biotechnology Information database, we determined the mouse Δ78 sequence using the basic local alignment search tool. Specific primers were designed using Primer Premier 5.0 software (PRIMER Biosoft, Palo Alto, CA, USA). The forward and reverse primers for α-pro-GDNF were located within the Δ78 sequence,while the upstream and downstream primers for β-pro-GDNF spanned the exon 2-exon 3 splice site. The primer sequences are shown in Table 1.
Real-time quantitative polymerase chain reaction (qPCR)
qPCR was performed using specific primers for α- and β-pro-GDNF using the Light Cycler 480 system (Roche Diagnostics, USA). RNA isolated from brain tissues wasreverse-transcribed into cDNA with the PrimeScript II first strand cDNA synthesis kit (Takara, Dalian, China). qPCR was conducted in a 20-μL PCR reaction volume containing 10 μL SYBR Premix Ex Taq (Takara), 1 μL cDNA template,8 μL RNase H2O, 0.5 μL forward primer and 0.5 μL reverse primer. The thermocycling conditions were as follows: 30 seconds at 95°C, 1 cycle; 5 seconds at 95°C, 20 seconds at 60°C, and 10 seconds at 72°C, 40 cycles. β-Actin was used as the internal reference. The relative expression levels were quantified using the 2-ΔΔCtmethod (Livak et al., 2001).
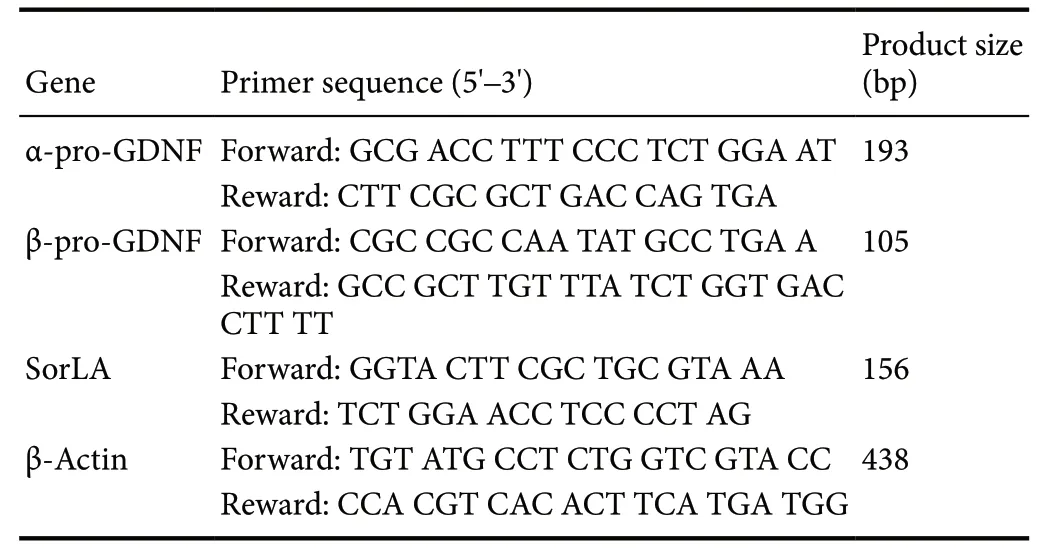
Table 1 Primer sequences for real-time quantitative polymerase chain reaction
western blot assay
The protein extracts (~50 μg/well) were separated by 10%SDS-PAGE and transferred onto nitrocellulose membranes(EMD Millipore, Billerica, MA, USA). The membranes were blocked in 5% bovine serum albumin for 2 hours at room temperature and incubated overnight at 4°C with the primary antibodies, including mouse β-actin polyclonal antibody (1:1000) and rabbit pro-GDNF polyclonal antibody(1:50), produced by GenScript Inc. (Nanjing, China). Both α-pro-GDNF and β-pro-GDNF can be detected using the anti-pro-GDNF antibody designed by our laboratory. The blots were washed with Tris-buffered saline/Tween and then incubated in the dark for 2 hours at room temperature with IRdye secondary antibodies, including goat anti-mouse and goat anti-rabbit antibodies (1:5000; LI-COR Biosciences,Lincoln, NE, USA). After three washes, the membranes were scanned with the Odyssey laser imaging system (LI-COR Biosciences). The gray values of the bands were analyzed with ImageJ software (version 1.48; National Institutes of Health, Bethesda, MD, USA) using β-actin as an internal reference.
Statistical analysis
Data, expressed as the mean ± SD, were analyzed with SPSS 16.0 software (SPSS, Chicago, IL, USA). Independent sample t-tests were performed to determine the statistical significances of α- and β-pro-GDNF expression levels in the same brain region and whole brain. One-way analysis of variance followed by the least significant difference test was conducted to evaluate significant differences in α- or β-pro-GDNF expression in different brain regions. To determine the relationship between GDNF splice variants and SorLA expression in the various brain regions, a simple linear regression(bivariate) was conducted to evaluate whether SorLA (a sorting protein) impacted the expression of the GDNF splice variants. P < 0.05 was considered statistically significant.
Results
Preparation of specific primers for α-pro-GDNF and β-pro-GDNF
The Cp values of α-pro-GDNF and β-pro-GDNF were 19.40 and 23.50 respectively, while the Cp values of total GDNF and water (the negative control) were 23.80 and > 35, respectively (Additional Figure 1A and B), indicating that the target PCR products could be amplified using the optimized primers. The homogeneity of the products was confirmed by the melting curves (Additional Figure 1C). Moreover,non-specific amplification products were not found, indicating high specificity of the primers.
Assessing Δ78 alternative splicing in the mouse brain
To assess alternative splicing (Δ78 locus) in the mouse brain,skin was used as the negative control (http://www.uniprot.org/uniprot/P48540). Agarose gel electrophoresis of the PCR products showed two fragments in the three brain tissues examined (Figure 1A), which confirmed the presence of the Δ78 alternatively-spliced transcript in the mouse brain.Furthermore, sequencing results demonstrated the alternative splicing occurred in the Δ78 locus (α-pro-GDNF and β-pro-GDNF splicing variants) during GDNF transcription in the mouse brain (Figure 1B).
Inverse expression of GDNF alternatively-spliced transcripts and proteins in the mouse brain
qPCR was conducted to further explore the mRNA expression of the GDNF splice variants in the various mouse brain regions. α-pro-GDNF mRNA was most highly expressed in the olfactory bulb, and least expressed in the hippocampus and substantia nigra/ventral tegmental area. β-pro-GDNF mRNA was most abundant in the olfactory bulb, corpus striatum and substantia nigra/ventral tegmental area, and least in the hippocampus (Figure 2A). Intriguingly, β-pro-GDNF was more abundant than α-pro-GDNF in every region(Figure 2A). Next, we assessed transcript levels in whole brain by combining the RNA samples from the seven brain regions. Expression of the β-pro-GDNF transcript was 6.2-fold higher than that of the α-pro-GDNF transcript (P < 0.01;Figure 2B).
Western blot assay was performed to evaluate α- and β-pro-GDNF expression at the protein level in the different mouse brain regions (Figure 3A). α-pro-GDNF protein was most abundant in the olfactory bulb, amygdala and corpus striatum, and least in the hypothalamus. β-pro-GDNF protein was most highly expressed in the pons and substantia nigra/ventral tegmental area, and least in the hypothalamus(Figure 3B). Protein samples from the seven brain regions were combined to measure expression in whole brain.Unexpectedly, β-pro-GDNF protein was significantly less abundant than α-pro-GDNF protein (P < 0.01; Figure 3C),inverse to the transcript expression results.

Figure 1 Measuring levels of the alternative splice variants using specific primers.

Figure 2 mRNA expression of α-pro-GDNF and β-pro-GDNF in the mouse brain.
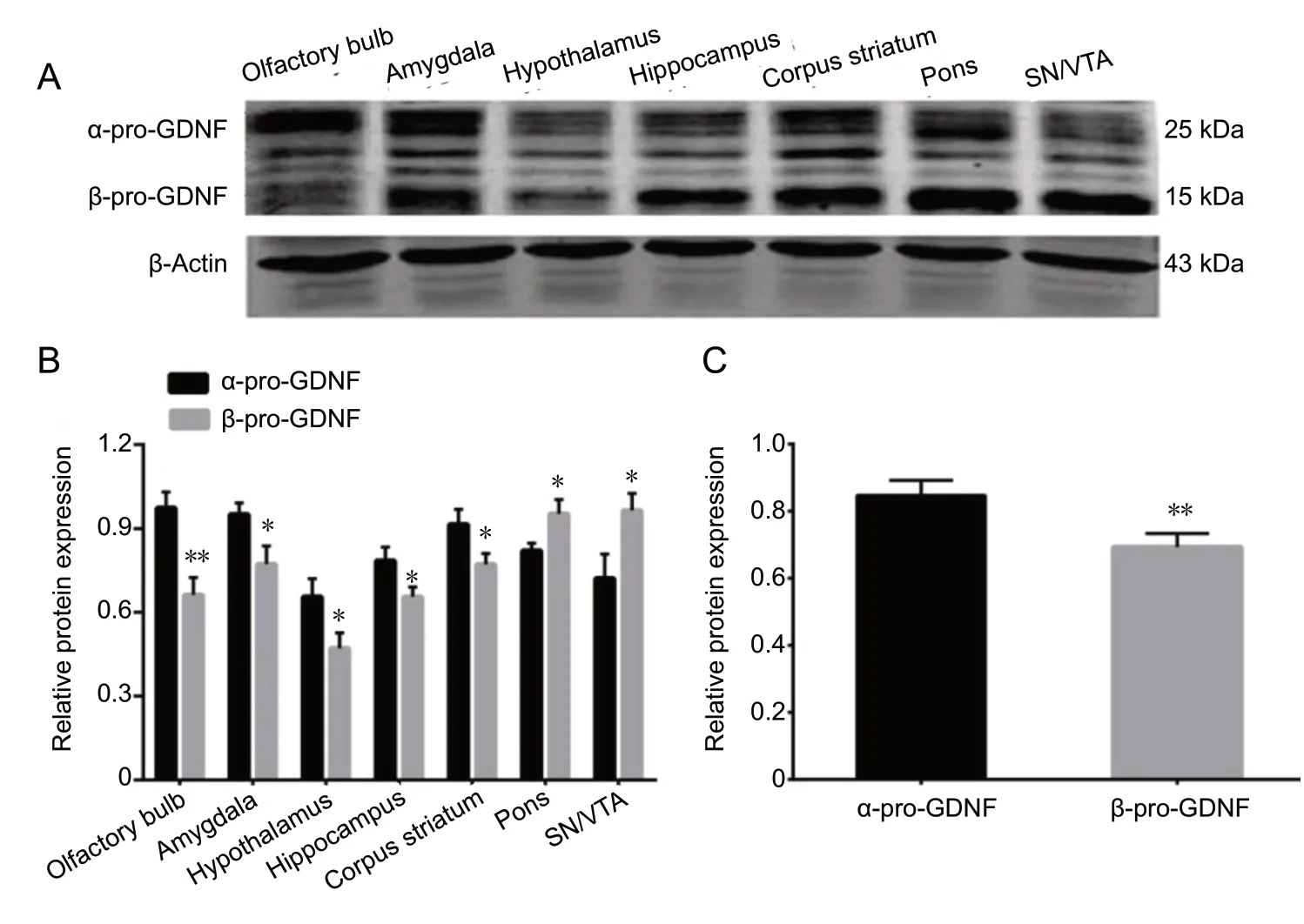
Figure 3 Protein expression of α-pro-GDNF and β-pro-GDNF in the mouse brain.

Figure 4 Relationship between GDNF splice variants and SorLA expression in the various brain regions.
SorLA is correlated with β-pro-GDNF, but not with α-pro-GDNF in the mouse brain
Given SorLA’s effects on amyloid precursor protein, we hypothesized that SorLA could affect pro-GDNF protein expression as well. Therefore, we examined the relationship between the expression of GDNF splicing variants and SorLA.SorLA was highly expressed in regions in which β-pro-GDNF was also highly expressed (Figure 4A). There was a linear relationship between SorLA and β-pro-GDNF expression levels. In contrast, the expression of SorLA was not associated with that of α-pro-GDNF (Figure 4B).
Discussion
GDNF, a member of the transforming growth factor β superfamily, is expressed and secreted in astrocytes, microglia and glioma cells (Ku et al., 2013; Liu et al., 2018; Fei et al., 2019;Li et al., 2019). It also enhances U251 cell migration and invasion (Tang et al., 2019), and promotes glioma progression(Ayanlaja et al., 2018). Additionally, GDNF promotes the regeneration and survival of dopaminergic neurons (Airaksinen et al., 2002; Andressoo et al., 2008; Saarenpaa et al., 2017;Chen et al., 2018), and is a promising neuroprotective therapy target for Parkinson’s disease (Dietz et al., 2006; Smith et al., 2007; Yue et al., 2017). Previous studies have reported two GDNF splice variants, with and without a 78 bp fragment, termed α-pro-GDNF and β-pro-GDNF, respectively(Lonka-Nevalaita et al., 2010). However, their expression in the different brain regions was unclear. Here, we examined expression of α-pro-GDNF and β-pro-GDNF mRNA and protein levels in seven mouse brain regions rich in dopaminergic neurons.
To date, there are no published reports on the mRNA expression of the GDNF splice variants using specific SYBR Green I qPCR primers. Given the lack of commercial reagents, we designed specific primers for α-pro-GDNF and β-pro-GDNF. Our results revealed differential expression of the GDNF splice variants in the various brain regions, consistent with previous studies (Trupp et al., 1995; Airavaara et al., 2011). Furthermore, β-pro-GDNF was significantly more abundant than α-pro-GDNF in each region at the mRNA level. Furthermore, consistent with these and previous findings (Suter-Crazzolara et al. 1994), the mRNA expression of β-pro-GDNF was 6.2-fold higher than that of α-pro-GDNF in whole brain. Unexpectedly, at the protein level, β-pro-GDNF expression was markedly lower than α-pro-GDNF expression. It is therefore necessary to investigate the mechanisms underlying the disparity in transcript and protein levels of the GDNF splice variants.
Early studies have shown that α- and β-pro-GDNF are secreted in a distinct manner (Lonka-Nevalaita et al., 2010).SorLA, a sorting protein, appears to be involved in the processing and secretion of GDNF precursor protein (Westergaard et al., 2004, Geng et al., 2011). The binding between GDNF Δ78 and SorLA is significantly weaker than that between full-length GDNF and SorLA (Geng et al., 2011),suggesting that SorLA has a stronger affinity for α-pro-GDNF than for β-pro-GDNF. Therefore, we hypothesized that SorLA could affect protein expression of the GDNF isoforms. Interestingly, we found a linear association between SorLA and β-pro-GDNF, while SorLA was not related to α-pro-GDNF, indicating that SorLA sorts GDNF in the mouse brain (Chen et al., 2005; Oh-hashi et al., 2009). Given that α-pro-GDNF is secreted constitutively and β-pro-GDNF is secreted activity-dependently (Lonka-Nevalaita et al.,2010), we speculated that β-pro-GDNF could be quickly transported and secreted into the extracellular space, leading to low intracellular β-pro-GDNF protein levels. Conversely,α-pro-GDNF secretion would likely be comparatively slow,resulting in α-pro-GDNF protein accumulation intracellularly. However, the mechanisms regulating α- and β-pro-GDNF secretion remain unclear.
In summary, we investigated α-pro-GDNF and β-pro-GDNF expression in the mouse brain, and examined the mechanism underlying the inverse expression of their transcripts and proteins. Differential expression of the GDNF splice variants was observed in brain regions containing dopaminergic neurons. α-pro-GDNF was less and more abundant than β-pro-GDNF at the mRNA and protein levels, respectively.Furthermore, we found that SorLA was related to β-pro-GDNF, but not α-pro-GDNF, suggesting that SorLA, as a sorting protein, might contribute to the converse expression of the mRNA and protein levels of the isoforms. However, how Sor-LA regulates pro-GDNF expression and secretion remains unknown. Furthermore, other mechanisms may also impact expression of the GDNF splice isoforms. In future studies, we will investigate in greater detail the mechanisms underlying the differential expression of α- and β-pro-GDNF. Nonetheless, this study provides new insight into α-pro-GDNF and β-pro-GDNF expression in the mouse brain.
Acknowledgments:We are very grateful to the staff from the Department of Anatomy and Neurobiology of Xuzhou Medical University, China for providing the experimental platform. We would also like to thank all contributors for their support and effort. We shall acknowledge the GenScript Inc Company (USA) for synthesizing the pro-GDNF polyclonal antibody.
Author contributions:Study design, experimental implementation, data collection, and manuscript writing: XHG, HL; experimental implementation, data analysis, manuscript writing: LZ; data interpretation and statistical analysis: TH, XC; manuscript review and modification: HW;substantial contributions to conception and design, fundraising and technical support: DSG. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This work was supported by the the National Natural Science Foundation of China, No. 81772688 (to DSG); the Postdoctoral Science Foundation of Jiangsu Province of China, No. 1202119C (to HL).The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:This study was approved by the Animal Ethics Committee of Xuzhou Medical University, China on July 14, 2016.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Figure 1:Assessing the specificity of the PCR primers.
- 中国神经再生研究(英文版)的其它文章
- Ethanol extract from Gynostemma pentaphyllum ameliorates dopaminergic neuronal cell death in transgenic mice expressing mutant A53T human alpha-synuclein
- Peripheral nerve injury induced changes in the spinal cord and strategies to counteract/enhance the changes to promote nerve regeneration
- Genetic targeting of astrocytes to combat neurodegenerative disease
- Pathological significance of tRNA-derived small RNAs in neurological disorders
- Applications of advanced signal processing and machine learning in the neonatal hypoxic-ischemic electroencephalography
- Protective effect of hydrogen sulfide on oxidative stress-induced neurodegenerative diseases

