Mesenchymal stem cell-derived exosomes:Toward cell-free therapeutic strategies in regenerative medicine
Zhan-Jun Ma,Jing-Jing Yang,Yu-Bao Lu,The Second Clinical Medical College,Lanzhou University,Lanzhou 730000,Gansu Province,China
Zhao-Yang Liu,Department of Medical Imaging,Shanxi Medical University,Jinzhong 030600,Shaanxi Province,China
Xue-Xi Wang,School of Basic Medical Sciences,Lanzhou University,Lanzhou 730000,Gansu Province,China
Abstract
Key words:Exosomes;Mesenchymal stem cells;Cell-free therapy;Regenerative medicine;Mesenchymal stem cell-derived exosomes;Extracellular vesicles
INTRODUCTION
Regenerative medicine,aimed at promoting the repair and regeneration of tissues and organs,is multidisciplinary.It can be understood as the use of biology and tissue engineering to find effective and feasible treatments that promote self-repair and regeneration or the generation of new tissues or organs to maintain,improve,and repair damaged bodies.Stem cell transplantation is the main method for tissue regeneration.Stem cells are immature tissue precursor cells that are capable of selfrenewal to form a cloned cell population and,thus,differentiate into multiple cell lineages[1,2].Stem cells can be classified as (1) Embryonic stem cells derived from early embryos;(2) Induced pluripotent stem cells;and (3) Adult stem cells,including hematopoietic stem cells,neural stem cells,and mesenchymal stem cells (MSCs).The therapeutic potential of stem cells can be attributed to three key mechanisms[3].The first is homing,the migration of stem cells to the site of injury;the mechanism is thought to be similar to that of leukocyte migration and to involve cell-surface receptors,such as chemotactic receptors.Integrins,vascular cell adhesion molecule 1,and G protein receptor signals are also likely to play important roles in this process.The second is differentiation into diverse cell types,enabling supplementation or replacement of damaged cells[4].The third is secretion of biologically active factors that affect surrounding tissues.Adult stem cells promote the maintenance and repair of adult tissues and organs[5].MSCs are one of the most important types of adult stem cell and have been used for cell-based therapy of diverse diseases[6].
MSCs were discovered in 1968 by Friedensteinet al[7],who described them as fibroblasts capable of secreting hematopoietic growth factors and cytokines.Later studies showed that MSCs are ubiquitous and can be isolated from a variety of tissues,including bone marrow,adipose tissue,dental pulp,umbilical cord,umbilical cord blood,placenta,amniotic fluid,Wharton’s jelly,the brain,spleen,liver,kidney,lung,thymus,and pancreas.Moreover,MSCs have self-renewal ability and can differentiate into multiple cell types[8,9].MSCs can be isolated and expanded from the stroma of many tissues,e.g.,bone marrow and subcutaneous adipose tissue[10].MSCs show promise for cell therapy because of the their ease of isolation,self-renewal andin vitroexpansion ability,low immunogenicity,multidirectional differentiation,and release of trophic materials that promote tissue renovation or direct cell replacement[11].However,the disadvantages of MSCs include the difficulty in producing cells with a stable phenotype,the deleterious effect of the presence of large cells in the pulmonary microvasculature,host cell rejection,ectopic tissue formation,and tumor formation.These disadvantages have restricted their clinical use[12-15].Thus,alternative MSC-based and complication-free therapeutic strategies are needed.The therapeutic potential of MSCs is determined by their paracrine secretion of a range of growth factors,chemokines,and cytokines[16-18].Therefore,finding a cell-free therapeutic strategy with the same output and efficacy seems to be necessary.
Research has focused on extracellular vesicles (EVs) secreted by MSCs as a possible non-cellular therapy[19].MSCs release numerous EVs,including microvesicles (MVs),exosomes,and apoptotic bodies,which may act as paracrine mediators between MSCs and target cells[20].MVs and exosomes exert a pro-regenerative effect,which is mediated by their protein,mRNA,and regulatory non-coding RNA (e.g.,microRNA[miRNA]) contents.Exosomes are the most prominent type of EV and have potential for cell-free therapy because of their biological activities and ability to mediate intercellular communication[21,22].MSC-derived exosomes (MSC-Exos) replicate the biological activity of MSCs and are thus an alternative to whole-cell therapy[23,24].In addition,the surface of exosomes can be modified to enable targeting of specific cell types,suggesting their promise for cell-free therapy.
MSC-Exos have potential for tissue engineering and regenerative therapy.In this review,we summarize the characteristics of MSC-Exos and highlight their functions and potential as a novel cell-free strategy for regenerative medicine.
CHARACTERISTICS AND BIOLOGICAL FUNCTIONS OF MSCS
Characteristics
MSCs are an undifferentiated adult stem cell population with self-renewal ability,low immunogenicity,and multilineage differentiation potential.MSCs have plastic adhesion properties and can be easily isolated from a variety of tissues,such as adipose tissue,umbilical cord blood,liver,amniotic fluid,placenta,and dental pulp[8,9].The International Therapeutic Association of MSCs established the recognition characteristics of human MSCs in 2006.These include maintenance of adherence under standard culture conditions;expression of CD105,CD73,CD90,STRO-1,CD29,and CD44;no expression of CD45,CD34,CD14,CD11b,CD79a,CD19,or HLA-DR;and the ability to differentiate into osteoblasts,adipocytes,and chondrocytesin vitro[25].The ease of isolation and biological functions of MSCs make them suitable in preclinical and clinical trials of cell therapy (Figure1).
Biological functions
Multilineage differentiation potential:MSCs can differentiate into multiple mesenchymal (such as osteoblasts,chondrocytes,adipocytes,endothelial cells,and cardiomyocytes) and non-mesenchymal (such as neurons,glial cells,and hepatocytes)lineages.These characteristics make MSCs good seed cells for tissue engineering and regenerative medicine (e.g.,bone and cartilage reconstruction,nerve regeneration,and vascular tissue repair)[26].
Promotion of tissue repair:After systemic adoptive transfer,MSCs may occur with lodging in non-specific tissues,homing to natural walls or migration into damaged and/or diseased tissues[27].MSCs can migrate to injured tissue and release cytokines,inflammatory mediators,extracellular matrix (ECM) components,and antibacterial proteins,thereby generating a suitable microenvironment for tissue repair.MSCs are suitable for repair of tissue injury and treatment of,for instance,diabetes,graft-vs-host,cardiovascular,inflammatory,liver,lung,kidney,nerve,autoimmune,and bone and cartilage diseases[28,29].In a rat model of lipopolysaccharide (LPS)-induced acute lung injury,allogeneic MSCs transplantation ameliorated the redox environment by upregulating heme oxygenase 1 and protected against lung injury[30].In a clinical trial,autologous bone-marrow-derived MSCs (BMSCs) ameliorated the motor disability and cognitive impairment in stroke patients[31].
Immunosuppression:The therapeutic effect of MSCs is mainly attributed to their immunoregulatory activity.MSCs exert immunomodulatory and anti-inflammatory effects by regulating lymphocytes associated with the innate and adaptive immune system[32].MSCs modulate the immune response by inhibiting a wide range of immune cells,including T,B,and natural killer (NK) lymphocytes,and affecting the function of myeloid cells such as monocytes,dendritic cells (DCs),and macrophages[11,33].Specifically,MSCs inhibit T-cell proliferation,activation,and secretion of inflammatory factors [such as interleukin (IL)-2,tumor necrosis factor (TNF)-α,and interferon-γ],reduce the Th1/Th2 ratio,and decrease the number of Th17 cells[33].Also,MSCs induce the generation of regulatory T cells (Tregs),including classic CD4+CD25+FoxP3+Tregs and non-classical Tregs (such as CD8+CD28-regulatory T cells),and IL-10+Tr1 cells[34,35].In addition,MSCs suppress the differentiation of B lymphocytes into plasma cells and their secretion of immunoglobulins[36].Furthermore,MSCs inhibit the cytotoxicity potential of NK lymphocytes,and promote the transformation of M1 macrophages (pro-inflammatory) to M2 macrophages (anti-inflammatory)[37,38].MSCs modulate antigen presentation by antigen-presenting cells by downregulating MHC and co-stimulatory molecules (CD40,CD86,and CD80) and suppressing the maturation of DCs[38].The immunomodulatory properties of MSCs suggest their therapeutic potential for a variety of diseases.
Neuroprotective effect:MSCs transdifferentiate into neural cells and secrete neurotrophic and anti-inflammatory factors following transplantation,thus exerting strong trophic and neuroprotective effects.The therapeutic role of MSCs has been evaluated in preclinical models of neurodegenerative diseases,including amyotrophic lateral sclerosis (ALS),Huntington disease,multiple sclerosis (MS),Parkinson disease,and spinal cord injury (SCI)[39].The neuroprotective effect of MSCs is mediated by production of neurotrophic factors,such as brain-derived neurotrophic factor (BDNF),ciliary neurotrophic factor,glial cell line-derived neurotrophic factor,nerve growth factor,and neurotrophin-3 (NT-3)[39,40].The BDNF and NT-3 released by MSCs act on neural progenitor cells in the lesion,improving neurogenesis[40,41].
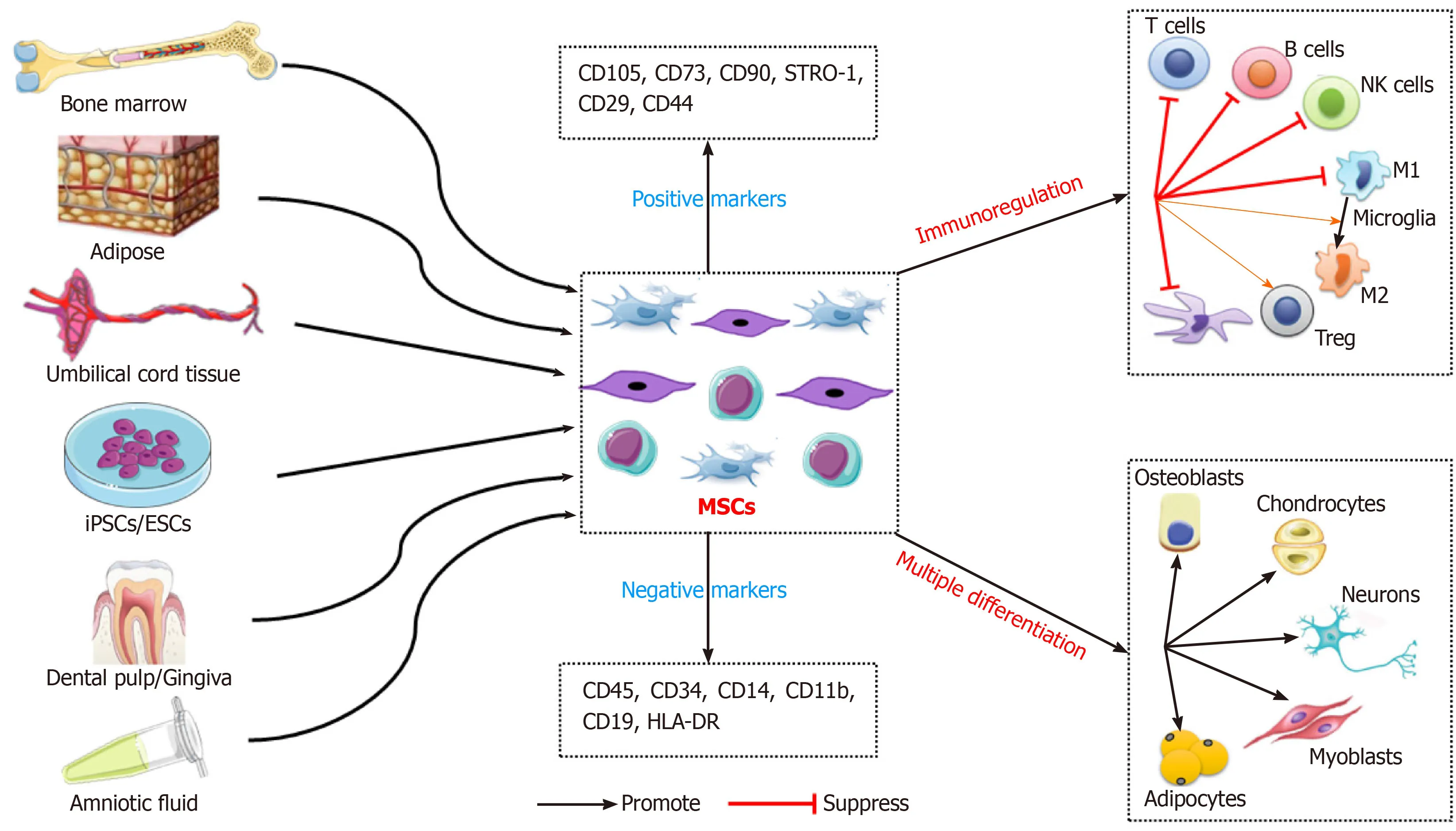
Figure1 Schematic diagram of mesenchymal stem cells-based regenerative medicine.Mesenchymal stem cells can be easily isolated from a variety of tissues,and the multiple differentiation and immunomodulatory properties of mesenchymal stem cells make them ideal candidates for cell therapy.ESCs:Embryonic stem cells;iPSCs:Induced pluripotent stem cells;CD:Cluster of differentiation;MSCs:Mesenchymal stem cells;DC:Dendritic cells;NK cells:Natural killer cells;M1:Microglia M1 phenotype;M2:Microglia M2 phenotype;Treg:Regulatory cell.
Mechanisms underlying MSC based therapy
MSCs have diverse functions but the underlying mechanisms are unclear.The therapeutic potential of MSCs is based mainly on their immunoregulatory activity and replacement of damaged tissue by differentiating into various cell lineages.It has long been thought that the effect of MSCs on damaged or diseased tissue is based on their immunoregulatory effect[11].However,the therapeutic benefit of MSCs is attributable not only to their differentiation capacity but also their secretion of soluble factors that exert immunoregulatory,angiogenetic,and ECM remodeling,and anti-apoptotic,antifibrotic,and antioxidant effects[8,16].In this way,MSCs directly or in a paracrine manner rescue damaged cells,reduce tissue damage and,ultimately,accelerate organ repair[42].Haynesworthet al[42]in 1996 first reported the paracrine effect of MSCs.MSCderived paracrine factors have been shown to promote angiogenesis,protect against acute renal,liver,and tissue injury,promote neovascularization,and enhance arteriogenesis[5,18,43].MSCs secrete mediators that directly activate target cells or stimulate neighboring cells to secrete active factors[43].Interestingly,MSC-derived EVs,including exosomes,exert other paracrine effects on tissue regeneration by transferring information to damaged cells or tissue and have biological activity similar to that of MSCs[19].Moreover,compared with MSCs,MSC-Exos can cross biological barriers,can be modified to load molecular drugs,have fewer side effects and less immunogenicity,and remain active during storage[44].Therefore,the regenerative potential of MSC-Exos as cell-free therapy has been evaluated.
EXOSOMES
Definition and morphological characteristics of exosomes
Exosomes are one of the main subclasses of EVs,which were discovered in sheep reticulocytes in 1983[45];the term “exosome” was coined in 1987[46].Exosomes are small lipid membrane vesicles,which are formed by endocytosis,integration,and efflux.Exosomes are secreted by a wide range of mammalian cell types,including MSCs,B cells,cytotoxic T cells,neurons,cancer cells,oligodendrocytes,platelets,epithelial cells,DCs,and mast cells[47].Exosomes are present in body fluids such as saliva,blood,bile,urine,semen,cerebrospinal fluid,ascites fluid,amniotic fluid,and colostrum[48].Morphologically,exosomes are described as cup-shaped or saucer-like when observed by transmission electron microscopy[48,49].Similar to other lipid vesicles,exosomes float in a sucrose gradient and have a density of 1.13 g/mL (B-cell-derived exosomes) to 1.19 g/mL (intestinal cell-derived exosomes)[49,50].B-cell exosomes are the most homogeneous in terms of size (60-80 nm)[50].
EVs are classified as exosomes,MVs,or apoptotic bodies,depending on their origin.Exosomes are 30-100 nm in diameter,MVs are 100-1000 nm in diameter,and apoptotic bodies are 1-5 μm in diameter[49,51,52].There are overlaps in the sizes of EVs,and the lack of standardization is an issue.The major EV subtypes currently recognized,together with their basic characteristics,are summarized in Table1.
Biogenesis of exosomes
Exosomes originate from the endocytosis-exogenous pathway,while other EVs are derived directly from the plasma membrane.Exosome biogenesis occursviathe endocytosis-ectopic pathway when cells absorb a small amount of intracellular fluid in specific membrane regions and form early endosomes.Those early endosomes begin to mature and expand into late endosomes,which undergo inward germination to form intraluminal vesicles (ILVs) with a diameter of 30 nm to 100 nm.Late endosomes,often referred to as multivesicular bodies (MVBs) due to their inclusion of ILVs,fuse with lysosomes,resulting in degradation of their contents,or fuse with the cell membrane and are released into the extracellular environment – these are defined as exosomes[48,52].The exosomes are subsequently taken up by recipient cells.Exosomes can be endocytosed or interact with recipient cells through ligand-receptor or direct binding[53](Figure2).Although the endosomal-dependent pathway is the main route of exosome biogenesis,direct budding of the plasma membrane can also produce exosomes.Two major MVB and ILV biogenesis pathways have been identified:The endosomal sorting complex required for transport (ESCRT)-dependent and ESCRTindependent pathways (Figure2).The ESCRT comprises four complexes and their associated proteins,ESCRT-0,ESCRT-I,ESCRT-II,and ESCRT-III,which are involved in identifying ubiquitinated proteins in the endosomal membrane,and budding and separating of the endosomal membrane then modulate the integration process that ultimately produces ILVs[54].In contrast,the ESCRT-independent pathway integrates cellular content into exosomesviabudding of ceramide-induced ILVs[55].The classification of other proteins is mediated by variations in the normative ESCRTdependent pathway[56].In addition,there are other mechanisms in exosome biogenesis,and this finding suggests that ILV formation requires sphingolipid ceramide.Moreover,neutral sphingomyelinase enhances ILV formation by promoting MVB budding[48].
Isolation of exosomes
Various exosome separation techniques,including ultracentrifugation-based separation technology,size-based technology,precipitation technology,and immunoaffinity capture,as well as novel combinations of these,are available or under development (Table2).
Ultracentrifugation:The method most commonly used to isolate exosomes is ultracentrifugation,frequently in combination with a sucrose density gradient or a sucrose cushion[57].Cells and larger particles are removed by increasing the centrifugal force,and exosomes are pelleted by centrifugation at ≥ 100000 ×gfor >2 h.This method is simple and cost-effective but requires specialized equipment and lacks specificity,so exosomes may be contaminated with other EVs of similar diameter[58].
Membrane filtration:Exosomes can be isolated by membrane filtration[58].After removing cell debris and macromolecules,the sample is ultrafiltered to remove contaminants.Membrane filtration is rapid and easy to perform.However,it can be difficult to separate exosomes from contaminants,such as apoptotic bodies or vesiclesof similar diameter,depending on the pore size of the filter[59].
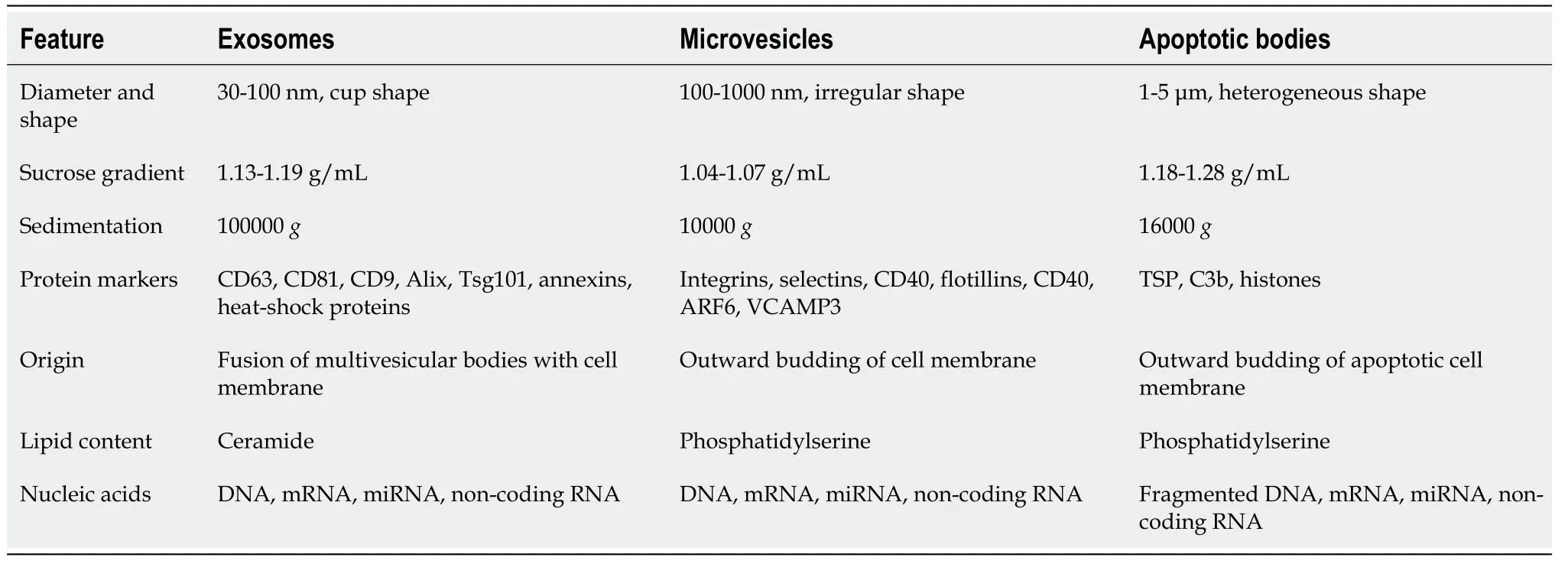
Table1 Characteristics of different types of extracellular vesicles

Table2 Summary of exosome isolation methods
Precipitation:Polyethylene glycols (PEGs) can be used for precipitation[60].ExtraPEG was adapted from a PEG-based virus isolation method and can be applied to various vesicle types and biological fluids[61].PEG-mediated exosome isolation involves lowspeed centrifugation followed by a single small-volume filtration purification step.This method is rapid and inexpensive[58],but the exosomes produced are of low purity and the technique is costly[57].
Size exclusion chromatography:Exosome isolation by size exclusion chromatography(SEC) involves a column packed with porous polymeric beads.SEC involves removal of cells and larger particles by low-speed centrifugation,followed by two filtration steps using 0.2 μm pore filters with a 100 kDa molecular weight cut-off and purification by SEC[62].High yield and no need for specialized equipment are the main advantages of this approach but the exosomes produced have low purity and clogging,vesicle capture,and exosome loss due to membrane attachment can occur[57,58].
Immunoaffinity capture:Immunoaffinity capture of exosomes involves antibodies against exosome markers (including CD81,CD63,or CD9) and specialized lectins targeting mannose[62,63].This method enables production of exosomes with high purity but is costly,has a low yield,and requires cell-free samples[57].
In addition,exosome isolation kits and precipitation solutions can be used to isolate exosomes.However,there is no one-size-fits-all technique,and it is impractical to separate exosomes completely from other components.Therefore,the most appropriate technique for isolating exosomes should be selected.After isolation,exosomes can be stored at -80 °C.
Characterization and identification of exosomes
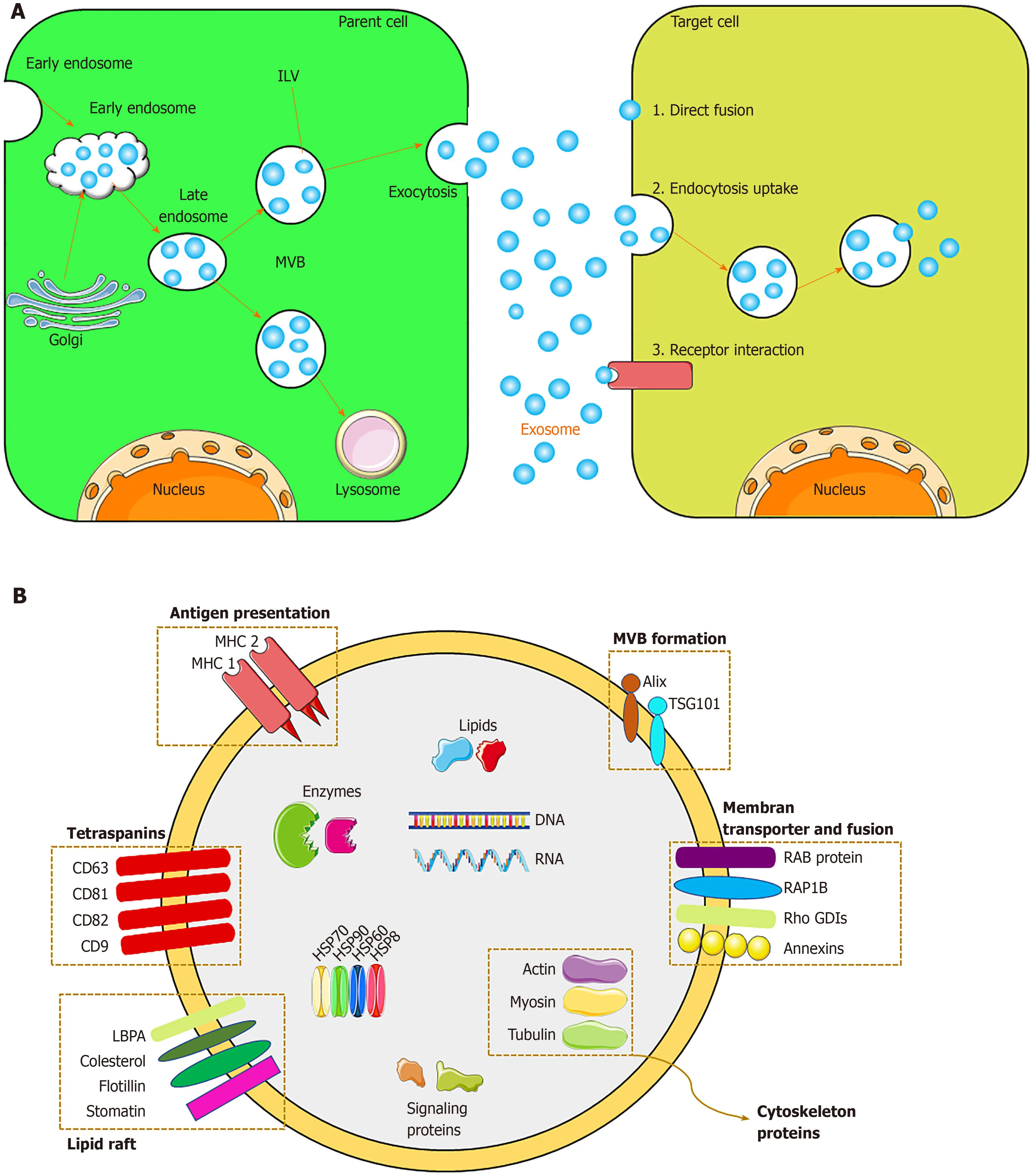
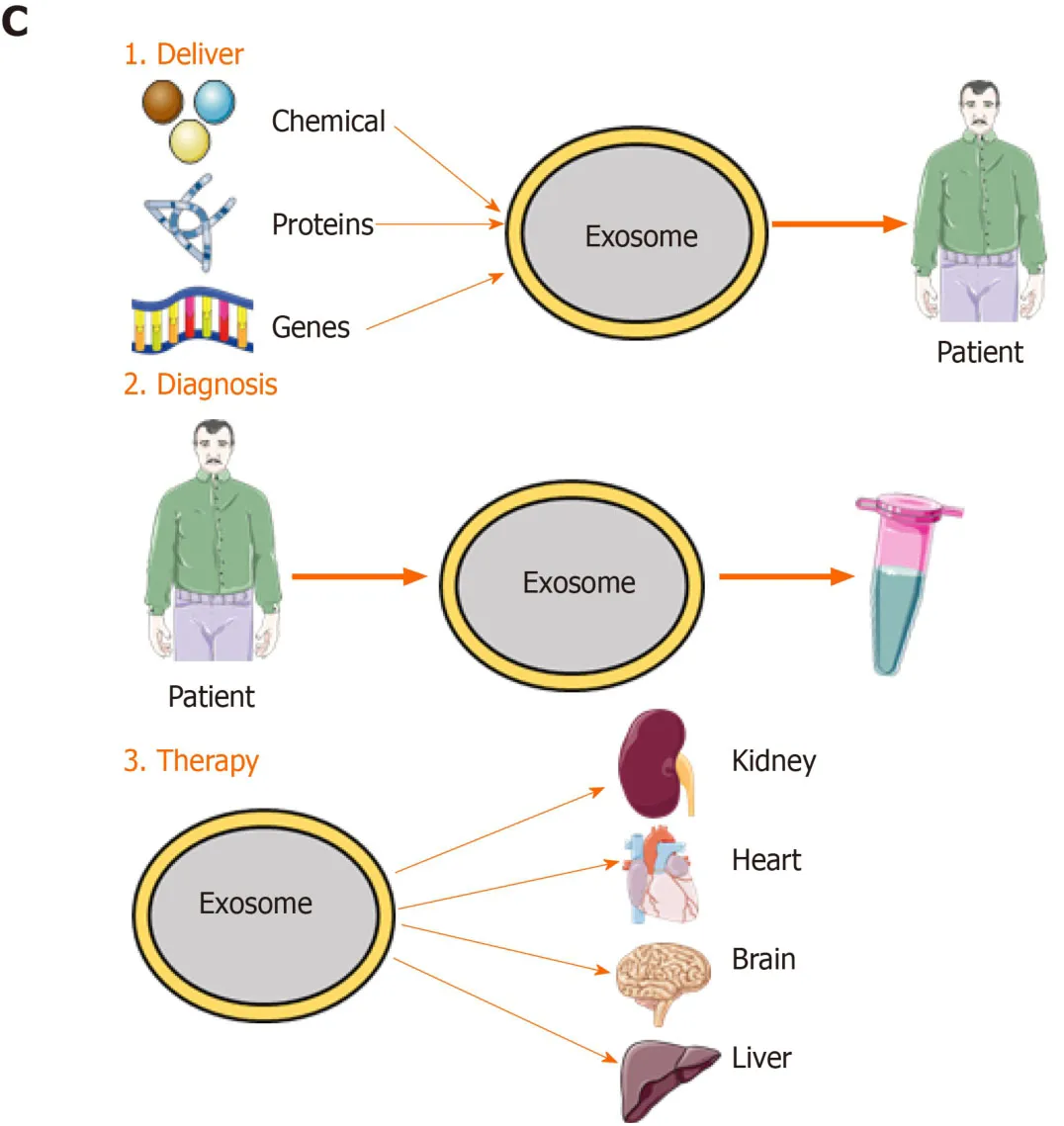
Figure2 Exosome biogenesis and its application.A:Exosome biogenesis and intercellular communication;B:Exosome components;C:Exosome application.The applications include:(1) Drug deliver.Therapeutic agents such as chemicals,peptides,and RNAs can be delivered into patients;(2) diagnosis:Exosomes derived from patients can be used for disease diagnosis;and (3) therapy:Exosomes derived from mesenchymal stem cells can be used for various diseases.MVB:Multivesicular body;ILV:Intraluminal vesicle;MCH 1,2:Major histocompatibility complex 1,2;TSG101:Tumor susceptibility gene 101;ALIX:ALG-2-Interacting Protein X;RAP1B:Member of RAS oncogene family.
Exosomes are identified based on their morphology,size,and marker proteins.Methods for identifying exosomes include transmission electron microscopy (TEM),scanning electron microscopy (SEM),atomic force microscopy (AFM),nanoparticle tracking analysis (NTA),flow cytometry analysis,Western blot,and enzyme-linked immunosorbent assay (ELISA)[47,62].TEM,SEM,and AFM are used to determine the size and morphology of exosomes;of these,TEM is the most frequently used[64].NTA is frequently applied to evaluate the size distribution and concentration of exosomes.Flow cytometry and Western blot can be used to identify exosome surface marker proteins[65],for example,CD9,CD63,CD81,and CD82.The proteins in exosomes can be quantified by Bradford or bicinchoninic acid assay or ELISA[65].Two or three of these methods are often used in combination to analyze exosomes.
Exosome contents and function
Because exosomes are formed by budding from early endosomes,they have a lipid bilayer membrane,which protects the resident genetic material (DNA,mRNA,miRNA,pre-miRNA,and other non-coding RNAs),lipids,and proteins during transportation to target cells[66].The most common exosomal surface proteins are members of the tetraspanin family,a group of scaffold membrane proteins including CD63,CD81,and CD9,which serve as markers.Other common proteins include membrane transporters and fusion proteins (such as GTPases and annexins),heat shock proteins (such as HSP60,70,and 90),MVB biogenic proteins (such as ESCRT complex,Alix,and TSG101),lipid-related proteins,and phospholipases[21,67].The exosome membrane also contains cholesterol,sphingomyelin,and ceramide in a large number of lipid rafts[68].Exosomes also contain mRNA and miRNA,which,upon endocytosis by the recipient cell,modulate protein synthesis and cell function[68].The protein,lipid,and nucleic acid contents of exosomes vary according to the identity and physiological condition of the source cell and the extracellular environment.Therefore,the content of exosomes serves as an indicator of their source cell.Unique exosomes containing different proteins and RNAs determine their various subpopulations and therefore exert different effects on recipient cells.
Exosomes have various functions.Depending on their characteristics,exosomes can be used for disease diagnosis,drug delivery,and as therapeutic agents (Figure2).Exosomes engage in specific interactions with the recipient cells,promoting information and material exchange between widely separated anatomic sites[69].Because they can cross the blood–brain barrier,exosomes have potential for drug delivery to the brain[70].The nanometer-scale size and stability of exosomes suggest their diagnostic potential.Finally,exosomes are a cell-free alternative to cellular therapy.Following injection,exosomes are safer and easier to control than live cells,which can undergo uncontrolled growth and tumor formation[24].The roles of exosomes in immunology and cancer biology have been established[71].
Unlike cells,exosomes do not undergo malignant transformation,do not replicate,and do not induce metastasis.In this review,we focus on the potential of MSC-Exos in regenerative medicine.
MSC-EXOS
MSCs have been isolated from a variety of sources.Because of their ease of isolation,no ethical considerations,and low immunogenicity,MSCs have therapeutic potential for various diseases.However,injection of MSCs may cause malignant transformation and spread of tumors.Also,differentiation of MSCs induces tissue ossification or calcification in animal models.MSC-Exos have the same functions as MSCs,without the above complications.
Properties and functions of MSC-Exos
MSC-Exos were first isolated by Laiet al[72]in 2010,and the purified exosomes reduced the infarct area in a mouse model of myocardial ischemia/reperfusion (I/R) injury.Therefore,exosomes represent novel biological agents for promoting tissue repair.MSCs are the most prolific exosome producers[73],and the exosomes produced by MSCs have similar morphological characteristics,isolation methods,and preservation conditions to those from other cell types.The composition of exosomes depends on their cellular origin.MSC-Exos harbor membrane-bound proteins such as CD44,CD73,and CD29;the surface protein profile of exosomes is dependent on the medium used to culture the source MSCs.mRNAs and miRNAs are encapsulated in MSC-Exos;the miRNAs participate in the exchange of information between cells and modulate the function and fate of the recipient cell[73].
MSC-Exos carry proteins,lipids,DNA,and RNA from MSCs,which is the basis for their therapeutic effect.MSC-Exos have biological functions similar to MSCs,but have a smaller volume,can penetrate biofilm,have low immunogenicity,and can be stored.The lipid bilayer of exosomes protects the contents and protects nucleic acids from RNases[74].Furthermore,exosomes transport a variety of biologically active components and reflect the physiological and pathological state of the source cell;they transmit information,remove intracellular components,and can transport drugs[73].Also,MSC-Exos can suppress apoptosis;promote cell regeneration and migration;regulate the immune and inflammatory responses;and promote angiogenesis,nerve regeneration,and tissue repair and regeneration (Figure3).MSC-Exos have potential for regenerative medicine,as shown in animal models of disease and injury[75].
Regenerative advantages of exosomes over MSCs
Ease of collection:Various types of MSCs secrete exosomes,and each produces 1000 to 10000 exosomes.Exosomes can be extracted from culture medium by,for example,ultracentrifugation.Exosomes can be produced on a large scale using specialized cell lines[76].Compared with MSCs,the production of MSC-Exos is simpler and less costly and time-consuming.
Stability for long-term storage:The volume of exosomes is about one millionth that of MSCs,and they are less complex,have a stable structure,and are easy to produce and store.Exosomes are unaffected by storage at -20 °C for 1 wk,and their activity is maintained during long-term storage at -80 °C[73].
Safety:MSC-based therapies have issues with cell survival,regenerative ability,immune rejection,and differentiation to tumors.These problems can be avoided by using exosomes as cell-free therapy.Due to the low content of exosome membranebound proteins,the possibility of immune rejection is very low even after allogeneic administration.In addition,exosomes do not proliferate,so there is no possibility of tumor formation[77].Therefore,MSC-Exos have better safety than MSCs for clinical applications.
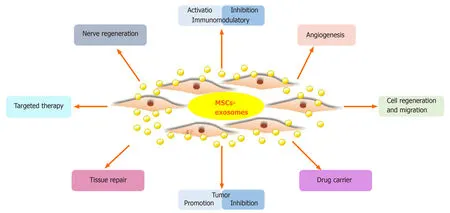
Figure3 Main functions of mesenchymal stem cell-derived exosomes.MSCs:Mesenchymal stem cells.
Exosomes as ideal carriers:Exosomes can transfer active substances into recipient cells for cell-to-cell information exchange.Therefore,exosomes can be used as carriers for drugs and biological macromolecules.Sunet al[78]in 2010 reported that curcumin transported in exosomes had a stable structure,improved dissolution ability,a higher blood concentration,and greater anti-inflammatory activity.In animal models,curcumin in exosomes protected mice from LPS-induced septic shock.Furthermore,unlike non-host vehicles,exosomes have low immunogenicity and so do not induce immune rejection or other complications.
Targeting:The exosome membrane harbors proteins with binding affinity for the target cell membrane or a ligand in the ECM.These membrane-bound molecules facilitate the targeting of exosomes to specific tissues or microenvironments.The bilayer membrane of exosomes can be modified with specific factors to enable targeting of cells and tissues[48].
THERAPEUTIC POTENTIAL OF MSC-EXOS IN REGENERATIVE MEDICINE
Based on the advantages of MSC-Exos as cell-free therapy,their regenerative and therapeutic potential has been exploredin vitroandin vivo.Below we will summarize recent studies on the effects of MSC-Exos on conditions of the kidney,liver,cardiovascular system,nervous system,skin,bone,and muscle (Figure4).
MSC-Exos in kidney diseases
Acute kidney injury:Renal I/R injury (I/RI) is one of the causes of acute kidney injury (AKI) and is caused by sudden obstruction of blood flow to the kidneys.It is associated with morbidity and mortality in patients with AKI[79].In addition,AKI is a potential risk factor for progressive chronic kidney disease (CKD),and there is no effective treatment[79].Wanget al[80]assessed the effect of human bone marrow-derived MSC-Exos in rats with I/R-induced AKI.MSC-Exos improved renal I/RI and renal function by reducing the urea and creatinine levels and inhibiting inflammation and apoptosis.In a mouse model of I/RI,C-C motif chemokine receptor-2 (CCR2)-enriched mouse bone marrow-derived MSC-Exos strongly bound extracellular CCL2 and reduced its concentration,inhibiting the recruitment and activation of peripheral monocytes/macrophages.Importantly,CCR2 knockdown MSC-Exos failed to bind CCL2 and did not protect against renal I/RI[81].Moreover,miRNAs in MSC-Exos also exert a reno-protective effect.Zhuet al[82]studied the effect of human bone marrowderived MSC-Exos containing miR 199a 3p on renal I/RI in a mouse model.Injection of MSC-Exos into mice with I/R injury induced recovery of renal function and histologic protection and reduced the cleaved caspase 3 and semaphorin 3A levels.Anin vitrostudy by the same group showed that MSC-Exos increased the expression of the anti-apoptotic protein Bcl-2 and decreased that of the pro-apoptotic proteins Bax and caspase 8 by activating the AKT and ERK pathways in oxygen-glucose deprivation (OGD)-induced HK-2 cells.Co-culture with miR 199a 3p knockdown MSC-Exos reversed these effects[82].Therefore,exosomal miR 199a 3p plays a crucial role in MSC-Exos mediated suppression of I/R induced apoptosis.
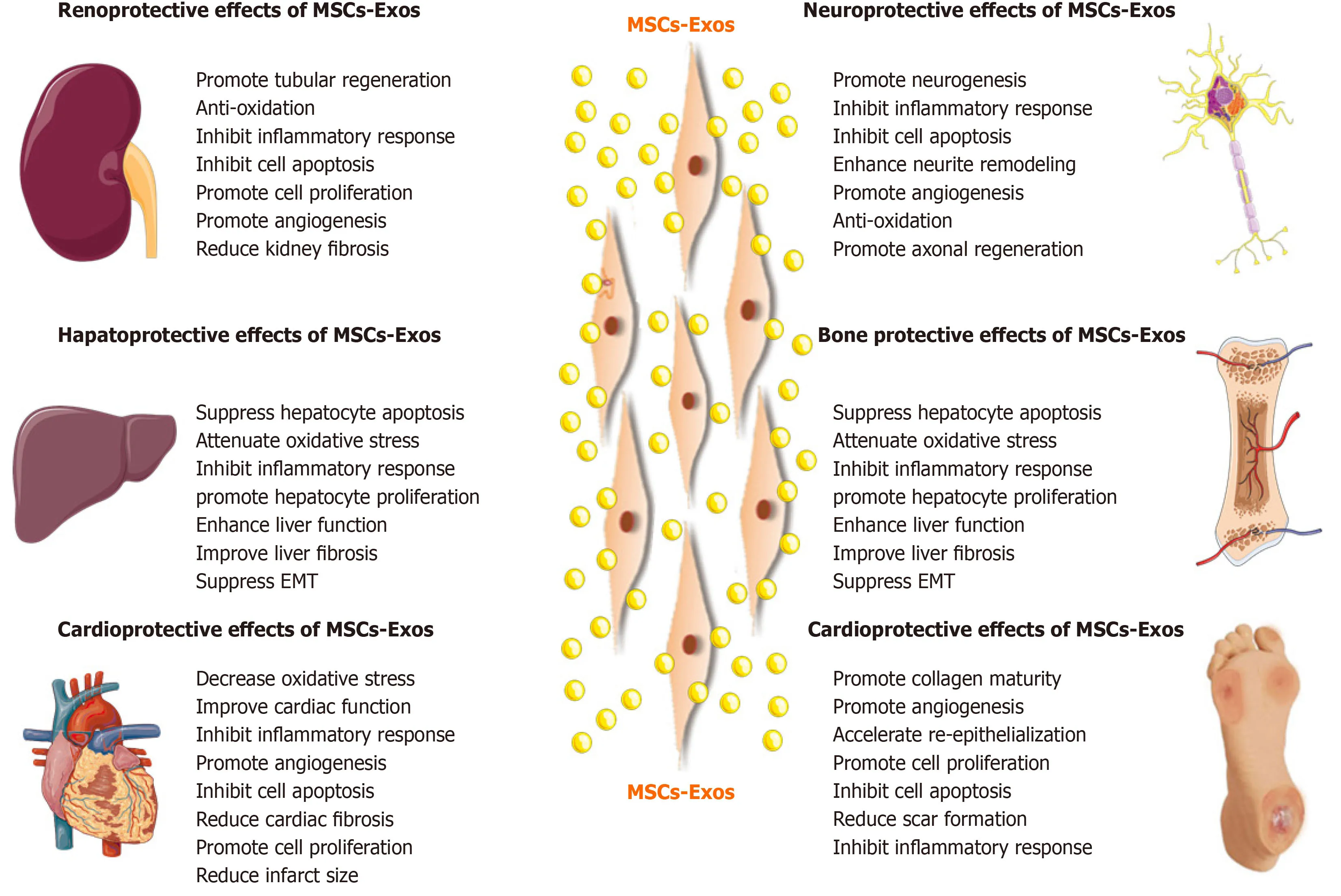
Figure4 Therapeutic effects of mesenchymal stem cell-derived exosomes in kidney,liver,cardiovascular,neurological,and musculoskeletal diseases,as well as cutaneous wound healing.MSCs:Mesenchymal stem cells.
Drug-induced nephrotoxicity is a common cause of AKI,with an incidence as high as 60%.The anticancer drug cisplatin can induce kidney disease and elevate the levels of BUN and creatinine,inducing oxidative stress and apoptosis[79].Zhouet al[83]showed that injection of human umbilical cord-derived MSC-Exos repaired cisplatin-induced AKI in rats and NRK-52E cell injury by ameliorating oxidative stress and apoptosis and promoting cell proliferationin vivoandin vitro.Human umbilical cord MSC-Exos promoted autophagy of renal tubule epithelial cells and in kidney tissue by inhibiting mTOR,thus alleviating apoptosis and inflammation[84].Jiaet al[85]reported that human umbilical cord-derived MSC-Exos-mediated delivery of 14-3-3ζ enhanced autophagy by modulating ATG16L,thus preventing cisplatin-induced AKI.
CKD:CKD is a progressive disease with complex symptoms and multiple causes.Several factors influence the severity and rate of progression of CKD.AKI is associated with an increased risk of development of CKD,and no effective treatment is available.Zhuet al[86]investigated the effect of human adipose tissue-derived MSC-Exos on the AKI-CKD transition.MSCs upregulated the expression of Sox9 in the renal tubules,promoted the regeneration of renal tubules,ameliorated AKI,and reduced renal fibrosis.These effects were reversed by an inhibitor of MSC-Exos release.Further,the MSCs activated tubular Sox9 and prevented TGF-β1-induced transformation of tubular epithelial cells (TECs) into a pro-fibrotic phenotypeviaexosome shuttlingin vitro.Therefore,MSC exosomes suppressed the AKI-CKD transition by TECdependent activation of Sox9[86].Furthermore,MSC-Exos-mediated delivery of miRlet7c to injured kidneys improved kidney architecture and reduced collagen accumulation in unilateral ureteral obstruction-injured mice,ultimately ameliorating renal fibrosis[87].
Diabetic nephropathy:Diabetic nephropathy (DN) is a serious complication of diabetes and a common cause of end-stage renal disease.Nagaishiet al[88]reported that BMSCs ameliorated DNviathe paracrine effect of renal trophic factors,including exosomes.Also,bone marrow-derived MSC-Exos markedly improved renal function,promoted histological restoration of renal tissue,significantly increased LC3 and Beclin-1 expression,and significantly decreased mTOR and fibrotic marker expression in renal tissue in a rat model of DN.These effects were in part abolished by the autophagy inhibitors chloroquine and 3-MA[89].These findings suggest the therapeutic potential of MSC-Exos for DN.
MSC-Exos in liver diseases
Liver injury:MSC-Exos can be used for treatment of liver injury.The liver injury caused by I/R affects liver function and increases mortality after liver transplantation and liver resection[90].Nonget al[91]evaluated the effect of human-induced pluripotent stem cell (hiPSC)-derived MSC-Exos on a rat model of hepatic I/R injury.MSC-Exos markedly suppressed hepatocyte necrosis,sinusoidal congestion,and the levels of markers of hepatocyte injury [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)],inflammation (TNF-α and IL-6),apoptosis (caspase-3 and Bax),and oxidative stress (GSH,GSH-Px,and SOD).Therefore,the hiPSC-MSC-Exos alleviated hepatic I/R injury,possibly by inhibiting inflammation,oxidative stress,and apoptosis.Additionally,hiPSC-MSC-Exos alleviated hepatic I/R injury by activating the sphingosine kinase and sphingosine-1-phosphate pathway in hepatocytes and promoting cell proliferationin vitroandin vivo[92].MiRNAs associated with MSC-Exos also exert a hepatoprotective effect.Zhanget al[90]reported that umbilical cord-derived MSC-Exos containing miR-20a alleviated liver I/R injury.Furthermore,MSC-Exos containing miR-20a stimulated the expression of miR-20a target genes,such asBeclin 1andFAS,in LO-2 cells.These target genes are involved in apoptosis and autophagy,which are implicated in the pathogenesis of liver I/RI.
Drug-induced liver injury accounts for more than 50% of acute liver failure (ALF)cases in the United States and has become a major clinical problem[93].Tanet al[93]found that human embryonic (HuES9.E1)-derived MSC-Exos exert a hepatoprotective effect inin vitromodels of acetaminophen and H2O2-induced hepatocyte injury and in a mouse model of carbon tetrachloride (CCl4)-induced acute liver injury.The effect was mediated by increasing hepatocyte proliferation,as demonstrated by upregulation of two proliferation factors (PCNA and cyclin D1) and an anti-apoptotic factor (Bcl-xL).Also,the antioxidant activity of human umbilical cord-derived MSC-Exos reportedly suppresses CCl4-induced liver injury[96].Importantly,MSC-Exos exerted a hepatoprotective effectviaantioxidant defenses in the progression from initial liver injury to fibrosis and liver tumor[94].Additionally,in a CCl4-induced liver injury mouse model,miR-455-3p-enriched exosomes from human umbilical cord MSCs attenuated macrophage infiltration and local liver damage and reduced the serum levels of inflammatory factors,thereby improving liver histology and ameliorating liver injury[95].
Tamuraet al[96]evaluated the effect of MSC-Exos on concanavalin-A-induced liver injury as a model of immune-induced liver injury.Bone marrow derived-MSC-Exos reduced the serum ALT level,decreased the hepatic necrotic area,apoptosis,and the production of proinflammatory cytokines,and increased the levels of antiinflammatory cytokines and regulatory T cells,suggesting an anti-inflammatory effect.
Liver fibrosis:Liver fibrosis is a common outcome of severe chronic liver injury and is characterized by excessive accumulation of the ECM or scar tissue in the liver.If liver fibrosis is not well controlled,it can progress to cirrhosis but,in principle,it is reversible[97].In a CCl4-induced liver injury model,transplantation of human umbilical cord derived-MSC-Exos reduced the surface fibrous capsules and softened their texture,alleviated hepatic inflammation and collagen production,and inhibited the epithelial-to-mesenchymal transition in the CCl4-induced fibrotic liver[98].MSC-Exos significantly restored serum AST activity and inactivated the TGF-β1/Smad signaling pathway by decreasing collagen type I/III and TGF-β1 and the phosphorylation of Smad2[100].Moreover,in vivoadministration of human bone marrow derived-MSCExos alleviated liver fibrosis by reducing collagen accumulation,enhancing liver functionality,inhibiting inflammation,and increasing hepatocyte regeneration,by inhibiting hepatic stellate cell (HSC) activation through the Wnt/β-catenin pathway[99].Also,exosomes containing miR-181-5p increased autophagy and ameliorated TGF-β1-induced liver fibrosis by inhibiting the STAT3/Bcl-2/Beclin 1 pathway in HST cells and a CCl4-induced liver fibrosis mouse model[100].Moreover,adipose tissue-derived MSC-Exos expressing miR-122 decreased the proliferation and activation of HSCs in a liver fibrosis model.Furthermore,MSC-Exos containing miR-122 stimulated the expression of miR-122 target genes such as insulin-like growth factor receptor 1,cyclin G (1),and prolyl-4-hydroxylase α1 in HSCs.These genes are involved in cell proliferation and collagen maturation[101].
Liver failure:ALF is a clinical syndrome caused by inflammation-induced hepatocyte injury,apoptosis,and necrosis,and is a major challenge worldwide.The LPS/D-GalNinduced mouse is generally used as a model of ALF and reflects human liver failure precisely;also,LPS and D-GalN are used to create an animal model of ALF[102].Jianget al[102]showed that human umbilical cord derived-MSC-Exos repaired damaged liver tissue and decreased the levels of ALT and AST and the expression of the NLRP3 inflammasome and downstream inflammatory factors in a LPS/D-GalN-induced mouse model of ALF.Moreover,pretreatment with exosomes from human umbilical cord derived-MSCs plus TNF-α alleviated ALF by inhibiting the activation of the NLRP3-related inflammatory pathway,at least in part,by increasing the expression of miRNA-299-3p[103].In a model of hepatocyte injury and apoptosis induced by LPS/DGalN,bone marrow derived-MSC-Exos increased the expression of the autophagy marker proteins LC3 and Beclin-1 and promoted the formation of autophagosomes.MSC-Exos significantly decreased the expression levels of the proapoptotic proteins Bax and cleaved caspase-3 and increased that of the anti-apoptotic protein Bcl-2.However,the autophagy inhibitor 3MA significantly reversed the inhibition of apoptosis by MSC-Exos.Therefore,MSC-Exos reduced hepatocyte apoptosis by promoting autophagy after ALF[104].
MSCs-Exos in cardiovascular diseases
Myocardial ischemia-reperfusion injury:Myocardial ischemia-reperfusion (MI/R)can induce apoptosis and necrosis of myocardial cells,and even cause cardiac arrest,thereby affecting the outcome of heart disease treatment.Practical and effective therapeutic modalities for MI/R injury are urgently needed.Laiet al[72]reported that human embryonic stem cell-derived MSC-Exos reduced infarct size in a mouse model of MI/R injury.A subsequent study by the same group showed that MSC-Exos increased the levels of ATP and nicotinamide adenine dinucleotide (NADH),decreased oxidative stress,increased phosphorylated-Akt and phosphorylated-GSK-3β (anti-apoptotic factors),and reduced phosphorylated-c-JNK (proapoptotic factor) in I/R hearts,ultimately preventing left ventricular dilatation and improving cardiac performance.MSC-Exos also reduced neutrophil and macrophage infiltration[105].Hence,MSC-Exos are a potential adjuvant to reperfusion therapy for myocardial infarction (MI).Liuet al[106]found that rat bone marrow-derived MSC-Exos significantly reduced apoptosis and the myocardial infarct size,upregulated myocardial LC3B expression,and improved cardiac function in rats with I/R injury.Also,in vitro,MSC-Exos reduced H2O2-induced ROS production and apoptosis and enhanced autophagyviathe AMPK/mTOR and Akt/mTOR pathways in rat H9C2 cardiomyocytes.Moreover,rat bone marrow-derived MSC-Exos reduced MI/R injury,possibly by inhibiting apoptosis and promoting autophagy[107].Cuiet al[108]reported that adipose-derived MSC-Exos significantly attenuated I/R-induced MI,decreased the serum levels of creatine kinase-myocardial band,lactate dehydrogenase,and cardiac troponin I in a rat model of MI/R.MSC-Exos antagonized I/R-induced myocardial apoptosis,upregulated Bcl-2,and downregulated Bax and caspase-3 activity in rat myocardium.Furthermore,MSC-Exos activated Wnt/β-catenin signaling by attenuating the I/R-induced inhibition of Wnt3a,p-GSK-3β (Ser9),and βcatenin expression.
MiRNAs associated with MSC-Exos are also important in protecting against MI/R injury.In an MI/R injury model in which H9C2 cells are subjected to hypoxia/reoxygenation,Sunet al[109]found that miR-486-5p carried by bone marrow-derived MSC-Exos suppressed PTEN expression,activated the PI3K/AKT signaling pathway,and inhibited the apoptosis of injured cardiomyocytes.MiR-125b reduced the myocardial infarct area and thus ameliorated MI/R.Chenet al[110]loaded miR-125 into bone marrow-derived MSC-Exos,and the resulting MSC-Exos-miR-125b significantly increased cell viability;decreased apoptosis;downregulated Bax and caspase-3;upregulated Bcl-2;decreased the levels of IL-1β,IL-6,and TNF-α in cardiomyocytes;and restored the cardiac function of I/R rats by regulating SIRT7.Also,miRNA-181a delivery by human umbilical cord blood-derived MSC-Exos suppressed inflammation and increased the Treg ratio by inhibiting c-Fos,thus exerting a therapeutic effect on MI/R injury[111].Therefore,MSC-Exos facilitate the targeted delivery of small RNAs to treat MI/R injury.
Myocardial infarction:MI typically results in irreversible loss of myocardial cells and heart failure due to limited blood supply and is a leading cause of death worldwide.Zhaoet al[112]used human umbilical cord derived-MSC-Exos in an acute MI (AMI) rat model.Administration of MSC-Exos significantly improved cardiac systolic function and reduced cardiac fibrosis.Moreover,MSC-Exos protected myocardial cells from apoptosis and promoted tube formation by,and migration of,human umbilical vein endothelial cells (EA.hy926 cells).Therefore,MSC-Exos improved cardiac systolic function by protecting myocardial cells from apoptosis and promoting angiogenesis.Subsequently,the same group reported that human umbilical cord derived-MSC-Exos promote Smad7 expression by suppressing miR-125b-5p to improve myocardial repair[113].In a follow-up study,adipose-derived MSC-Exos alleviated MI-induced cardiac damage by inhibiting cardiac dysfunction,apoptosis,fibrosis,and inflammationin vitroandin vivoby activating the S1P/SK1/S1PR1 signaling pathway and promoting M2 macrophage polarization[114].Xuet al[115]reported that exosomes from adipose tissue,bone marrow,and umbilical cord blood derived-MSCs inhibited cardiomyocyte apoptosis and promoted angiogenesis,thereby improving cardiac function and protecting the myocardium in rats with MI.Notably,adipose tissue derived MSC-Exos stimulated the production of cardioprotective factors[115].
MSC-Exos have been genetically modified to enhance their protective effect against MI.Kanget al[116]produced CXCR4-enriched exosomes from rat bone marrow derived MSCs overexpressing CXCR4,which promoted cardiac functional recovery by increasing angiogenesis and cell survival,reducing infarct size,and improving cardiac remodeling by activating the PI3K/Akt signaling pathway following MI.Tissue matrix metalloproteinase inhibitor 2 (TIMP2) is a member of the tissue inhibitor family of metalloproteinases.Because TIMP2-mediated inhibition of matrix metalloproteinases is an important determinant of post-MI remodeling,Niet al[117]analyzed the therapeutic effects of exosomes from TIMP2-overexpressing human umbilical cord derived-MSCs (MSC-ExosTIMP2) in a rat model of MI.MSC-ExosTIMP2improved cardiac function by alleviating MI-induced oxidative stress and cardiomyocyte apoptosis,and promoting angiogenesis and ECM remodeling,in partviathe Akt/Sfrp2 pathway.Macrophage migration inhibitory factor (MIF),a proinflammatory cytokine,plays a key role in regulating cell homeostasis.Liuet al[118]found that bone marrow derived-MSC-ExosMIFare superior to MSC-Exos for ameliorating MI injury;the effect was mediated by enhancing cardiac function and reducing cardiac remodeling,cardiomyocyte mitochondrial fragmentation,reactive oxygen species generation,and apoptosis.
MiRNAs associated with MSC-Exos also protect against MI.Lutheret al[119]demonstrated that bone marrow-derived MSC-Exos expressing miR-21a-5p downregulated the expression of the pro-apoptotic gene products PDCD4,PTEN,Peli1,and FasL,and reduced cardiomyocyte death in an AMI model.Moreover,miR-301 in exosomes secreted by bone marrow derived MSCs protected against MI by inhibiting myocardial autophagy.In a follow-up study,exosomes from human MSCs transfected with the lncRNA KLF3-AS1 were injected into rats with MI.The overexpression of KLF3-AS1 in exosomes reduced the MI area,apoptosis,and pyroptosis,and attenuated MI progression by acting as a competing endogenous RNA(ceRNA) to sponge miR-138-5p that can regulate Sirt1 so as to suppress cell pyroptosis and attenuate MI progression[120].Moreover,human umbilical cord derived MSC-Exos protected cardiomyocytes from AMI injury by transferring miR-19a,targeting SOX6,activating AKT,and inhibiting JNK3/caspase-3 activation[121].Also,bone marrow derived exosomal miR-185 suppressed ventricular remolding,myocardial injury,and cardiomyocyte apoptosis,and improved the cardiac function of MI mice by inhibiting SOCS2[122].
In summary,MSC-Exos improve cardiac function and myocardial remodeling by transporting specific factors with anti-apoptotic,anti-inflammatory,antioxidant,and pro-survival effects.
MSC-Exos in neurological diseases
Traumatic brain injury:Traumatic brain injury (TBI) is characterized by functional and structural impairment.There is a need for modalities that improve the recovery rate.Zhanget al[123]found that rat bone marrow derived MSC-Exos improved functional recovery by promoting neurovascular remodeling (angiogenesis and neurogenesis) and by reducing inflammation in rats with TBI.Thus,MSC-Exos may be beneficial for TBI and possibly other neurological diseases.Subsequently,Niet al[124]investigated the neuroprotective role of rat bone marrow derived MSC-Exos on earlystage controlled cortical impact (CCI)-induced TBI.Administration of MSC-Exos reduced the lesion size and improved neurobehavioral performance;they also inhibited the expression of a pro-apoptotic protein (Bax) and proinflammatory cytokines (TNF-α and IL-1β) and increased the expression of an anti-apoptotic protein(Bcl-2).MSC-Exos also decreased the activation of microglia/M1 macrophages and increased that of M2 macrophages after TBI.Therefore,MSC-Exos exert a neuroprotective effect by inhibiting early neuroinflammation in mice with CCIinduced TBIviamodulating microglia/macrophage M2 phenotype polarization[124].Furthermore,human bone marrow derived MSC-Exos exerted a neuroprotective effect and improved the long-term neurologic outcomes in a porcine model of TBI[125].
Stroke:Stroke is one of the leading causes of death and disability worldwide.Stroke can cause highly dynamic changes in neurovascular units and promote the development of brain injury.MSC-Exos play an important role in neurological and function recovery from stroke.Xinet al[126-128]investigated the effect of rat bone marrow derived MSC-Exos on stroke.The intravenous administration of MSC-Exos improved functional recovery and enhanced neurite remodeling,neurogenesis,and angiogenesis[126].Also,miR-133b in the exosomes released from MSCs is transferred to neural cells,leading to regulation of gene expression,promotion of neurite remodeling,and improvement of functional recovery in a rat model of stroke[127].Also,Xinet al[128]reported that MSC-Exos enriched with the miR-17-92 cluster increased neural plasticity and functional recovery from stroke in rats,possibly by inhibiting GSK-3β activity and targeting PTEN to activate the PI3K/AKT/mTOR signaling pathway.In a follow-up study,adipose-derived MSC-Exos promoted angiogenesis by brain microvascular endothelial cells after OGDviathe miR-181b-5p/TRPM7 axis,suggesting their therapeutic potential for stroke[129].Similarly,in the OGD induced rat oligodendrocyte (OL) injury model,miR 134 in rat bone marrow-derived MSC-Exos prevented OL apoptosis by negatively regulating the caspase 8 dependent apoptosis pathway and so have therapeutic potential for ischemic stroke[130].Moreover,mouse BMSC-Exos promoted the proliferation,and inhibited the apoptosis of,astrocytes injured by OGD,accompanied by inhibition of the expression of inflammatory factors by downregulating lipocalin 2.More importantly,MSC-derived exosomal miR-138-5p reduced neuronal injury following stroke in mice[131].
Spinal cord injury:SCI is a severe central nervous system (CNS) injury for which few efficacious drugs are available.Huanget al[132]reported that systemic administration of rat bone marrow-derived MSC-Exos significantly attenuated lesion size,apoptosis,and inflammation,and promoted angiogenesis,thus enhancing functional recovery from SCI in rats.Therefore,MSC-Exos show potential as a cell-free therapeutic strategy for SCI.Sunet al[133]reported that human umbilical cord-derived MSC-Exos significantly promoted locomotor functional recovery and reduced inflammation after SCI,possibly inducing macrophage polarization from the M1 (proinflammatory) to the M2 (anti-inflammatory) phenotype.In a rat model of traumatic SCI,Liuet al[134]showed that injection of rat bone marrow-derived MSC-Exos attenuated neuron apoptosis,suppressed glial scar formation and inflammation,and promoted axonal regeneration and angiogenesis,ultimately enhancing functional behavioral recovery after traumatic SCI.Administration of MSC-Exos suppressed the activation of A1 neurotoxic reactive astrocytes.Furthermore,rat bone marrow-derived MSC-Exos ameliorated SCI by inhibiting complement mRNA synthesis and release and inhibiting activation of NF-κB signaling by binding to microglial cells[135].Additionally,rat bone marrow-derived MSC-Exos reduced tissue damage,promoted recovery of motor function,and inhibited neural cell apoptosis after SCI by activating the Wnt/β-catenin signaling pathway[136].Accordingly,bone marrow-derived MSC-Exos show therapeutic potential for acute SCI.
MSC-Exos have been genetically modified (mainly by miRNAs) to enhance the protective effect against SCI.Liet al[137]found that systemic injection of exosomes from miR-133b-modified rat bone marrow-derived MSCs (MSC-ExosmiR-133b) resulted in transfer of miR-133b into the injured spinal cord,promoting functional recovery after SCI.Also,tail vein injection of MSC-ExosmiR-133bsignificantly improved the recovery of hindlimb function,reduced lesion volume,preserved neurons,and promoted axon regeneration after SCI,which was attributed in part to the activation of ERK1/2,STAT3,and CREB,and the inhibition of RhoA expression.Moreover,exosomes secreted from miRNA-29b-modified rat bone marrow-derived MSCs relieved SCI in rats,possibly by regulating proteins involved in neuronal regeneration,such as NF200,GAP-43,and GFAP[138].Two consecutive studies assessed the effect of exosomes secreted from miRNA-126-modified rat bone marrow-derived MSCs on SCI in rat[139,140].Huanget al[139]indicated that MSC-ExosmiR-126induce angiogenesis and neurogenesis,inhibit apoptosis,and promote functional recovery after SCI.Yuanet al[140]indicated that MSC-ExosmiR-126protect the neurons of rats with SCI,stimulate axon regeneration,and improve the recovery of limb motor function after SCI,in part by activating ERK1/2,STAT3,and CREB and inhibiting RhoA expression.Therefore,exosomes from miRNA-modified MSCs is a novel therapeutic approach for SCI.
Neurodegenerative diseases:MSC-Exos play a pivotal role in neuroprotection and neuroregeneration in diverse neurodegenerative diseases.Alzheimer disease (AD) is one of the most common neurodegenerative diseases and causes cognitive and memory disorders.Amyloid-β (Aβ) peptide induces neuroinflammatory processes in the CNS of AD patients,leading to excessive Aβ accumulation.Leeet al[141]reported that human adipose-derived MSC-Exos reduced β-amyloid pathology,and reduced apoptosis of AD neurons.In an AD mouse model,human umbilical cord-derived MSC-Exos reversed cognitive impairment and cleared Aβ deposits.Also,MSC-Exos modulated microglial activation,alleviating neuroinflammation[142].Moreover,MSCExos stimulated neurogenesis in the subventricular zone and alleviated beta amyloid 1-42-induced cognitive impairment in a mouse model of AD[143].Taken together,these findings demonstrate the therapeutic potential of MSC-Exos for AD.Huntington’s disease (HD) is a hereditary neurodegenerative disease caused by the aggregation of mutant Huntingtin (mHtt).Leeet al[144]investigated the therapeutic role of exosomes from human adipose-derived MSC-Exos in anin vitromodel of HD.MSC-Exos significantly decreased mHtt aggregates and reduced mitochondrial dysfunction and apoptosis in R6/2 mouse-derived neurons.ALS is a fatal neurodegenerative disease characterized by selective degeneration and death of upper and lower motor neurons.Treatment of neurons from G93A ALS mice with human adipose-derived MSC-Exos alleviated aggregation of superoxide dismutase 1,and normalized the cellular phenotype,restoring to normal the levels of mitochondrial proteins including p-CREB and PGC-1α[145].Subsequently,two studies by the same group showed that mouse adipose tissue-derived MSC-Exos exerted an anti-apoptotic effect and rescued the function of mitochondria in anin vitromodel of ALS[146,147].MS is a chronic demyelinating disease caused by CNS inflammation and immune dysfunction,which can result in severe physical disability.Liet al[148]reported that in a model of immuneinduced demyelination,rat bone marrow-derived MSC-Exos improved motor function and reduced demyelination and neuroinflammation in rats by regulating M2 polarization of microglia.Thus,bone marrow-derived MSC-Exos have therapeutic potential for MS.
MSC-Exos in musculoskeletal diseases
Osteoarthritis:Osteoarthritis (OA) is the most common chronic degenerative OA disease.Because of the limited self-healing ability of cartilage,there is no cure for OA.Exosomes secreted by MSCs show therapeutic potential for OA.Zhuet al[149]compared the effect of exosomes secreted by induced pluripotent stem cell-derived MSCs (iMSCExos) and those secreted by synovial membrane MSCs (SMMSC-Exos) on OA.Injection of iMSC-Exos and SMMSC-Exos attenuated OA in the collagenase-induced mouse model – iMSC-Exos had a superior therapeutic effect.Wanget al[150]examined the therapeutic potential for OA of exosomes from human embryonic stem cellinduced MSCs.In vitro,MSC-Exos maintained the phenotype of IL-1β-induced primary mouse chondrocytes by increasing collagen type II synthesis and reducing ADAMTS5 expression.In a mouse model of destabilization of the medial meniscus induced-knee joints,MSC-Exos prevented cartilage destruction[150].Also,mouse bone marrow-derived MSC-Exos re-established chondrocyte homeostasis,prevented chondrocyte apoptosis,and stimulated macrophage polarization toward an antiinflammatory phenotypein vivo.Moreover,MSC-Exos protected against cartilage and bone degradationin vivo[151].Zhanget al[152]demonstrated that human embryonic stem cell-derived MSC-Exos alleviated subchondral bone deterioration,suppressed inflammation,and restored matrix homeostasis in a model of temporomandibular joint OA and,ultimately,promoted temporomandibular joint repair and regeneration.Lumbar facet joint OA (LFJ-OA) is a common cause of lower-back pain (LBP).Liet al[153]evaluated the effect of mouse bone marrow-derived MSC-Exos in an LFJ-OA mouse model.MSC-Exos relieved pain by abrogating aberrant CGRP positive nerves and abnormal H type vessel formation in the subchondral bone.Also,MSC-Exos attenuated cartilage degeneration and suppressed tartrate resistant acid phosphatase expression and RANKL RANK TRAF6 signaling activation to facilitate subchondral bone remodeling.Therefore,bone marrow-derived MSC-Exos can ameliorate LBP and LFJ-OA.
MiRNAs and long noncoding RNAs (lncRNAs) associated with MSC-Exos also protect against OA.Taoet al[154]overexpressed miR-140-5p in human synovial MSCs,and the resulting MSC-ExosmiR-140-5ppromoted chondrocyte proliferation and migration and restored ECM secretion by rescuing SOX9,by inhibiting RalA.In an OA rat model,MSC-ExosmiR-140-5pprevented OA and the severe damage to knee articular cartilage caused by instability of the knee joint.Also,human bone marrow-derived MSC-Exos increased the expression of the chondrogenic genes type II collagen alpha 1 and aggrecan and decreased that of the chondrocyte hypertrophy markers matrix metalloproteinase-13 and Runx2 (runt-related transcription factor 2) in chondrocytes from mice with OA.Furthermore,MSC-Exos attenuated the IL-1β-induced inhibition of chondrocyte proliferation and apoptosisviathe lncRNA-KLF3-AS1/miR-206/GIT1 axis[155].Liuet al[156]investigated the effect of human MSC-Exos on IL-1β-induced OA chondrocytesin vitroand in a collagenase-induced rat model of OAin vivo.The lncRNA KLF3-AS1 was markedly enriched in MSC-Exos,which ameliorated IL-1βinduced cartilage injury and suppressed IL-1β-induced apoptosis of chondrocytesin vitro.Also,the exosomal lncRNA KLF3-AS1 promoted cartilage repair and chondrocyte proliferation in a rat model of OAin vivo.Infrapatellar fat pad (IPFP)-derived MSC-Exos ameliorated OAin vivoand inhibited apoptosis,enhanced matrix synthesis,and reduced the expression of catabolic factorsin vitro[157].In addition,IPFPMSC-Exos partially inhibited mTOR and significantly enhanced autophagy in chondrocytes.However,intra-articular injection of miR-100-5p antagonists significantly suppressed the IPFP-MSC-Exos-mediated protection of articular cartilagein vivo.In summary,IPFP-MSC-Exos improve OA by maintaining cartilage homeostasis,which is likely to be mediated by inhibiting miR-100-5p-regulated mTOR-dependent autophagy[157].Moreover,human bone marrow-derived MSC-Exos carrying miR-26a-5p inhibited inflammation,proliferation,and migration and promoted apoptosis,thus attenuating OA progression[158].
Osteoporosis:Osteoporosis is an age-related disease that results from an imbalance between bone formation and resorption and is characterized by systemic damage to bone mass and microstructure,ultimately increasing the risk of fragile fractures.Osteoporosis is particularly associated with postmenopausal estrogen deficiency.Qiet al[159]reported thatin vitro,human induced pluripotent stem cell-derived MSC-Exos enhanced cell proliferation and alkaline phosphatase activity and upregulated the mRNA and protein levels of osteoblast-related factors in bone marrow MSCs from ovariectomized rats.In vivo,MSC-Exos stimulated bone regeneration and angiogenesis in critical-sized calvarial defects in ovariectomized rats.Zhaoet al[160]investigated the effect of rat bone marrow-derived MSC-Exos on osteoblastsin vitro.Co-culture with MSC-Exos promoted the proliferation of hFOB 1.19 osteoblasts cellsviathe MAPK signaling pathway,alleviating the progression of osteoporosis.Rescue experiments indicated that MSC-Exos promoted the growth and cell cycle of hFOB 1.19 cells;these effects were reversed by p-JNK knockdown.Yanget al[161]showed that the human bone marrow-derived MSC-derived exosomal lncRNA MALAT1 enhanced osteogenic activity and alleviated symptoms of osteoporosis in a mouse model by acting as a miR-34c sponge to upregulate SATB2 expression.Radiotherapy for cancer causes damage to normal tissue,including bone.Radiation-induced bone marrow-derived MSC damage is the main cause of radiation-induced bone loss.Zuoet al[162]investigated the ability of bone marrow-derived MSC-Exos to restore the function of recipient bone marrow-derived MSCs and alleviate radiation-induced bone loss.MSC-Exos attenuated radiation-induced bone loss in a rat model by reducing oxidative stress,accelerating DNA damage repair,promoting proliferation,and increasing the levels of senescence-associated proteins.
MSC-Exos in cutaneous wound healing
Skin wound healing is a complex pathophysiological process involving multiple cells and cytokines.MSC-Exos can accelerate skin healing and reduce excessive scar formation.Zhanget al[163]investigated the use of human induced pluripotent stem cellderived MSC-Exos in cutaneous wound healing.Transplanting MSC-Exos to wound sites accelerated re-epithelialization,reduced scar width,and promoted collagen maturity.In addition,MSC-Exos not only promoted the formation of new blood vessels but also accelerated the maturation of the skin wound in a rat model.Also,MSC-Exos stimulated the proliferation and migration of human dermal fibroblasts and human umbilical vein endothelial cells and promoted the secretion of types I and III collagen and elastin[163].Moreover,human umbilical cord-derived MSC-Exos significantly accelerated re-epithelialization,and increased expression of CK19,PCNA,and collagen I (compared to collagen III)in vivoin a rat model of skin burn.In vivostudies confirmed that MSC-Exos-mediated activation of Wnt/β-catenin promotes wound re-epithelialization and cell proliferation.Disruption of Wnt4 expression in MSC-Exos reduced the therapeutic effectin vivo[164].Huet al[165]investigated the roles of human adipose-derived MSC-Exos in cutaneous wound healing.MSC-Exos were taken up and internalized by fibroblasts and stimulated their migration,proliferation,and collagen synthesis in a dose-dependent manner;also,the expression of Ncadherin,cyclin-1,PCNA,and collagens I and III was increased.Systemic administration of MSC-Exos increased collagens I and III production during the early stages of wound healing,while MSC-Exos inhibited collagen expression to reduce scar formation in the later stages.Therefore,MSC-Exos promote cutaneous wound healing by optimizing the characteristics of fibroblasts[167].Maet al[166]exposed HaCaT keratinocytes to H2O2to establish a skin lesion model.Human adipose-derived MSCExos promoted the proliferation and migration of HaCaT cells and inhibited their apoptosis.In addition,activation of Wnt/β-catenin signaling was confirmed by an increased β-catenin protein level.Therefore,MSC-Exos promote cutaneous wound healing by modulating Wnt/β catenin signaling[168].Heet al[167]found that human bone marrow-derived MSC-Exos accelerated cutaneous wound healing by inducing M2 polarization of macrophages in part by transferring donor exosome-derived miRNAs.Therefore,the miRNAs in MSC-Exos could be applied to enhance the healing of cutaneous wounds.
Diabetic foot ulcer (DFU) is a catastrophic medical problem caused by diabetes,which affects 15% of people with diabetes and increases the risk of amputation.MSCExos reportedly accelerate cutaneous wound healing in DFU.In the study of Dalirfardoueiet al[168],a full-thickness excisional wound was established on the dorsal skin of streptozotocin induced diabetic mice.Menstrual blood derived MSC-Exos enhanced neoangiogenesis by upregulating vascular endothelial growth factor A,inhibited inflammation by inducing M1-M2 macrophage polarization,accelerated re epithelialization,and reduced scar formation by decreasing the Col1:Col3 ratio.Therefore,menstrual blood derived MSC-Exos ameliorated cutaneous nonhealing DFUs.Moreover,Liet al[169]showed that bone marrow-derived MSC-Exos carrying lncRNA H19 promoted wound healing in mice with DFU by promoting fibroblast proliferation and migration and suppressing apoptosis and inflammation by inhibiting miR-152-3p and promoting PTEN expression.
CLINICAL STUDIES WITH MSC-EXOS
Although MSC-Exos had shown good clinical application prospects in preclinical studies,a limited number of human clinical studies are already available on the use of MSC-Exos products according to the ClinicalTrials.gov (Table3).Among them,determining the optimal dose,the appropriate time window for MSC-Exos administration,and the route of administration to achieve maximum efficacy without side effects are the most important issues[170].A preliminary study demonstrated that increasing dosage of MSC-Exos in a patient with severe treatment-refractory graft-vshost grade IV disease,affecting the skin and intestinal tract,was well tolerated and showed a significant and sustainable improvement of symptoms,which remained stable for 5 mo[171].Another clinical trial applied umbilical cord-blood-derived MSCExos for improving β-cell mass in type 1 diabetes mellitus patients (NCT02138331).Many more studies are expected to be initiated shortly (Table3).
LIMITATIONS OF MSC-EXOS
MSC-Exos are effective and safe for regenerative medicine.However,MSC-Exos have several limitations that should be mentioned[44,172].First,ultracentrifugation can damage or destroy exosomes and the product is typically of low purity.There is no standardized technique for the isolation,quantification,and purification of MSC-Exos.The difficulty of extracting and purifying exosomes increases the cost of their application.Second,the potential of MSC-Exos in tissue repair and regeneration is unclear,as are the components/properties of MSC-Exos that promote tissue regeneration.Also,how to determine the amount of MSC-Exos needed for treatment is unknown,as is whether excess MSC-Exos cause irreversible tissue damage.Third,there is no guidance,supervision,or safety assessment of MSC-Exos.Fourth,it is worth exploring whether MSC exosomes are effective when administered systemically by the intravenous,subcutaneous,or intramuscular route[173].As an emerging therapeutic agent,the safety,challenges,and risks of MSC-Exos need to be evaluated for their use in tissue repair and regenerative medicine.
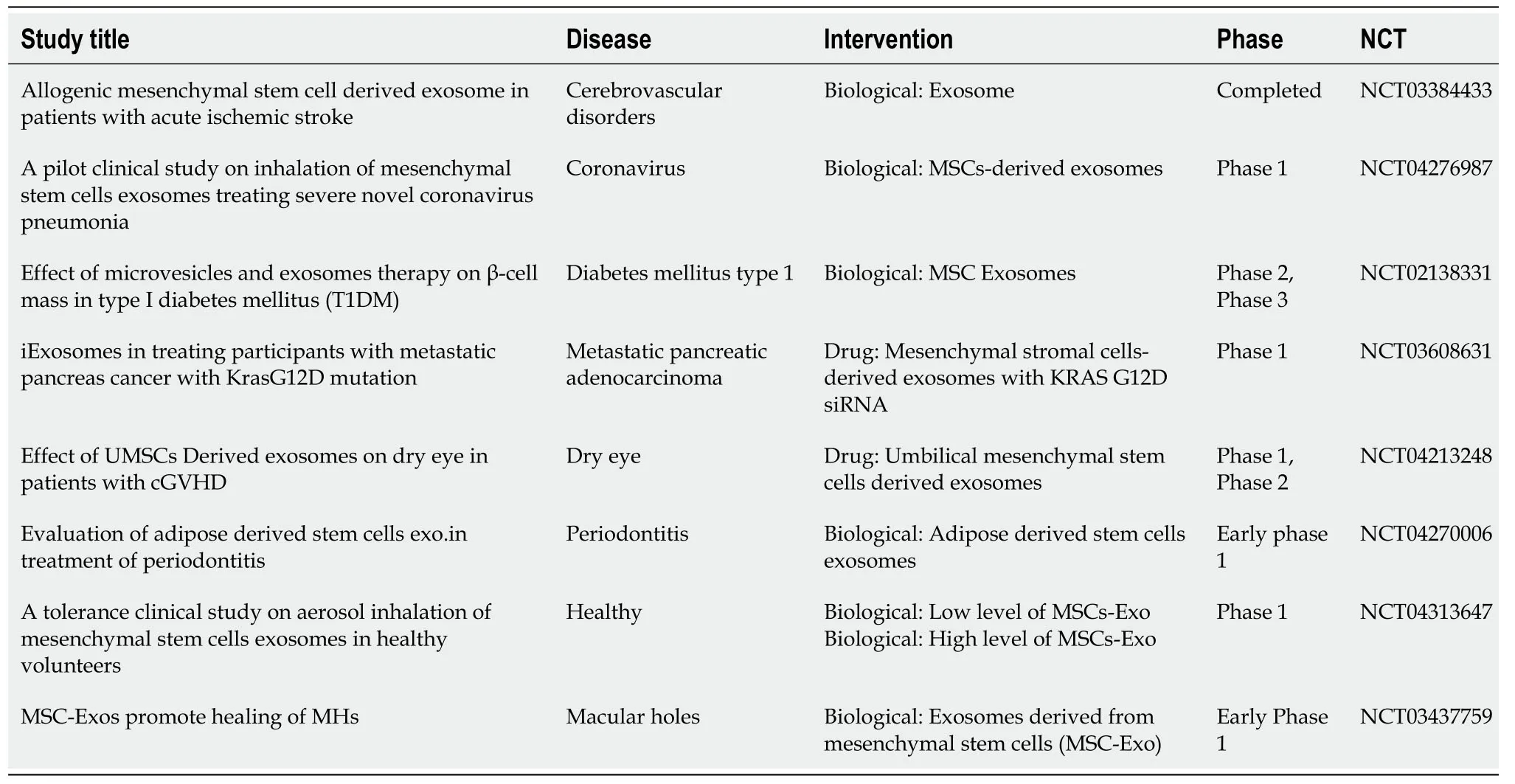
Table3 Clinical studies with mesenchymal stem cell-derived exosomes
CONCLUSION
MSC-based therapies are widely used worldwide,and the mechanisms underlying their effects may include induced differentiation,immune regulation,cell fusion,paracrine effects,carriage of mRNA or miRNA,and mitochondrial metastasis.MSCExos have therapeutic potential because they have most of the therapeutic effects of MSCs themselves.Exosomes can cross biological barriers,can be modified to load molecular drugs,have few side effects and are relatively non-immunogenic,and maintain their activity during storage.MSC-Exos have received attention because of their ability to,for example,promote tissue regeneration,suppress inflammation,and regulate the immune system.In addition,MSC-Exos do not have the safety implications of injecting live cells.The therapeutic efficacy of MSC-Exos against diseases of the kidney,liver,heart,brain,muscle,and skin has been demonstrated,and further research will enable their large-scale production.
The following issues related to MSC-Exos need to be overcome:(1) Lack of standardization of molecular characteristics,comparability,and reproducibility,and difficulty in obtaining high-purity exosomes of defined size;(2) Biodistribution,toxicity,and clearance of MSC-Exos after injection,and verification of their safety;and(3) Most studies of MSC-Exos are short-term,and so their long-term therapeutic effect is unknown,as is the safe dose for humans.
To promote the clinical application of exosomes,we suggest that:(1) Guidelines and standards for use of MSC-Exos are needed;(2) The pathways and recognition signals of MSC-Exos for target cells and organs need to be identified.Exosomal surface molecules can be modified to target MSC-Exos to particular cell types;(3) A standard method of collecting and enriching exosomes is needed,and the purity of MSC-Exos needs to be increased to enhance their therapeutic efficacy;and (4) Because they can pass biological barriers,loading of genes or drugs in MSC-Exos facilitates their targeted delivery.
In summary,MSC-Exos are theoretically superior to intact MSCs for regenerative medicine.However,a series of challenges and difficulties need to be addressed so that the therapeutic potential of exosomes can be realized.
 World Journal of Stem Cells2020年8期
World Journal of Stem Cells2020年8期
- World Journal of Stem Cells的其它文章
- Hunting down the dominating subclone of cancer stem cells as a potential new therapeutic target in multiple myeloma:An artificial intelligence perspective
- Role of mesenchymal stem cell derived extracellular vesicles in autoimmunity:A systematic review
- Human embryonic stem cell-derived mesenchymal stem cells improved premature ovarian failure
- Assessment of tobacco heating system 2.4 on osteogenic differentiation of mesenchymal stem cells and primary human osteoblasts compared to conventional cigarettes
- Autophagy in fate determination of mesenchymal stem cells and bone remodeling
- Human embryonic stem cells as an in vitro model for studying developmental origins of type 2 diabetes
