Recent advances regarding tumor microenvironment and immunotherapy in hepatocellular carcinoma
Wei Qin, Zhen-Yu Cao, Si-Yuan Liu, Xun-Di Xu
Hunan Provincial Key Laboratory of Hepatobiliary Disease Research, Division of Hepatobiliary & Pancreatic Surgery, Department of Surgery, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan, China.
Abstract Hepatocellular carcinoma (HCC) is one of the most common malignant tumors of the liver, with poor prognosis and high mortality. Traditional treatments for patients with HCC have shown poor efficacy especially for advanced liver cancer. Compared with other organs, the liver has more natural immune cells such as Kupffer cells, natural killer cells and natural killer T cells. Immunotherapy for liver cancer has become the focus in current research. The theoretical basis of immunotherapy rests on immune tolerance and suppression in the tumor microenvironment. Common immunotherapy methods include vaccines, cytokines, adoptive cell therapies, immune checkpoint inhibitors, and oncolytic viruses. Compared with traditional treatment, immunotherapy can enhance the body’s immune function, delay tumor progression, and prolong survival. This article reviews the HCC microenvironment and immunotherapy both in the clinical and basic research aspects.
Keywords: Immunotherapy, hepatocellular carcinoma, microenvironment, immune
INTRODUCTION
Epidemiological data indicate that hepatocellular carcinoma (HCC) ranks fifth among malignancies, but it is the third most common cause of cancer-related death in the world. One of the major causes of HCC is persistent infection with hepatitis B virus (HBV) or hepatitis C virus (HCV). Aflatoxin exposure is also a crucial risk factor. In addition, excessive drinking, smoking, obesity, genetic factors and dietary habits are also important factors that promote the occurrence of HCC[1].With the development of surgical techniques, HCC may be cured by surgical resection or liver transplantation in the early stage. In addition, some early malignancies may be cured by prompt local treatment such as radiofrequency ablation (RFA) or percutaneous ethanol injection treatment. Unfortunately, due to the concealed and rapid progress of HCC, most patients have lost the chances of best treatment by the initial visit. Only a fraction of patients with HCC have the opportunity for these treatments. As for the patients who are not suitable for surgery or RFA, stereotactic body radiation therapy, portal vein embolization and other liver-directed therapies have become the standard treatment means[2]. In recent years, with the great scientific and technological advances, systemic therapy has been adopted by most hepatobiliary surgeons under conditions of appropriate liver function and good physical condition.
In the past several decades, many genetic alterations in HCC have been confirmed by previous mechanism studies, such as aberrant activation of oncogenes and the inactivation of anti-oncogenes. Apart from gene mutations, changes in epigenetics such as chromatin modification, DNA methylation, gene recombination, histone modification, RNA interference and copy number variation have been proven to play a critical part in the development of primary liver cancer[3].
The tumor microenvironment plays a vital role in tumorigenesis and cancer development and metastasis, which can be divided into an immune microenvironment characterized by immune cells and nonimmune microenvironment characterized by fibroblasts. The majority of immune cells of the tumor microenvironment include T lymphocytes, B lymphocytes, macrophages, natural killer (NK) cells and antigen-presenting cells (APCs)[4]. In the microenvironment of the liver tumor, the composition and proportion of immune cells play vital roles in the progress of tumorigenesis [Figure 1]. Compared to other organs, the liver is richer in immune cells, including natural killer T cells (NKT cells), Kupffer cells and NK cells[5]. Moreover, inactivation of immunosuppressive cytokines (such as IL-4, -5, -8, -10,etc.) and immune activating cytokines (such as TNF, IL-1,etc.) tends to produce an immunosuppressive environment. Constant exposure to antigen at higher concentrations from the gastrointestinal tract induces the liver to develop intrinsic immune tolerance and immune evasion to ward off autoimmune injury[6]. Since intrinsic immune tolerance and immune escape are often connected with HCC tumorigenesis, a growing number of studies point to immunotherapies (such as vaccines, adoptive cell therapies, immune checkpoint blockade, cytokines,etc.) targeting the microenvironment as a new strategy against hepatic cancer to bring new hope to patients with HCC. In this article, we reviewed the important role of the microenvironment in HCC tumorigenesis and the recent advances of immunotherapy in HCC.
ROLE OF IMMUNE CELLS IN LIVER TUMOR MICROENVIRONMENT
The tumor microenvironment contains a series of immune cells including NK cells, dendritic cells (DCs), macrophages, regulatory T cells (Tregs), neutrophils, T cells and eosinophils [Figure 1]. The function and number of immune cells both matter in the liver immune microenvironment, and related changes are frequently observed in the development of HCC. Multiple immune cells will promote tumor occurrence and development by activating or inhibiting various complex signaling pathways.
Tumor-associated macrophages
Previous works have established that there are monocyte-derived macrophages divided into M1 and M2 phenotypes[7]. Tumor-associated macrophages (TAMs) are a significant component of the liver microenvironment, being very important to tumor development and thus resulting in the poor outcome of HCC patients. Preclinical studies have shown that TAMs suppress the immune system and promote tumor progression through the expression of chemokines and cytokines[8,9]. It has been reported that CCL17, CCL18 and CCL22 can block the activation of cytotoxic T cells by attracting Tregs to tumor sites[10-12]. TAMs interact with bone myeloid-derived suppressor cells (MDSCs) to lower major histocompatibility complex II (MHCII), IL-6 and IL-12 levels and increase IL-10 production[13]. IL-10 produced by TAMs increases the expression of FoxP3+Tregs and then blocks CD4+CD25-T cell expression, at least promoting the progression of HCC[14].

Figure 1. HCC microenvironment components and linkage. A variety of immune-related cells in the tumor microenvironment can promote or inhibit the development of hepatocellular carcinoma through various mechanisms. HCC: hepatocellular carcinoma; VEGF: vascular endothelial growth factor; NK: natural killer; DC: dendritic cell; PDGF: platelet-derived growth factor; TGF: transforming growth factor; HGF: hepatocyte growth factor; EGF: epidermal growth factor; FGF: fibroblast growth factor; MPPs: matrix metalloproteinases; NO: nitric oxide; TNF: tumor necrosis factor; NKT: natural killer T cells; ROS: reactive oxygen species
The chemotactic migration and selective activation functions of TAMs are achieved by osteopontin expressed by HCC cells through the CSF1-CSF1R pathway[15]. Many studies indicate that TAMs release many important cytokines, including TNF-α, IL-6, IL-1β, and IL-23 and that they promote the expansion of Th17 cells, which protects against antitumor immunity by activating several markers[16,17]. In addition, TAMs are able to induce angiogenesis by producing angiogenic factors and matrix metalloproteinases[18]. Recently, more studies have determined that autophagy plays a key role in the functional control and immunosuppression of TAMs[19].
Tumor-associated neutrophils
Similar to macrophages, tumor-associated neutrophils (TANs) have different effects on the biological behavior of tumors due to different degrees of polarization[20]. Recent evidence suggests that TANs can be classified into N1 (suppress tumor development) and N2 (promote tumor development) phenotypes on the basis of TGF-β expression[19,20]. Some cytokines such as type I interferons and TGF-β can modulate the activity of TANs[21]. Studies have confirmed that the impact of neutrophils on tumor development is regulated by CD8+T cells[22].TANs are one of the key factors for HCC progression and poor prognosis, and neutrophil-lymphocyte ratio closely correlates with tumor progression, which is a significant independent factor predicting survival after hepatectomy in patients with liver cancer[23,24]. In peripheral blood, the highest levels of the cytokines CCL17 and CCL2 are produced by peripheral blood neutrophils activated by HCC cells and TANs. The newest discovery shows that TANs mediate the intratumoral infiltration of TAMs and regulatory T cells by overproducing CCL2 and CCL17, which then contributes to HCC progression and metastasis[25]. Zhouet al.[25]collected and analyzed HCC patients’ clinical and pathological data, and the results showed that the number of CCL2+or CCL17+TANs was related to tumor progression and differentiation. Moreover, these authors also found that TANs triggered the secretion of microRNA 301b-3p by releasing bone morphogenic protein 2 and transforming growth factor beta 2 (TGFβ2) in HCC cells, and then increased the expression of HCC stem cell-like cells by inhibiting the expression of the limbic system-associated membrane protein and CYLD lysine 63 deubiquitinase genes. From this research, a positive feedback loop governing cancer stemlike cells and TANs in HCC were identified[26]. TANs play a significant role in immunosuppression in HCC, unfortunately, the exact underlying mechanisms between TANs and other molecules in HCC are not quite clear.
Innate lymphocytes
The liver is a non-lymphoid organ although possessing anti-tumor capabilities, due to containing large populations of natural lymphocytes including NK cells, NKT cells,etc.[27-29]. The activation of NK cells rely on a cascade of multiple activated and inactivated receptors[30]. In the oxygen-deficient liver environment, the function of NK cells will be impaired due to hypoxia-inducible factor 1α (HIF-1α), which induces changes in MHC class I polypeptide-related sequence A (MICA)[31]. HIF-1α is important for regulates metabolism, cell proliferation and apoptosis of in HCC microenvironment[31,32]. In the presence of HIF-1α, proangiogenic genes including vascular endothelial growth factor (VEGF) gene are frequently overexpressed in immune cells[33].
It is reported that α-fetoprotein (AFP) can directly influence the functions of NK cells. Short exposure of NK cells to AFP can promote the IL-2 hyperresponsive phenotype and contribute to the release of IL-6, IL-1β and TNF-α, which is related to a low recurrence rate and better overall survival of HCC patients with HBV[34,35]. Moreover, NK cell functions can be impaired via TGF-ß1, IL-8 and IL-10 released by Tregs[36].
Tregs
Tregs belong to the subset of CD4+T cells, which do not merely suppress autoimmune response, but also impair the immune response against tumors[37,38]. Gaoet al.[39]demonstrated that Tregs represent an independent predictor of HCC recurrence and survival, and is related to invasiveness and intratumoral homeostasis. They also found that the combination of Treg removal and simultaneous stimulation of effector T cells was an effective immunotherapy to decrease recurrence and prolong postoperative survival. CD4+CD25+Tregs were used to contribute to tumor prevention in HCC patients through various contactdependent and contact-free mechanisms. Fuet al.[40]further demonstrated that CD4+, CD25+and FoxP3+Tregs impaired the function of CD8+T cells effector and that the number of circulating Tregs correlated with progression in HCC patients. In addition, another study showed that that compared with normal tissues, where CD4+CD25+T cells were significantly increased in the area around the tumor[41].
CD8+ cytotoxic T lymphocytes
CD8+T cells are critical for pathogen clearance, where they contribute to the resolution of HBV and HCV infections in the liver[42,43]. Guoet al.[42]study confirmed that Fas/FasL interactions might suppress antitumor immune responses via turnover of CD8+T cells. In addition, a series of immunoregulatory elements such as IL-10, IL-2, VEGF and indoleamine-2,3-dioxygenase (IDO) play an important role in inhibiting tumorassociated antigen (TAA)-specific CD8+T cell responses[42,44,45]. It has been proven that CD14+DCs inhibit thein vitroresponse of CD8+T cells by affecting IL-10 and IDO[46]. Furthermore, CD4 helper T cells are substantially important in generating functional memory CD8 T cells by inducing costimulatory molecules and promoting the expression of extracellular cytokines in DCs[47,48].
DCs
NK cells are another participant in innate immunity, capable of initiating T cells against TAAs that are specialized APCs involved in HCC progression[49].
In HCC, DCs can produce chemotactic cytokines, and take the initial T cells as the point of action to promote their aggregation and proliferation[50]. Moreover, DCs can promote the maturation of B lymphocytes, enhance the immune response mediated by antitumor antibodies, and suppress immune escape[51]. The available evidence suggests that DC maturation disorder, function decrease and reduction in peripheral blood are often observed in the HCC microenvironment, often leading to tumor development[52,53].
In HCC, DC function is inhibited due to the production of some factors (IL-10, IL-6,etc.) in the tumor microenvironment[54]. Beckebaumet al.[55]confirmed that IL-10 has a considerable immunosuppressive effect on circulating DCs in HCC patients. In addition, previous studies have shown that DCs can promote immunosuppression by producing IL-10 and IDO in HCC, but that depends on the expression of CTLA4[46].
In general, in recent years, DCs have been widely used as new vaccines in the treatment of solid tumors such as liver cancer, prostate cancer, kidney cancer, melanoma,etc.[56,57].
MDSCs
Myeloid-derived suppressor cells MDSCs comprise a mixture of macrophages, monocytes, granulocytes and DCs, which are a heterogeneous population of immature myeloid cells[58]. Some previous investigations revealed that MDSCs participate in immunosuppressive networks and are potential immunotherapy targets for HCC[59-62]. A study by Zhouet al.[63]showed that immunosuppressed CD11b+CD33+HLA-DR-MDSCs were stimulated and amplified by hepatic CCRK by increasing IL-6 expression in human peripheral blood mononuclear cells. In addition, they also found that tumor-infiltrating CD11b+CD33+HLA-DR-MDSCs potently inhibited autologous CD8+T cell proliferation in HCC patients. MDSCs express galectin-9 and bind to TIM-3 on T cells to induce T cell apoptosis[64].
In HCC patients, MDSC can also impair function of NK cells by inhibiting their cytotoxicity and cytokine release[65]. Furthermore, Huet al.[66]showed that MDSCs can inhibit the production of IL-12 induced by TLR-ligand via the expression of IL-10 and can inhibit T lymphocyte activity. In summary, MDSCs play a variety of immunosuppressive roles in HCC.
Given that MDSCs play a variety of immunosuppressive roles in HCC, the immunotherapy targeted MDSC has become a research hotspot. Some previous research has shown that the combination of radiotherapy and IL-12 (RT/IL-12) may reduce accumulation of tumor-infiltrating MDSCs and reverse the intratumoral immunotolerant state and improve the immune level in HCC[67]. In addition, targeting MDSCs and combining anti-PD-1/PD-L1 can synergistically enhance the cure of HCC[63,68].
Hepatic stellate cells, endothelial cells and kupffer cells
In addition to that described above, hepatic stellate cells (HSC) are an important element in the microenvironment of liver tumor. Previous research has shown that HSC can secret hepatocyte growth factor/cytokines, which lead to decreased antitumor immunity function[69]. In addition, hepatocyte growth factor (HGF) secreted by activated HSC promotes the invasiveness and tumorigenicity of HCC[70,71].
Compared with normal tissues, the endothelial cells of HCC are significantly different in molecular and functional aspects. Endothelial cells are involved in tumor neovasculature and play a significant role inmalignant invasion and metastasis. Previous studies have shown that various angiogenic receptors are expressed in endothelial cells, such as epidermal growth factor homology domains-2 (Tie-2), VEGF receptors, platelet-derived growth factor receptor, epidermal growth factor receptor and C-X-C chemokine receptors, which promote the formation of new blood vessels[72,73]. Liver sinus endothelial cells are special endothelial cells that can collect sample portal venous blood and act as APCs to cross-prime T cells[74]. In addition, the synergistic induction of tumor-derived VEGF-A and prostaglandin E2 (PGE2) can reduce antitumor immune response through excessive turnover of CD8+T cells[45].
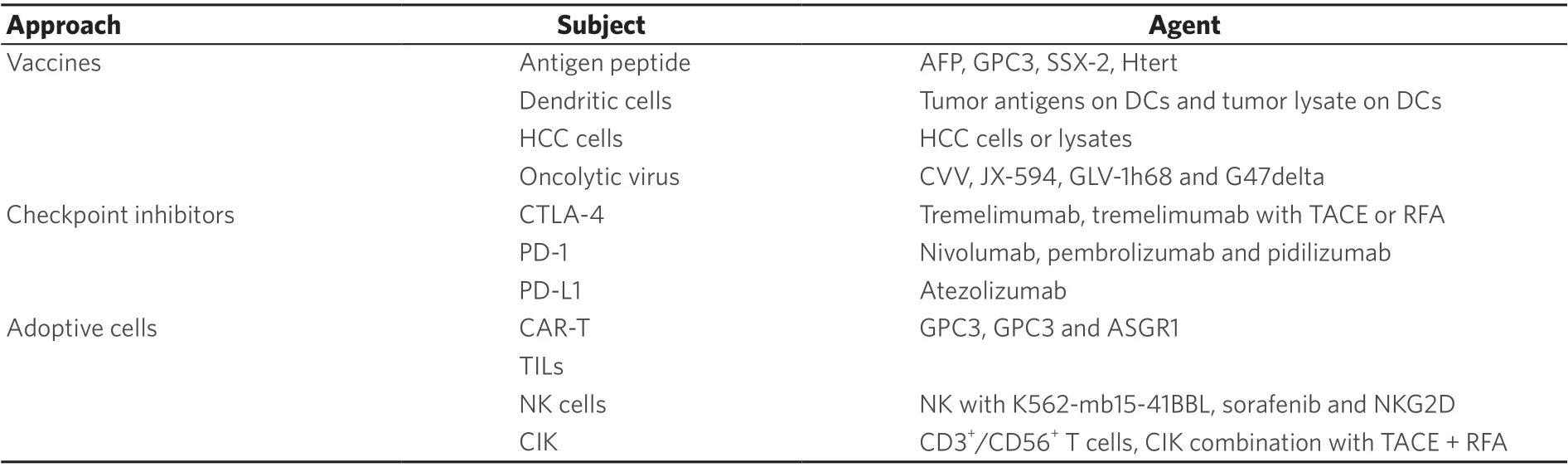
Table 1. Major immunotherapeutic approaches for HCC
Kupffer cells are hepatocyte macrophages that form the first line of defense against pathogens and promote local tolerance[75]. When stimulated by inflammatory cytokines, Kupffer cells are also stimulated (TNF-α, IL-1, PDGF) to produce excess osteopontin, which plays an important part in different cellular signaling pathways, promoting inflammation, tumor invasion and metastasis[76]. Kupffer cells produce large amounts of IL-6 in response to hepatocyte death, which contributes to compensatory proliferation of hepatocytes[77]. In addition, IL-10 inhibits the expression of Kupffer cell-derived inflammatory TNF-α, and works in concert with NO to reduce liver inflammation[78].
CURRENT STAGES OF IMMUNOTHERAPY
In view of the important role of the immune microenvironment in liver tumors, immunotherapy for liver cancer has become the focus in current research. According to the present study, immunotherapeutic approaches are divided into vaccines, cytokines, adoptive cell therapies, immune checkpoint inhibitors, and oncolytic viruses [Table 1].
Vaccines in HCC management
The essence of cancer vaccination is the body’s own immune system activated by an antigenic substance and then attacks the tumor. With the progress of immunology and technology, tumor vaccine therapy has become the frontier in tumor treatment research. Recently, some studies showed that HCC vaccines mainly include vaccines based on antigen peptides, DCs, cancer cells and DNA.
Antigen peptide vaccines
Antigen peptide vaccines are accurate targets for HCC and are based on TAAs. Recently, the most frequently reported peptide vaccines have been AFP peptide vaccines[79,80]. AFP is a glycoprotein belonging to the albumin family, and is normally expressed in abundance in fetal blood while aberrantly expressed on the surface of HCC cells. However, due to the immune tolerance of the liver, the immune response to AFP is limited[81]. It is difficult for AFP to produce an effective immune response when it is synthesized in the liver. Some clinical trials based on AFP have been initiated[82]. Zhanget al.[82]showed that cellular immune responses may be responsible for the antitumor activity against AFP-positive tumor cells in the mouse HCC model. Unfortunately, the AFP peptide vaccine also has certain limitations, where it only targets the AFPspecific immune response.
Carcinoembryonic antigen glypican-3 (GPC3) is another important antigen that targets liver cancer[83]. In 2012, a phase I clinical trial was been initiated. Thirty-three advanced HCC patients were injected with GPC3 peptide, and nineteen HCC patients showed stable disease after 2 months treatment. The study results showed that the GPC3-derived peptide vaccination not only has good immune tolerance, but also has a clear immune response and antitumor efficacy[84].
Studies indicate that human telomerase reverse transcriptase (hTERT) is a catalytic enzyme necessary for telomere extension[85,86]. Mizukoshiet al.[87]showed that hTERT is an important target for T cell-based immunotherapy in HCC. Another study found that 71.4% of liver cancer patients acquired TERT-specific immunity and 57.1% of patients had no HCC recurrence after vaccination with hTERT461 peptide-specific T cells[88]. SSX-2, a cancer-testis antigen, has been shown to be overexpressed in HCC patients. Recent evidence suggestw that a large number of SSX-2- and MAGE-A-specific CD8+T cells can be found in HCC[89].
DCs
DCs are the immune cells with the strongest antigen-presenting ability in the human immune system. Some cytokines such as recombinant human interleukin 4 (rhIL-4) and recombinant human granulocyte macrophage colony stimulating factor (rhGM-CSF) can activate the function of DCs, and DCs can be sensitized via the lysis of liver cancer cells. Some research has shown that immature DCs do not cause serious immune response but suppress CD8+T cell immunity[90]. CD8+T cells and polarized CD4+T cells are induced by mature DCs. Therefore, mature DCs should be selected as the tumor vaccine and their maturity should be evaluated[91]. In the body’s immune system, the CD4+T/CD8+T ratio is used to evaluate the antitumor immunity. Encouragingly, the proportion of CD4+T/CD8+T has been shown to be significantly increased after DC-based immunotherapy. In addition, the latest study demonstrated that the survival rate and survival time of HCC patients were increased after DC-based immunotherapy when analyzing 1276 cases in 19 clinical trials[92]. In addition, DC-derived exosomes are a novel class of vaccines for cancer immunotherapy. AFP-rich derived exosomes can elicit a strong antigen-specific antitumor immune response, providing a cell-free vaccine option for HCC immunotherapy[93].
However, there are many challenges in immunotherapy with DC vaccines. To resolve the problems better, some researchers combine DC-based vaccines with checkpoint inhibitors to improve efficacy. Wilgenhofet al.[94]combined DC vaccines with an immune checkpoint cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) inhibitor for advanced melanoma in phase II clinical studies. The experimental results showed that the combination of tumor vaccine and immune checkpoint inhibitor has significant advantages over single drug treatment. Furthermore, a phase I/IIa clinical study achieved encouraging results showing that the adjuvant DC vaccine for HCC was safe and well tolerated[95].
HCC cell vaccines
HCC cells or lysates that are physically or chemically disposed to eliminate pathogenicity could be used as immunogens for tumor-specific immune responses. Nemunaitiset al.[96]conducted a phase I trial of cellular immunotherapy using autologous whole-cell tumors (FANGTM). They observed that FANG manufacturing was successful in 7 of 8 attempts in this study. However, HCC vaccines are still in the early stage of clinical research and more studies are needed to prove their efficacy.
Oncolytic virus vaccines
The basic principle of antitumor oncolytic viruses is to expand them inside cancer cells and lyse them, eventually killing the cancer cells, and can selectively replicate and lyse in tumor cells without damagingnormal tissue. Pexa-vec (Jx-594) is an oncolytic vaccinia virus that selectively replicates in tumor cells that overexpress thymidine kinase, thereby ensuring that the virus specifically infects HCC cells, avoiding damage to normal cells. Moreover, the combined application of JX-594 and nivolumab in the treatment of liver cancer is also being evaluated (NCT03071094). Unfortunately, the phase III trial of Pexa-Vec/Nexavar combined therapy for liver cancer failed to show efficacy (NCT02562755)[97]. Moreover, the combined application of JX-594 and nivolumab in the treatment of liver cancer is also being evaluated (NCT03071094)[98].
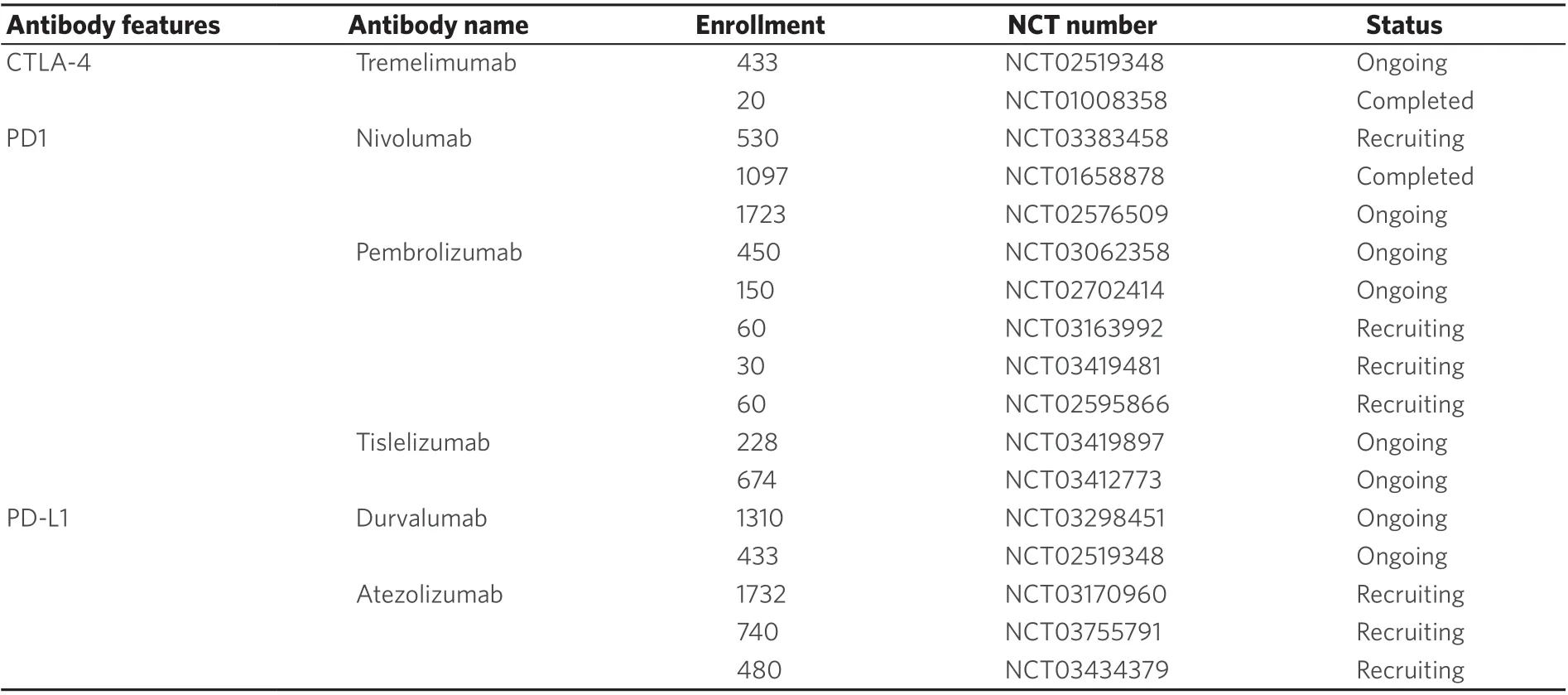
Table 2. Representative clinical trials of immune checkpoints inhibitor in hepatocellular carcinoma
The efficacy of cowpox virus (CVV) with an evolutionary tendency for cancer was studied in an animal model of metastatic HCC. The results showed that CVV may be a promising virus against metastatic HCC[99]. In addition, GLV-1h68 and G47delta have also been used for the treatment of HCC[100,101].
Twumasi-Boatenget al.[102]commented, “As key control points in the antitumor response continue to be deciphered, OVs will provide an increasingly important platform for bioengineers to re-wire antitumor immunity”.
Immune checkpoint inhibitors in HCC management
Previous studies confirmed that immune checkpoints are frequently activated in tumor tissues compared with normal tissues and contribute to evasion of immune surveillance by tumor cells to[103]. Tumor-associated T cells are reactivated by immune checkpoint inhibitors, and their antitumor function is increased. At present, the immune checkpoints PD-1, CTLA-4, TIM-3, VISTA and LAG-3 are most studied[104-109]. Among them, inhibitors of PD-1 and CTLA-4 have been approved for treating melanomas by the FDA. In addition, in the treatment of HCC, great progress has been made in recent years [Table 2].
CTLA-4 inhibitors
CTLA-4 is a transmembrane receptor for T cells, and its expression is closely related to T cell activation. CTLA-4 has a higher affinity with CD80 and CD86 compared with CD28. CTLA-4 can suppress the activation of T cell by competitive antagonism of CD28 binding to CD80 and CD86[110-112]. Blocking the binding of CTLA-4 to its ligands can stimulate the activation and proliferation of immune cells to induce or enhance the immune response.
Tremelimumab is a monoclonal antibody that targets CTLA4. In 2013, a clinical trial of tremelimumab enrolled 21 patients with chronic hepatitis C with Child-Pugh A or B cirrhosis and advanced HCC[105]. In this study, the HCC patients were administered tremelimumab intravenously at 15 mg/kg every 90 days until tumor development or severe toxicity. The results showed a partial response rate of 17.6% and disease control rate of 76.4%, and 45% of the patients had stable disease for more than 6 months after treatment with tremelimumab[105]. In a phase I/II trial, tremelimumab combined with TACE or RFA were used to treated HCC. Thirty-two patients with HCC were enrolled, patients received different doses of tremelimumab (3.5 and 10 mg/kg i.v.) every 4 weeks for 6 doses and infusion for the 3 months until non-treatment criteria were reached. Subtotal RFA or chemical ablation was performed after tremelimumab. The results showed that tremelimumab combined with RFA can result in the accumulation of intratumoral CD8+T cells, and doselimiting toxicities were not observed[113].
PD-1 and PD-L1 inhibitors
Some investigators have found that DCs, NK cells, B cells and mononuclear cells often show increased expression of PD-1[114]. PD-1, a member of the CD28 superfamily plays a key role in delivering co-inhibitory signals to TCR receptors[115]. Receptor binding to PD-L1 and PD-L2 were blocked by PD-1 inhibitors, resulting in T cells exerting normal efficacy against tumors[116]. In cancer cells, PD-L1/PD-1 signals are activated by PD-L1 or PD-L2, allowing them to evade immune surveillance[117].
Nivolumab, a monoclonal antibody targeting PD-1, has been investigated for HCC treatment. Recently, a phase 1/2 study in patients with HCC was used to assess the safety and efficacy of nivolumab in treating HCC. A total of 212 patients received nivolumab, 3 mg/kg every 2 weeks. The statistical results showed that the objective response rate was 16%, while disease control rate was 68%, and the 6-month survival rate was 82.5%[118]. In advanced HCC patients, the safety and effectiveness of nivolumab were also assessed. In the 49 advanced HCC patients involved in a clinical trial, an objective response rate of 10% and disease control rate of 55% were seen after treatment with nivolumab for 7 months[119]. Therefore, nivolumab shows good safety for patients with advanced HCC and has the potential to treat patients with advanced HCC. In view of those results, nivolumab was approved for the treatment of HCC patients with poor treatment after sorafenib.
Pembrolizumab is an IgG4 monoclonal antibody that targets the PD-1 receptor. In a phase II trial (KEYNOTE-224), 104 advanced HCC patients were enrolled, who were given 200 mg pembrolizumab injected every 3 weeks for about 2 years or until inappropriate. The results showed that there was objective response in 18 patients and stable disease rate of 44%, in the 104 patients studied. Statistical analyses indicated that pembrolizumab was also effective in advanced HCC patients treated with sorafenib[120]. A randomized phase III trial was conducted in 413 patients with advanced HCC[121]. In this study, improvement of overall survival, progression-free survival (PFS), overall response rate, and duration of response were observed in patients with pembrolizumab compared with the KEYNOTE224 study. The results showed that a good risk-benefit ratio for pembrolizumab in advanced HCC was also supported.
In 2008, 26 HCC patients who could not be resected or who showed metastasis were studied to determine the safety of atezolizumab, which targets PD-L1[122]. Atezolizumab (1200 mg) and bevacizumab (15 mg/kg) were injected via i.v. every 3 weeks. The results demonstrated a response rate of 62% and that this atezolizumab + bevacizumab combination was safe and well tolerated. The basic finding of this research was that the effects of atezolizumab were enhanced by anti-VEGF therapy. In addition, MEF2D can increase the expression of PD-L1 and inhibit antitumor immunity mediated by CD8+T cells[123].
Although immune checkpoint inhibitors have shown good efficacy in the treatment of HCC, they have limited efficacy in advanced HCC patients. The combined use of immune checkpoint inhibitors is expected to produce a synergistic effect and achieve better results. Selective clinical trials of combination immunotherapy agents in HCC are described in Table 3. In HCC patients with chronic HCV infection, the amount of CD8+T cells increased, but cell activity remained unchanged after blocking PD-L1/PD-1 or CTLA4. However,combined use of CTLA4 and PD-L1 inhibitors can reverse the instability of CD8+T cells and enhance intrahepatic HCV-specific CD8 and CD4 T cell cytokine response. Moreover, in the acute hepatitis C phase, the combination of PD-1/CTLA-4 inhibitors can reverse HCV-specific CD8+T cell dysfunction[124]. Interestingly, after the clearance of HCV, CD8+T cell proliferative ability can be restored preferentially under the action of immune checkpoint inhibitors[125]. In addition, Steinet al.[126]combined the PD-L1 monoclonal antibody atezolizumab and bevacizumab to treat patients with advanced HCC (NCT02715531). Moreover, the efficacy of immune checkpoint inhibitors (ipilimumab and nivolumab) combined with liver resection is being evaluated (NCT03682276, NCT03510871)[98]. As new adjuvant therapies, the curative effect of checkpoint inhibitors is also being evaluated in HCC patients undergoing surgical resection (e.g., NCT03859128 and NCT03847428)[98].

Table 3. Selective clinical trials of combination immunotherapy agents in HCC
Although many studies have reported that immune checkpoint inhibitors are showing encouraging results in the treatment of HCC, there are still potentially serious adverse reactions. PD-1 inhibitors block the interaction between PD-1 and PD-L1 and also inhibit T cells and APCs. PD-1/PD-L1 monoclonal antibodies cannot promote T cells to attack tumor cells[127,128]. Existing studies suggest that it is only effective for some patients, and a part of patients will get worse after this kind of treatment, so the treatment strategy for advanced HCC also needs to be optimized.
Adoptive cell immunotherapy
Adoptive cell immunotherapy is a special therapy including active specific immunotherapy and passive immunotherapy. In this type of therapy, cancer cells are killed by using patients’ own lymphocytes. Chimeric antigen receptor T cell immunotherapy (CAR-T), NK cells, tumor-infiltrating lymphocytes (TILs) and cytokine induced killer (CIK) cells constitute adoptive cell immunotherapy in HCC.
CAR-T
CAR-T cells are a special type of transgenic T lymphocytes that specifically recognize TAAs and improve the targeting of effector T cells and break host immune tolerance[129]. A previous study confirmed that an intracellular signaling domain, extracellular antigen-binding domain, and an extracellular hinge area constitute the basic structure of CARs[130]. The intracellular signaling domain and extracellular antigenbinding domain are connected by the extracellular hinge area. It is the hinge area that confers high activity on the extracellular antigen-binding domain. Although CAR-T cell immunity has become a research hotspot in the application of solid tumors, more studies on HCC are still in the basic research stage, and the key point of technology lies in the selection of tumor-specific antigens. GPC3 is located on the cell surface and is a member of the glypican family of heparan sulfate. A previous study reported that GPC3 is a TAA and overexpressed in HCC compared with normal tissues. Gaoet al.[131]found that GPC3-positive HCC cells could be lysed by GPC3-targeted CAR-T cells and that the number of lysed tumor cells correlated with the expression of GPC3. Liuet al.[132]recently stated, “The inducibly expressed IL-12-armored GPC3-CAR-T cells could broaden the application of CAR-T-based immunotherapy to patients intolerant of lymphodepletion chemotherapy and might provide an alternative therapeutic strategy for patients with GPC3+cancers”.
Asialoglycoprotein receptor 1 (ASGR1) mediates the transport of targeted therapeutic molecules to the liver, specifically expressed on liver parenchymal cells. GPC3 and ASGR1 make a suitable target combination for dual-targeted CAR-T cells. Chenet al.[133]surveyed data and found that the risk of on-target, off-tumor toxicity may be reduced when using T cells with two complementary CARs against GPC3 and ASGR1. Recently, 13 GPC3+HCC patients were enrolled a phase I trial, where all patients who received GPC3 CAR-T treatment had no dose-limiting toxicity and tolerated the treatment well. The study results showed that GPC3 CAR-T treatment was feasible and safe for Chinese patients with GPC3+HCC (NCT02395250)[134]. With the application of CAR-T cells in the clinic, the safety of CAR-T cells has begun to attract people’s attention. MacKayet al.[135]noted, “Immune-cell failure is a major challenge for CAR therapies, and its mechanism remains to be investigated”. In addition, immune rejection and off-target effects of CAR-T cells also need to be resolved[136].
CIK cells
CIK cells are a new type of non-MHC-restricted immunocompetent cells mainly including CD3+/CD56-cells, CD3-/CD56+NK cells, CD3+/CD56+T cells,etc., among which CD3+/CD56+T cells are the main effector cells[137,138]. Preclinical trials have shown that CIK cells have high activity against HCC cellsin vitro. In addition, Panet al.[139]investigated the effect of CIK as adjuvant therapy on overall survival and relapsefree survival of HCC patients who received surgical treatment. Survival analysis showed that compared with hepatectomy alone, median overall survival and PFS were clearly prolonged in the hepatectomy/CIK combination group (41, 16 monthsvs.28, 12 months, respectively). The safety and efficacy of CIK cell immunotherapy were investigated in a phase III clinical trial in which 230 HCC patients treated with RFA, surgical resection or percutaneous ethanol injection were randomly injected with 6.4 × 109autologous CIK cells (16 times during 60 weeks) or had no immunotherapy. The median time of recurrence-free survival in the immunotherapy group was clearly longer than control group (44 monthsvs.30 months). Cancer-related death was also lower in the CIK group than in the no immunotherapy group (P= 0.02); 17% of patients experienced adverse reactions related to CIK cytokines, but there was no difference in severe adverse events[140].
In addition, the efficacy and safety of the combination of CIK cells with liver-directed therapies in HCC were also evaluated. In a study that evaluated CIK cells combined with TACE + RFA in HCC patients. overall efficacy in the TACE + RFA + CIK group was better than in the TACE + RFA group (76.5%vs.79.8%). Kaplan-Meier analysis indicated that the overall survival rate of the TACE + RFA + CIK treatment group was significantly prolonged (56 monthsvs.31 months,P= 0.001)[141]. In another study, HCC patients with Child-Pugh scores of A or B and without prior treatment were enrolled. One group (n= 66) received CIK treatment and standard treatment, while another group (n= 66) received standard treatment only. The results showed that overall survival and PFS were significantly prolonged after treatment with CIK cells in patients who were not suitable for surgery[142]. Furthermore, one study found that targeting MDSCs is a good strategy to enhance the antitumor efficacy of CIKs for the treatment of patients with HCC[143].
Although more and more studies have shown that CIK cells makes up a significant part of HCC treatment, only a portion of T cells can provide a full antitumor function because of immune escape mechanisms and lack of specific tumor antigens.
TILs
TILs are isolated from tumor tissue and induced by IL-2in vitro. TILs are relatively rare in HCC, but they play an important role in tumor recurrence in patients. A phase I clinical trial indicated that immunotherapy with autologous TILs could be successfully performed with low toxicity[144]. In another randomized clinical trial, 76 HCC patients were enrolled and the ratio of postoperative recurrence was significantly lower in the group using IL-2 to activate lymphocytes compared to the control group[145]. Furthermore, the functions of tumor-infiltrating T cells were restored via the antibodies targeting immune checkpoints[146]. Until now, the widespread use of TIL immunotherapy has been limited, mainly because of difficulties in purification and amplification.
NK cells
NK cells belong to innate immune cells, and the main feature is that they can directly destroy tumor cells without prior stimulation. Preclinical experiments showed that CXCR6 can inhibit hepatocarcinogenesis by promoting natural killer T cell- and CD4+T cell-dependent control of senescence[147]. Another study also confirmed that expanded activated NK cells are highly cytotoxic to HCC cells. The function of NK cells against HCC were enhanced by the expression of NKG2D-CD3ζ-DAP10[148]. Tanet al.[149]reported that LrNK cells were observed to be present in liver cancer, often showing abnormal function. Moreover, this dysfunction is caused by Tim-3-mediated PI3K/mTORC1 interference. However, there is still a lack of clinical trials of NK cells in the treatment of HCC, so more clinical studies are urgently needed.
SUMMARY AND FUTURE EXPECTATIONS OF IMMUNOTHERAPY IN HCC
With the development of molecular biotechnology and tumor immunology, immunotherapy has been an important part in the mode of combined therapy of tumors. Along with the gradually deepened study of molecular biology and molecular immunology, T cells have an important influence on tumor immunity in the hepatic microenvironment. Recently, there have been a large number of studies on HCC immunotherapy, and some of them have achieved important positive results. At present, many preclinical studies demonstrate that vaccine therapy, adoptive cell therapy and immune checkpoint inhibitors make up a significant part in inhibiting the growth and development of HCC. Moreover, more clinical trials of immunotherapy for liver cancer are being conducted. In early clinical trials, tremelimumab, nivolumab, pembrolizumab and atezolizumab are targeting PD-1/PD-L1 or CTLA-4, and there have been encouraging results for a variety of tumors. However, phase III clinical research is still lacking, and more clinical research is urgently needed.
In addition, the results of the present study indicate that the effect of single immunotherapy on liver cancer remains limited. The indication of immunotherapy in HCC may depend on combination therapies. Combination of liver-targeted therapy and immunotherapy can enhance tumor and systemic immune response. In conclusion, the advent of immunotherapy revolutionized cancer therapy and brought treatment for HCC to a brand-new period.
DECLARATIONS
Authors’ contributions
Study concept and design: Xu XD
Literature search: Qin W
Drafting of the manuscript: Qin W, Cao ZY, Liu SY
Critical revision of the manuscript for important intellectual content: Xu XD
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study was funded by the National Natural Science Foundation of China (grant number 81670111).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
- Hepatoma Research的其它文章
- The advancement of immunotherapy in hepatocellular carcinoma
- Mechanisms and immunotherapies of HBV- and NAFLD-related hepatocellular carcinoma
- Direct-acting antivirals and risk of hepatocellular carcinoma: from genetic signature to metabolic risk factors
- Treatment options for recurrence of hepatocellular carcinoma after surgical resection: review of the literature and current recommendations for management
- Hepatocellular carcinoma and hepatitis C virus infection in Latin America: epidemiology, diagnosis and treatment

