Why natural killer cells in triple negative breast cancer?
Mustafa Abdel-Latif,Biotechnology Program,Faculty of Pharmacy and Biotechnology,German University in Cairo,Cairo 11835,Egypt
Rana Ahmed Youness,Pharmaceutical Biology Department,Faculty of Pharmacy and Biotechnology,German University in Cairo,Cairo 11835,Egypt
Abstract
Key words:Triple negative breast cancer;Natural killer cells;Immune checkpoint blockades;Programmed death-ligand 1;Cytotoxic T-lymphocyte-associated protein 4;Natural killer lectin-like group 2 member D
INTRODUCTION
As the leading type of cancer in women worldwide comprising 24.2% of all female cancer patients as of 2018,breast cancer (BC) is a prime target of oncological research.As of 2018,626679 deaths due to BC have been documented[1]with the actual mortality rates showing great variance according to stage of detection.This variance is demonstrated by the fact that a 99% survival rate is estimated for patients with the cancer still localized only to the breast,whereas patients in which the cancer has metastasized to more distant parts of the body show a much less encouraging survival rate of just 27%[2].
Diversity of BC
BC is by no means a uniform disease and is in fact represented by a number of different subtypes.Approximately 80% of BCs are estrogen receptor-positive (ER+)with the majority of these (65%) also being progesterone receptor-positive (PR+)[3,4].This means that in these subsets of BC their proliferation is stimulated by the hormones progesterone and/or estrogen due to their characteristic overexpression of these hormone receptors.As such,the typical method of therapy for ER+/PR+BC is based on targeting these receptors using blockers such as Tamoxifen™or by curtailing synthesis of the hormones themselvesviaaromatase inhibitors such as Anastrazole™and Letrozole™.About 20% of BCs are also human epidermal growth factor receptor 2(HER2) positive.The HER2 pathway is a proliferative one,meaning its overexpression results in uncontrolled cell division.The HER2+BC subtype is characterized as more aggressive in terms of tumor growth and spreading than others.However,despite its poor prognosis in relation to ER+and PR+subtypes,there remains a viable treatment strategy for HER2+BC,which relies on the targeting of HER2 using monoclonal antibodies such as trastuzumab (commercially known as Herceptin™),which abrogates the aforementioned proliferative activity of these cells and consequently attenuates malignancy,both through the direct effects of receptor blockade as well as recruitment of several immune cells through antibody-dependent cellular cytotoxicity (ADCC)[3,5].However,in roughly 10%-20% of BC cases,tumor cells are classified as negative for both hormone receptors and HER2.This case,known as triple-negative breast cancer(TNBC),is well recognized as the subtype with the poorest prognosis due to the lack of targeted therapeutic options[6,7].TNBC survival rates are comparatively lower than non-TNBC ones as demonstrated by a study published in 2018 by Gonçalves Jret al[8]that showed 5-year survival rates of 80.8% and 62.1% for non-TNBC and TNBC patients,respectively.
TNBC patients:Worst prognosis and poorest survival rates
As mentioned,TNBC provides the bleakest outlook of all BC subtypes.Dentet al[9]painted a picture of this in 2007 in an 8-year follow-up study of 1601 BC patients.Whilst a vast minority were TNBC patients (180;11.2%),a significantly worse prognosis was demonstrated by their higher mortality rate (42.2% in TNBCvs28% in other BC subtypes),disease recurrence (33.2%vs20.4%),with all TNBC-related deaths occurring within 10 years of initial diagnosis as opposed to regular BC mortalities stretching up to 18 years post diagnosis[9].A further study was conducted 1 year later on the same cohort investigating the metastatic effects of TNBC.Results were yet again discouraging:TNBC patients had a 23% risk factor of developing visceral metastasis within 10 years as opposed to just 9% of other BC patients[10].To this effect,the relative lack of therapeutic options for TNBC is an undoubtedly grave issue.
Chemotherapeutic insufficiency in TNBC
Despite its ominous implications,TNBC responds quite well to traditional chemotherapy.Response rate to neoadjuvant therapy has actually been found to be significantly higher in TNBC patients in comparison with other subtypes,with one comprehensive study by Liedtkeet al[11]on 255 TNBC patients (out of a 1118-BC patient cohort) clocking this difference at 22%vs11%.The real issue of TNBC is the poor survival rate of those who do not respond to such chemotherapies adequately,mainly due to the lack of secondary therapeutic options that would otherwise be available to PR+,ER+or HER2+patients.In an attempt to alleviate this dilemma,researchers identified the defective DNA repair pathways characteristic of TNBC as a potential target.The enzyme poly (ADP-ribose) polymerase,normally known to contribute to base-excision DNA repair,has been shown to be dysfunctional in TNBC and contributes to the genetic instability of the disease[12].As such,the poly (ADP-ribose)polymerase inhibitor iniparib has been tested in a combinatorial capacity with the chemotherapeutics gemcitabine and carboplatin.Whilst phase II trials were promising[13],phase III trials showed no considerable difference between combined therapy and sole chemotherapy[14].This yet again underlines the immense struggle to find targeted therapies in TNBC.
WHY IMMUNOTHERAPY IN TNBC?
However,TNBC is associated with a high degree of chromosome instability and mutation,such as that of the tumor suppressor gene TP53[15,16].Owing to this,mutant proteins produced by TNBC are hypothesized to be recognized by the immune system as unfamiliar antigens (i.e.neoantigens).This is one possible explanation to the characteristic and notable elevation of tumor-infiltrating lymphocyte (TIL) levels within the tumor microenvironments of TNBC patients,whereby lymphocytes are aggressively recruited to the site of malignancy.It is this observation of elevated TILs that prompted the reasoning that immunotherapy (i.e.stimulation of TILs or induced overexpression of neoantigens) would be an effective strategy to combat TNBC.This is further reinforced by the finding that higher TIL levels in TNBC are correlated to better prognoses amongst patients following the administration of immunestimulating chemotherapeutic agents such as anthracyclines.The heterogeneity of TNBC cells has in recent years been thoroughly examined to identify any potential candidates for targeted therapy[17].This has proven difficult,despite the identification of TNBC-specific antigens such as MAGE-A and NY-ESO-1.Usage of such antigens is merely confined to cancer “vaccines,” which enhance tumor immunogenicity by improving the action of immunotherapeutic agents such as immune checkpoint blockers (ICB)[18].
ICB in TNBC
The era of immuno-oncology has borne with it fresh hope for TNBC patients.As previously mentioned,the abundance of TILs in the TNBC microenvironment is indicative of immunotherapy being a promising treatment approach due to the disease’s evident immunogenicity.Naturally,evasion measures are undertaken by the disease that allows it to prosper even under the immune-heavy climate in which it grows.Upon analysis of expression patterns,the immune checkpoint ligand programmed-death ligand 1 was found to be significantly overexpressed amongst TNBC cell surfaces as one such evasion measure[19].As such,the programmed death-1/programmed-death ligand 1 axis has risen as the chief target of TNBC immunotherapies with a number of programmed-death ligand 1-directed monoclonal antibodies are currently in clinical trial phases[20].As is apparent from Table 1,the immunotherapeutic strategies of TNBC treatment are overwhelmingly biased towards ICB,with full approval still far off for most.Despite the promise of ICB in TNBC (well indicated by the progress into later trials for most monoclonal antibodies),some TNBC patients could yet find themselves short of options.
Limitations of ICB
Adverse inflammatory reactions:From a purely therapeutic standpoint,the effects of ICB treatment are extremely promising.Its positive response rates through the elevation of cytotoxic T-lymphocyte levels and efficacy as well as increased generation of T-helper cells are widely recognized.However,ICB by no means represents an anticancer “magic bullet” and poses issues of its own.Apart from the astronomical prices of ICB agents,a worrying problem commonly rears its head during ICB administration in the form of autoimmune events.Seeing as immune checkpoints normally serve as natural brakes to prevent prolonged or excessively severe immune responses,their blockade has predictably resulted in adverse inflammatory reactions in several cases.These responses vary greatly in terms of localization as well as severity;hypophysitis,gastroenteritis,enterocolitis,hepatitis and many other immune-mediated side effects have been observed in clinical studies,with theirintensities ranging from grade 2 (moderate) to grade 5 (life-threatening or fatal)[21].
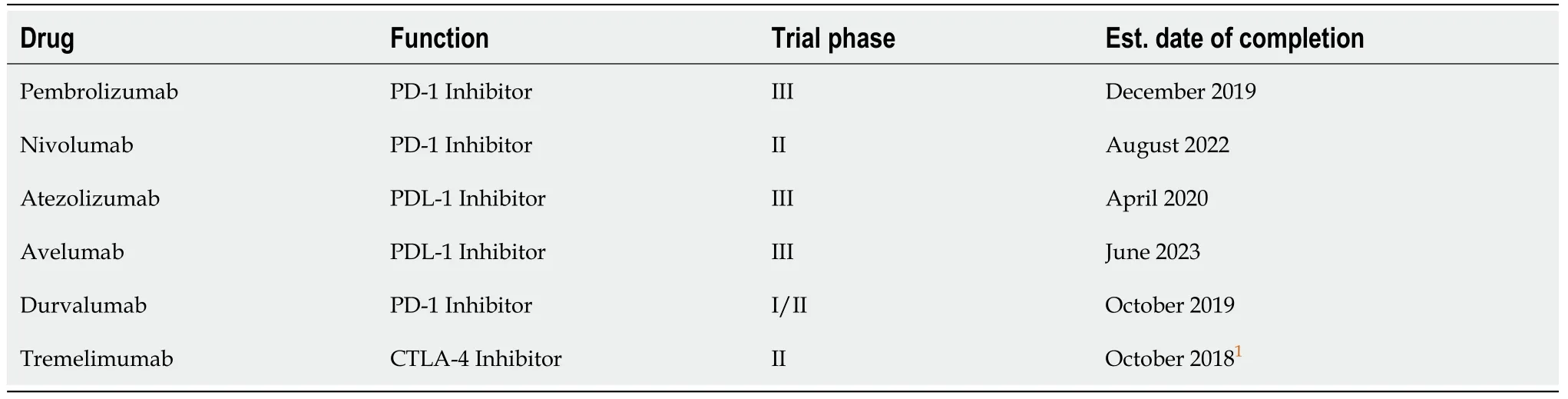
Table1 List of immunotherapeutic agents in various clinical trial phases and earliest expected completion dates of most significant trials[20]
Primary and/or secondary resistance:In addition to autoimmune tendencies,another issue with ICB therapy is the emergence of resistance against it amongst treated individuals.According to clinical trial data,in addition to responders and innate nonresponders,a third category of patients who initially respond to ICB therapy but subsequently acquire resistance to it has been defined.ICB is based mainly on the regeneration of exhausted T-cells and thus largely relies on the three elements of T-cell expansion,T-cell potency,and T-cell memory formation.All three of these mechanisms have been found to be impaired in cases of ICB resistance[22].
While unnaturally mutated neoantigens serve as the basis for immune recognition of malignant cells,the selection pressure applied by the immune system results in a remainder of cancer cells that have never innately expressed such antigens or have adapted to effectively “conceal” themselves from TILs either by shedding their neoantigens or the ability to present them through mutational loss[23].The downregulation of major histocompatibility complex (MHC) class I elements such as the β2-microglobulin domain has been documented amongst patients who acquired ICB therapy resistance.The lack of a proper antigen presentation apparatus in malignant cells in such cases greatly decreases the potential for antigen recognition and subsequent priming and clonal expansion of the T-cell pool,rendering ICB administration redundant whereby there is a numeric shortage of T-cells to be targeted[24].
The efficacy of cytotoxic T-cells has been found to be lacking within resistant individuals.Even after antigen recognition and proper expansion/activation of the Tcell population,the tumor microenvironment is far from an ideal place for T-cells to exert their effector functions.A cocktail of immunosuppressive cytokines,alternate immune checkpoints,inhibitory receptors and immunosuppressive leukocytes are all characteristic of the tumor microenvironment and contribute to the attenuation of Tcell effector functions even after ICB therapy.Most notably,a trend of loss-of-function mutations to the genes encoding Janus kinases 1 and 2 has been highlighted amongst the tumors of resistant patients.These two proteins are components of the Janus kinase/STAT pathway,which in an immune context is crucial to the production of numerous stimulatory cytokines necessary for proper T-cell efficacy[24].
The formation of memory antitumor CD8+ cells is one of the hallmarks of ICB therapy despite the unelucidated mechanism.Also characteristic of resistant patients is the impairment of this memory formation and consequent short-lived nature of reactivated cytotoxic T-lymphocytes[25].This could possibly be attributed to epigenetic factors that influence exhausted cells away from memory cell formation and thereby result in long-term resistance to ICB[26].
As has been mentioned,ICB represents a new source of hope for problematic cancers such as TNBC.However,the combined prominence of autoimmunity and/or resistance in clinical studies of various malignancies indicates that TNBC will not behave any differently and will indeed display these same tendencies;once again leaving TNBC patients bereft of options.Henceforth,it is necessary to look into tertiary methods of TNBC treatment in tandem with ICB research in order to provide alternative pathways for cases in which both chemotherapy and ICB should fail.
HUMAN IMMUNE SYSTEM
Innate and adaptive immune arms
The three major types of lymphocytes that comprise the mammalian immune system are T-cells,B-cells and NK cells;each playing its own indisputable role in the eternal fight between host and foreign bodies or malignancies.Whilst T-cells and B-cells are members of the adaptive immune system that is triggered by the detection of foreign antigens in the blood,NK cells belong to the innate immune system[27].This in turn implies a nonspecific mode of action whereby NK cells are not armed against particular antigens but are instead primed to fight any unnaturally altered cell.This is mainly achieved through immunoglobulin-like MHC class I-specific receptors on NK cell surfaces that phosphorylate internal inhibitory immunoreceptor tyrosine-based inhibitory motifs upon recognition of normally expressed MHC class I on functional host cells.When MHC class I is lacking or defective,as is common in virally infected and malignant cells,positive transcriptional signals are fed back to the NK cell that triggers their active cytolytic state.Other modes of activation,such as the recognition of stress-induced NK cell ligands or ADCC,also exist.It is because of this search and destroy mode of action that NK cells are often referred to as the “first line of defense”with regards to mammalian immunity[28].
Intricate cross talk between the innate and the adaptive arms of the immune system
The innate and adaptive immune systems are by no means secluded from one another;a constant flux of crosstalk between the two arms is characteristic of any properly functioning immune system as shown in Figure 1.An example of this is the “beacon”function of NK cells upon their encounter with malignant cells and consequent secretion of interferon-gamma.Interferon-gamma is noted for its activation of antigenpresenting cells such as macrophages and dendritic cells,which subsequently induce the effector functions of T-helper cells initiating a comprehensive cascade of adaptive immune responses[29].ADCC,where NK cells are recruited to constant regions of antibodies (derived from adaptive B-cells) coating malignant cells,is a demonstration of how the adaptive arm can potentiate the innate arm (and not only vice versa) in what has grown to be an attractive immunotherapeutic strategy[30].However,despite this evident harmonious relationship between the two arms,it has been demonstrated that,to a degree,the innate immune system can work alone in an antitumor capacity.O’Sullivanet al[31]illustrated this in a 2012 study involving groups of mice that were either:(1) Wild type;(2) Lacking an adaptive immune system;or (3) Deficient of both the adaptive and innate immune systems.Upon tumor induction,wild type mice naturally displayed the lowest rate of tumor growth.However,of the two remaining groups,mice in group 2 showed significantly more impaired tumor growth than group 3 mice,indicating a notably prominent role for innate level effector functions in antitumor immunity[31].
EXPLOITATION OF THE INNATE IMMUNE SYSTEM:A GAP IN THE IMMUNO-ONCOLOGICAL LANDSCAPE
Why innate-mediated immunotherapy?
As discussed previously,the modern immuno-oncology scene is dominated by ICB,whereby our understanding of immune checkpoints has led to the commercial release of various cytotoxic T-lymphocyte-associated protein 4 and programmed death-1 blockers such as ipilimumab,pembrolizumab and nivolumab[32,33].Despite the evident focus of research on ICB and the resounding progress made over the past decade,only a small fraction (approximately 20%) of patients enjoy long-term benefits from these therapies[34].This could,in part or in full,be attributed to the previously discussed resistance mechanisms of malignancies against ICB.Most of these mechanisms,such as the shedding of the antigen-presenting apparatus on malignant cell surfaces,the upregulation of alternative immune checkpoints or the reduction of memory cell formation,are evolutionary measures directed against T-cells in the tumor microenvironment,i.e.the adaptive immune system[23,33,35].
The incidence of resistance amongst cancer patients triggers the need to investigate on a molecular level.Whilst potential biomarkers for ICB efficacy have been investigated in a predictive sense[36],little progress has been made in elucidating the molecular bases by which acquired resistance in individual patients comes about[23].Henceforth,it is imperative that these intricacies be clarified with a view of adopting different immunotherapeutic strategies.

Figure1 Adaptive/innate intricate relationship.
The attractiveness of innate-mediated immunotherapy comes from the fact that,whilst not fully understood,ICB resistance mechanisms may be adaptive-specific and leave the door open for an innate approach.An example of this is the wellcharacterized shedding of human leukocyte antigen (HLA) molecules in cancer[37].This disarming of tumor cells’ antigen-presenting capability results in their significantly decreased immunogenicity with regards to the adaptive immune system.However,given NK cells’ inherent tendency to fight off cells with improper MHC function,a gateway is opened for alternative treatment for patients whose cancer has evaded ICB through this adaptive-specific mutation.It is in scenarios such as this that the innate immune system should be explored as a viable treatment option.
With regards to autoimmunity,NK cell stimulation poses a much lower risk than their T-cell counterparts.NK cells are conventionally short-lived in their active form,indicating that therapies that rely on their stimulation and recruitment are unlikely to result in any prolonged inflammatory reaction leading to autoreactivity.Interestingly,an inverse relation was in fact proposed.This was brought about by the observation of NK cell deficiency or dysfunction in patients of autoimmune disorders[38].One hypothesis for this is an ongoing “battle” between NK cells and autoreactive T-cells,which would indeed result in NK cell clearance during T-cell overstimulation.As such,therapies that involve NK cell stimulation could and have indeed in some studies shown positive effects on autoimmunity by the induced clearance of autoreactive T-cells[39].
NK CELLS:NATIVE SENTINELS OF THE INNATE IMMUNE ARM
Human NK cells are the natural guards of the innate immune system.They originate from hematopoietic stem cells and undergo maturation in the bone marrow[40].NK cells are categorized as the third largest lymphocyte population[41].NK cells represent 2%-18% in human peripheral blood[41];they are also found in peripheral tissues like the liver,peritoneal cavity and placenta[42,43].NK cells are phenotypically defined by the expression of CD56 and/or CD16 and the absence of the T-cell receptor CD3[44].“Natural cytotoxicity” describes an effectively contributing phenomenon of NK cells as a first line of defense against viral infections and more in general against pathogens without prior sensitization as previously mentioned[28,45-47].NK cells are also involved in immune surveillance against tumors and prevent dissemination of metastatic tumors[48,49].These effector functions are mediated through cellular cytotoxicity (mainly through perforins and granzymes),in addition to secretion of several noncellular mediators such as chemokines and cytokines[49].NK cells cross-talking among immune cells also play a regulatory control in mediating the anti-tumor adaptive immunity of T- and B-cells that in contrast require initial priming for the expression of their activity[50-52].
Subsets of NK cells
Human NK cells are classified according to the relative expression of the surface markers CD16 and CD56 into two major subsets/classes:CD56 dim and CD56 bright.These subsets differ in their function,phenotype and tissue localization[53].The low density CD56 (CD56dim) subset comprises the majority (almost 90%) of peripheral blood NK cells and are characterized by their high expression of CD16,killer Ig-like receptors (KIRs) and perforins,making them more potent cytotoxic lymphocytes as illustrated in Figure 2.However,CD56brightNK cells are rarely present in peripheral blood but are predominant in lymph nodes,inflamed tissues and decidua[54,55].The latter subset has a lower cytotoxicity as it expresses low levels of perforin and KIRs[56].CD56brightNK cells are known as immunoregulatory NK cells as its functions are mainly mediated through the cytokine production such as interferon-gamma,tumor necrosis factor-α and transforming growth factor-β as shown in Figure 2[57,58].
REPERTOIRE OF RECEPTORS ORCHESTRATING NK CELL SIGNALS
NK cells have a unique feature of discriminating infected or malignant cells from normal “self” cellsviaa complex balance between activating and inhibitory receptorligand interactions[59].In addition,the resting inactivated NK cell surface constitutively expresses a wide range of receptors,which upon their activation by different ligands initiate several downstream signaling pathways and result in boosting NK cytotoxicity and cytokine production[60].
Inhibitory receptors
Upon recognition of their respective ligands,inhibitory receptors on NK cells phosphorylate associated immunoreceptor tyrosine-based inhibitory motifs,which in turn recruit the phosphatases SHP and SHIP.They dephosphorylate transcriptional signaling molecules Lck,Syk,ZAP70,Vav1 and Fyn,which results in prevention of active-form transcription patterns.This pathway,along with the simultaneous dephosphorylation of activating immunoreceptor tyrosine-based activator motif domains,ensures that NK cells are not converted into an active state upon healthy host cell encounter and thus avoids events of autoimmunity.The aforementioned MHC class I-specific receptors are commonly referred to as KIRs and are almost all inhibitory in nature.KIRs are essential for tolerance towards host cells expressing classical MHC molecules (namely HLA-A,HLA-B and HLA-C).As for nonclassical MHCs,a heterodimer of CD94 and natural killer group 2 member A is responsible for detecting molecules such as HLA-E,which despite not being an archetypal MHC can be expressed amongst healthy host cells[61].
Activating receptors
Natural killer lectin-like group 2,member D (NKG2D) is a hexameric transmembrane protein that acts as the principal activating receptor on NK cell surfaces.Ligands of NKG2D,termed NKG2DLs,act as triggers to the cytolytic mode of NK cells.As can be seen in Figure 3,NKG2D is associated with two DAP10 homodimers that each harbor two YINM motifs.Upon association with NKG2DLs,these motifs are phosphorylated and proceed to recruit the Grb2/Vav1/SLP-76 signaling complex and/or the p85 subunit of PI3-kinase.Taken together with the recruitment of the Syk/Zap70 complex by the alternate activating receptor Ly49D,these comprise the initial steps of three different cascades that have the downstream additive effect of amplifying both cytokine and cytotoxic molecule production within NK cells.Effectively “armed” for attack,NK cells proceed to kill their target either directly through apoptotic mechanisms such as the tumor necrosis factor,Fas/FasL,TRAIL and perforin/granzyme pathways or indirectly through stimulation of both innate and adaptive immune responseviaoverproduction of cytokines such as IL-7,IL-12,and IL-15,which serve to upregulate NKG2D expression both on fellow NK cells and adaptive CD8+ cells[61].

Figure2 Natural killer cell subsets differ both functionally and phenotypically.
NK cell ligands
NKG2DLs are mainly divided into two classes:MHC class I-related proteins(comprising MIC-A and MIC-B) and UL16 binding proteins (comprising ULBPs 1-6).Given the prominence of NKG2D amongst NK cells,the most integral aspect for the effective facilitation of tumor immunosurveillance is the differential expression pattern of NKG2DLs.To this effect,NKG2DLs are commonly overexpressed amongst malignancies.Indeed,studies have shown evidence for the increased expression of MIC-A and ULBPs 1-5 in all of colorectal cancer,ovarian cancer and BC along with further evidence of MIC-B expression in BC.As prognostic factors,NKG2DLs tend to be inconsistent.An example of this being ULBP 5 overexpression indicating a positive prognosis in colorectal cancer whereas ULBPs 2 and 4 correlate with a negative prognosis in ovarian cancer.The prognostic effects of various NKG2DLs on BC were elucidated in a comprehensive 2012 study by de Kruijfet al[62]of Leiden University.The results of the study yielded the finding that high MIC-A/B and ULBP 2 expression correlated with significantly more favorable prognoses as opposed to low expression with regards to tumor size,tumor grade and relapse rates.
NK CELLS IN CANCER
As shown in Figure 4,NK cells are equipped with these thorough mechanisms of host and nonhost recognition and therefore are important mediators of tumor immunosurveillance and eradication.This point is underlined by the higher susceptibility to cancer yielded by mice that are NK cell-deficient in comparison with wild type mice[28]as well as the higher susceptibility of mice lacking both adaptive and innate immune components in comparison with those lacking just the adaptive arm[31].Consistent with these findings,the increased risk of cancer within humans with lower NK cell counts has been demonstrated through an 11-year follow-up study in 2000[63].This is further corroborated by the observation of NK cell cytolytic impairment in various forms of cancer,such as non-small cell lung carcinoma and BC.
NK CELLS IN IMMUNO-ONCOLOGY
Given their favorable characteristics in terms of resistivity and autoimmunity in comparison with adaptive methods,NK cells are a prime candidate for novel immunotherapeutic research,and their exploitation has long been appealing.Attempts to achieve this include the upregulation of stimulatory receptors,downregulation of inhibitory ones,ADCC mediation,manipulation of cytolytic pathways such as Fas/FasL and TRAIL and NK cell activation through external agents such as vaccines and chemotherapeutic drugs[38].In adoptive NK cell treatment involving external cell transfusions,it has indeed been demonstrated that increased NK cell count correlates to a favorable outcome in diseases such as non-Hodgkin lymphoma[64].With NK cells having been proven integral to anticancer immunity,recent significant attention has turned to manipulating the tumor microenvironment in an NK cell-stimulatory capacity in ways such as induced expression of activating NKG2DLs on cancer cell surfaces.
Despite such promise,NK cell-based therapies have hardly made any clinical progress as opposed to more popular immunotherapeutic strategies such as ICB.Given ICB’s limitations,it is imperative that more attention be turned to this scope of research in the near future as a means to provide patients of aggressive and difficult-t
 World Journal of Clinical Oncology2020年7期
World Journal of Clinical Oncology2020年7期
- World Journal of Clinical Oncology的其它文章
- Mechanisms and anatomical risk factors of pneumothorax after Bevacizumab use:A case report
- Complete response in anaplastic lymphoma kinase–rearranged oncocytic thyroid cancer:A case report and review of literature
- Is there a role for treatment-oriented surgery in liver metastases from gastric cancer?
- Circulating cell-free nucleic acids as prognostic and therapy predictive tools for metastatic castrate-resistant prostate cancer
- MUTYH:Not just polyposis
- Role of imaging biomarkers in mutation-driven non-small cell lung cancer
