Hepatocellular carcinoma and hepatitis C virus infection in Latin America: epidemiology, diagnosis and treatment
Marina Galicia-Moreno, Hugo Christian Monroy-Ramirez, Marina Campos-Valdez, Jaime Sanchez-Meza, Laura Sanchez-Orozco, Juan Armendariz-Borunda,2
1University of Guadalajara, Institute of Molecular Biology in Medicine and Gene Therapy, Department of Molecular Biology and Genomics, Health Science University Center (CUCS), Jalisco 44340, Mexico.
2Tecnologico de Monterrey, Campus Guadalajara, Jalisco 45138, Mexico.
Abstract Hepatocellular carcinoma (HCC) is the most common cancer associated with chronic liver disease and cirrhosis. The most common cause of HCC is chronic hepatitis C virus infection and many studies in Europe, Asia and North America have focused on its etiology, epidemiology, diagnostic tools, and therapeutic options. However, little is known about these issues in Latin America. The aim of this review is to address these aspects of HCC in Latin America. The main risk factors associated with developing HCC in this region are: age, concomitant cirrhosis, hepatitis C infection, obesity and hereditary disease such as hemochromatosis. On the other hand, screening tests and diagnostic methods of HCC are mostly serum alpha fetoprotein quantification, liver ultrasound, computed tomography, magnetic resonance, and histopathology. Novel diagnostic methods include gut microbiota analysis and the use of nanotechnology and they continue to be tested. Finally, according to the Barcelona Clinic Liver Cancer, curative treatments used in HCC patients are mainly liver resection, liver transplantation, and local ablation, each with advantages and disadvantages. In conclusion, clear strategies are urgently needed to understand the extent of HCC and related problems in this part of the world. This review provides greater knowledge of HCC for the proper design of preventive programs by taking into consideration specific characteristics of our population. Also, this review allows for an understanding of individualizing treatments according to the patient’s needs.
Keywords: Liver, hepatitis C, epidemiology, diagnosis, treatment, hepatocellular carcinoma, Latin America
INTRODUCTION
Latin America is one of the most urbanized regions in the world, made up of 20 countries and 13 departments with an estimated population of 626 million[1].
In this and other regions with large populations, access to health care is the main impediment for early diagnosis and correct treatment of HCC; and therefore, for the implementation of surveillance programs[2]. HCC is the most common cancer associated with chronic liver disease and cirrhosis, and is the second leading cause of cancer-related deaths worldwide[3]. Etiology factors for HCC varies according to its geographical area[4], being the most reported causes of HCC around the world chronic hepatitis C and B viruses (HCV, HBV) infections, and alcohol consumption[5]. Recently, it has been considered that nonalcoholic fatty liver disease could also be an important risk factor for HCC development[6].
Globally, liver cancer is the sixth cause of incidence and fourth in cancer-related mortality. New cases of liver cancer in 2018 were 841,080 that correspond to 4.7% of all registered cancer cases. Worldwide in that same year, there were 781,631 deaths caused by liver cancer, number that represent 8.2% of deaths in that year[7].
In 2018, Latin America registered an incidence of 38,400 HCC cases; 11,229 of which corresponded to Central America, 24,248 to South America and 2,923 to the Caribbean, being more affected the masculine gender. Brazil was the country with most patients with HCC, followed by Mexico, Argentina and Peru[7]. On the other hand, regarding mortality data, HCC was the main cause of death in Latin America and Caribbean countries during 2018, affected mainly masculine gender, Brazil and Mexico were the countries in the LAC region with the highest mortality[7]. The incidence, mortality, cumulative risk data and prevalence for the countries belonging to Latin America are shown in Table 1.
At present, many studies have been focused on studying the etiology, epidemiology, diagnosis tools, and therapeutic options in Europe, Asia and North America. However, little is known about these issues in Latin America. The aim of this work is to provide a comprehensive review about the current situation of HCC of viral origin (HCV) in Latin American, showing information about diagnosis and therapeutic strategies employed in this region.
EPIDEMIOLOGY OF CHRONIC HCV INFECTION IN RELATION TO HCC: PREVALENCE AND MORTALITY
The main causes of HCC in different geographical areas around the world have been related to infective agents such as HCV[8]. Of the total number of cancer cases, HCV caused 160,000 cases of HCC in 2018, which is equivalent to 7.1% of cases worldwide caused by infectious agents. Of these, 3900 were reported in Central America, 7400 in South America and 782 cases in the Caribbean[7].
Other data show that in 2016, of 7.2 million people worldwide who were living with chronic hepatitis C, 57% corresponded to Latin America and 3% were from the Caribbean [Figure 1 and Table 2][9,10]. The number of people diagnosed and treated for chronic HCV infection in the Americas is very low - only around 25% of all cases are diagnosed but just 14% are from Latin America and the Caribbean. Approximately 301,000 were treated in 2016, which is equivalent to only 16% of the diagnosed population in America, whereas in Latin America and the Caribbean, just 5% were under treatment. Determining the precise number of patients with known HCV status and receiving care has been difficult[9].
In 2013 in America, of 125,700 deaths due to HCV and HBV, 80% were attributable to HCV of which 39% occurred in the Americas. Compared to 1990, the number of deaths has increased by 134%, and 8% since 2010[9].From the available data, ranking of the prevalence of HCV (% of total population) in the LAC countries in 2015 were: Puerto Rico (1.0%), Brazil (0.9%), Argentina and Colombia (0.8%), Dominican Republic (0.5%), Peru (0.5%), México and Venezuela (0.4%), and Guadeloupe, Panama, Chile and Cuba (0.3%)[10].

Table 1. Incidence, mortality and prevalence of HCC in Latin America and the Caribbean in 2018
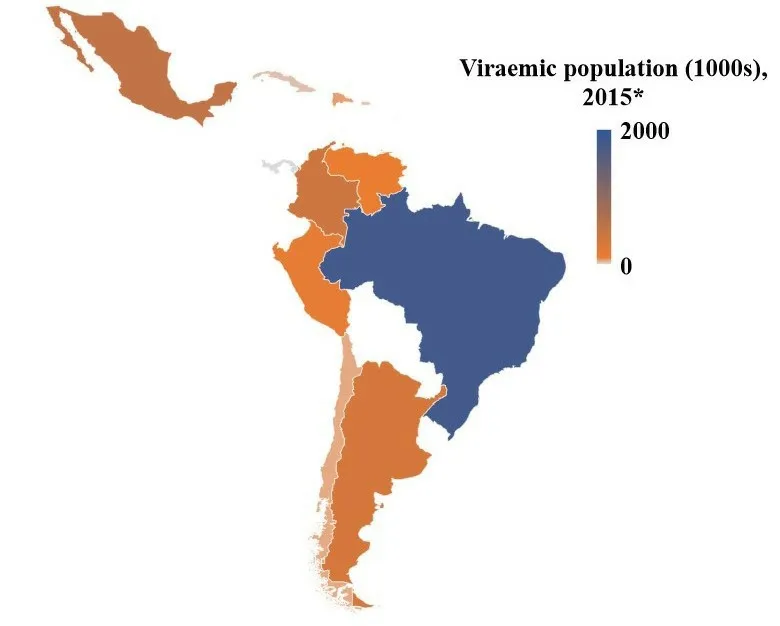
Figure 1. Map of the estimated hepatitis C virus viremic population in countries from Latin America and the Caribbean. Countries that are not shown or are in white color do not have available data
RELATIONSHIP BETWEEN HCV CHRONIC INFECTION AND HCC
In 2015, the WHO estimated that viral hepatitis accounted for 1.34 million deaths. These deaths resulted from chronic liver disease (720,000 due to cirrhosis) and primary liver cancer (470,000 cases). Each year, the number of deaths related to viral hepatitis has been growing and is represented by the increase in mortality related to viral hepatitis by 22% since 2000[11].
Around 75% of people exposed to HCV infection will not be able to eradicate the virus - 60%-70% of them will develop chronic liver diseases and of the remainder, 5%-20% will develop cirrhosis over a period of 20-30 years and 1%-5% will die from cirrhosis or HCC[12].Globally, the estimated annual percentage change in liver cancer due to age-standardized HCV incidence rate has increased 0.57 (95%CI: 0.48-0.66) between 1990 and 2016. This pattern is heterogeneous across regions and countries. This rate was also higher in low- and middle-income countries than in high-income countries[13].

Table 2. Estimated number of viremic people in Latin America and the Caribbean in 2016

Figure 2. Choropleth maps related to HCV related to disease and death in Latin America. A: HCV prevalence in 2015; B: estimated population with cirrhosis related to HCV in 2015; C: HCC mortality rate caused by HCV by 100,000 inhabitants, 2013. HCV: hepatitis C virus; HCC: hepatocellular carcinoma
In 2016, the percentage contribution of HCV to absolute liver cancer incidence (in both sexes) in Tropical, Southern, Central and Andean Latin America were 37.4%, 47.9%, 34.2% and 13.6%, respectively[13]. Deaths due to HCC related to HCV infection (in 2013) had the highest incidence in the Dominican Republic at 3.27 per 100, 000 people, followed by Chile with 3.22, Cuba with 2.96, Argentina with 2.84, and Mexico with 2.68[9,10][Figure 2 and Table 3].
DISTRIBUTION OF HCV GENOTYPES IN LATIN AMERICA
Studies on HCV genotypes related to different stages of liver disease have been reported even though the results are controversial. Nevertheless, cofactors such as age, sex, obesity, diabetes and alcohol consumption must be taken into consideration since they have an impact on the progress of chronic liver disease[14]. Worldwide, genotype 1 is the most prevalent, causing 44% of all infections, followed by genotype 3 with 25% and then genotype 4 with 15%. HCV genotype 1 is also the most prevalent in Latin America [Figure 3] and genotype 1b is predominant in the LAC[15].
According to Maucort-Boulchet al.[16], HCV was the cause of HCC in 58.7% of cases in Mexico, 50.0% in Brazil and 35%-38.8% in the rest of the LAC countries that were analyzed[3,10,16][Figure 3].
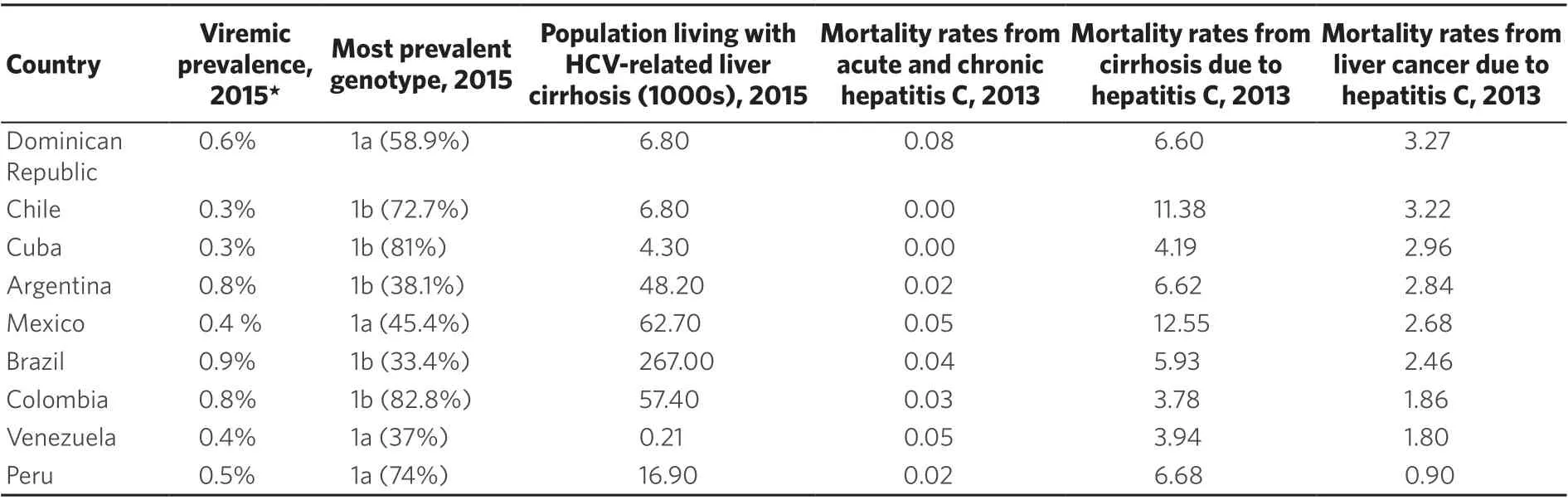
Table 3. HCV infections, genotypes and mortality rates in Latin America and the Caribbean
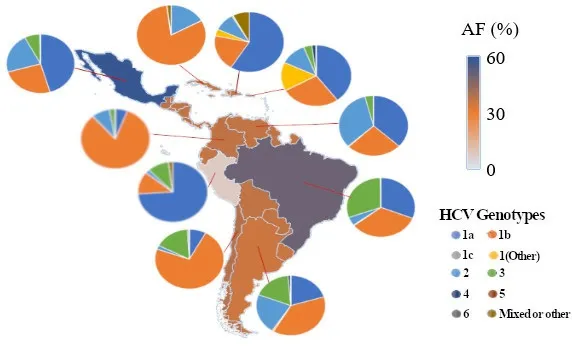
Figure 3. Choropleth map of the fraction of liver cancer attributable to HCV in some Latin America and the Caribbean countries, 2012. Pie charts represent the genotypes present in some of those countries and the proportion of cases that are related to each genotype. Genotype distribution data are either taken from the literature or based on regional averages in the absence of country-specific data. HCV: hepatitis C virus; AF: attributable fraction
ETIOLOGY AND RISK FACTORS IN HCC DEVELOPMENT
The etiology of HCC depends on the geographic location. For example, in countries where HCC is endemic such as Africa, Asia and Alaska, the most common cause is HBV infection. In countries where the risk of HCC is low, cirrhosis is the main cause of HCC in spite of the etiology[17].
The main risk factors associated with developing HCC are as follows.
Age
The risk of developing HCC is higher during the seventh decade of life. Nevertheless, HCC tends to affect people in their sixties in Mexico[18].
Cirrhosis
This is the main risk factor for HCC[18]. Several etiologic agents are implicated with cirrhosis development including chronic viral hepatitis, alcohol consumption, hemochromatosis, metabolic diseases such as nonalcoholic fatty liver disease,etc.In Mexico, the main risk factor for cirrhosis is alcohol consumption[19]. Long term studies have reported that 1%-8% of cirrhotic patients will develop HCC (3%-8% in patients infected with HCV)[2].
Hepatitis C
HCV infection is another important risk factor for developing HCC. New cases of HCC develop in 3%-5% of patients with cirrhosis due to HCV per year[20]. HCV Genotype 1b has been identified as a high risk factor for HCC development. Studies conducted in Latin America and the Caribbean have reported several other risk factors for HCV infection and eventual HCC in specific social minority groups such as drug users[21], prison inmates[22], and sex workers[23].
Aflatoxin
This is an important risk factor related to HCC development, mainly in Africa and Asia. Aflatoxin is produced byAspergillus flavusand is found in maize and peanuts, which causes modifications in the DNA of hepatocytes[17].
Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis
Due to the growing obesity epidemic, nonalcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease which includes a clinic-pathologic spectrum of disease ranging from isolated hepatic steatosis to nonalcoholic steatohepatitis (NASH), which can progress to cirrhosis or HCC. Once cirrhosis has developed, NASH pathology may be difficult to evaluate because fatty deposition and inflammation often disappear. Between 40% to 60% of patients with NASH-induced cirrhosis may develop a complication such as HCC after a period of 5 to 7 years. A meta-analysis conducted by Singalet al.[24], showed an association between the PNPLA3 variant and an increased risk for HCC, especially in patients with NAFLD-related cirrhosis. According to the Centers for Disease Control, the incidence of HCC was higher in Latinos than in non-Latinos. Latinos with HCC also have shorter survival rates than non-Latinos[25].
Obesity
This is yet another important risk factor that leads to an increase in the incidence of HCC. In subjects with a body mass index of 35 or above, tumor development is more frequent[26].
In Latin America and the Caribbean, a heterogeneous obesity pattern across the countries has been found. However, the prevalence of obesity has been increasing, not only among rural populations, the poor and least educated, but also the urban populations, the rich and highly educated[27]. Chile and Mexico have the highest prevalence of overweight and obese boys at 11.9% (9.6-14.3) and 10.5% (8.8-12.4) respectively, while for girls, the prevalence in Uruguay and Costa Rica are 18.1% (14.9-21.9) and 12.4% (10.0-15.1) respectively[28]. In Mexico, mortality data regarding obesity has been calculated and consequently, NAFLD will be the second most important cause of liver disease in the future, around 2050[29]. These data and evidence for the association between obesity and NAFLD as the third cause of HCC in Latin America, predict an increase in the incidence of HCC incidence in the near future. Thus, the implementation of measures to incorporate healthy diets and physical activity in the general population is urgent, in order to achieve healthy body weights to reduce the incidence of cancer among other chronic diseases such as diabetes mellitus.
Hereditary hemochromatosis
This condition is related with a 200-fold increase in risk for HCC. The formation of free radicals and lipoperoxidation products produce iron toxicity in the liver that eventually, can cause cirrhosis and HCC[30].
Wilson’s disease
This is a heritable disease that is the result of a mutation in theATP7Bgeneand causes alterations in plasma copper circulation. A high content of free copper in circulation induces cytoplasmic cell damage, cirrhosis, and eventually HCC[30].
SCREENING AND DIAGNOSTIC METHODS FOR HCC
The diagnosis of HCC includes screening tests, histopathological and imaging methods. The most widely used tests for screening are as follows.
Serum alpha-fetoprotein
Produced by the fetal liver and yolk cells as well as regenerating hepatocytes. By itself, serum alpha-fetoprotein (AFP) quantification is more sensitive than other markers. AFP has a cut off point of 10.9 ng/mL[31]but its sensitivity is low (25%-48%) since its concentration depends on tumor size. It is important to mention that some patients with HCC (30% to 50%) do not have high levels of AFP even in advanced stages. Due to this, it is not recommended as the only screening test[32].
Liver ultrasound
Liver ultrasound (LU) is considered the first choice screening test for HCC detection because it has a sensitivity between 60%-80%, and its specificity is above 90%[33]. According to international guidelines (EASL and AASLD), a LU every six months is suggested for early detection of HCC in cirrhosis patients[34,35]. This test is able to detect lesions larger than 1 cm in diameter, is safe, low-cost and does not have secondary effects.
LU + alpha-fetoprotein
Together, both strategies add only 6 to 8% of cases of previously undetected HCC. Combining both markers actually increases the number of false positive results. These diagnostic tests are suggested in subjects with a high risk of HCC at a frequency of 6 to 12 months.
Others methods used in diagnosing HCC are as follows.
Computed tomography and magnetic resonance imaging
The diagnosis and prognosis of HCC depends on the stage at which the tumor is detected. If detected at an early stage of HCC, long-term patient survival is more probable. Noninvasive imaging tests, including computed tomography (CT) and magnetic resonance imaging (MR) have been recommended by several clinical practice guidelines as the first-line diagnostic tools for the screening, diagnosis, staging and surveillance of HCC[35-37]. One characteristic about a liver nodule that suggests dysplasia is decreased hepatic artery flow, and the maintenance of portal venous flow. The presence of new unpaird arteries not accompanied by bile ducts is also a classic characteristic for differentiated neoplastic nodules from typical, regeneratiing nodules[38,39]. This, and other changes can be evaluated with current imaging techniques. The hemodynamic alterations that occur in HCC represent pathological markers for current, noninvasive diagnosis of HCC through CT and MR. Through the evaluation of dynamic images, the diagnosis of HCC is established by the detection of contrast-hyperenhancement in the arterial phase, and hypoenhancement in the portal venous phase. This response is defined as the “HCC radiological hallmark”. This vascular pattern allows HCC diagnosis with almost 100% specificity and positive predictive value for nodules with diameters of at least 1 cm[40-42]. According to recent meta-analyses, the sensitivity of CT and MR were 63%-73% and 77%-90% respectively, with a specificity of 87%-98% and 84%-97%, respectively[43-46]. Today, new imaging techniques have been developed to improve the non-invasive evaluation of HCC. The most significant techniques are diffusion weighted imaging (DWI) and hepatobiliary contrast agents.
DWI is a functional magnetic resonance technique that consists of quantifying proton diffusion in tissues[44]. There is cellular increase in HCC and this cellular proliferation restricts water proton diffusion[47]. It is important to mention that DWI quantification demonstrates restricted specificity for HCC because some lesions can show restricted diffusion on DWI[48,49].
On the other hand, hepatobiliary contrast agents such as gadobenate dimeglubine (Gd-BOPTA) and gadoxetate disodium (Gd-EOB-DTPA) can provide information about tumor vasculature and hepatocyte function in a single examination[50].
Histopathology
This diagnostic method can only be considered when evaluating nodules greater than 2 cm, or if radiological findings are not compatible with HCC. However, liver biopsies can yield false negative results[51]. Histological evaluation is considered positive if the sample is positive for at least two of glypican 3, heat shock protein 70 and glutamine synthase, which represents a sensitivity of 72% and a specificity of 100%[52].
Finally, we will review two novel, unconventional, diagnostic methods of HCC.
Gut microbiota analysis
The gut microbiota has an important role in the maintenance of homeostasis in humans. Evidence demonstrates connections between gut microbiota and HCC development. Ponzianiet al.[53]demonstrated that translocated bacterial elements from the gut to the liver can start an inflammatory process through toll-like receptors (TLRs). Lipopolysacharides from Gram (-) bacteria can bind TLR-4; TLR-2 recognizes the bacterial triacylated lipopeptide, and TLR-5 can recognize flagellin, a protein component of bacterial flagella[54,55]. In all cases, the final effect is the production of inflammatory cytokines such as TNF-a, IL-1b and IL-6 from the NF-kB pathway[56]. The gut microbiota and the development of HCC is also linked directly via the JAK or STAT3 pathway, which are mainly activated by IL-6[57]. Due to this, gut microbiota evaluation could improve diagnostic reliability. Pre- clinical and clinical trials have shown a direct correlation between Gram (-) bacteria and inflammatory changes related to the development of HCC. All these observations suggest that evaluation of the proportion of harmful and beneficial bacteria could be considered as a promising tool for the early diagnosis of HCC. Some limitations have been considered for these purposes as sometimes, HCC patients are subjected to antibiotic treatment, which may alter the composition and function of their microbiota, limiting diagnostic use.
Nanotechnology for HCV diagnosis
HCV infection is the main etiologic agent of hepatic cirrhosis and HCC. There are 8 main genotypes and 86 subtypes described in the last few years[58]. These antiviral agents are effective in reducing the probability of developing cirrhosis and HCC. However, these are highly expensive drugs and inaccessible to most patients, especially in low-income countries. Another important aspect to consider is that only a few highlyspecialized laboratories perform molecular diagnosis for HCV, which is essential for the best diagnosis and treatment[59].
Nanotechnology is a new, interesting and promising diagnosis tool for infectious diseases that uses nanoparticles and specific nanoscale tests directed at the pathogen genome[59]. There are different types of nanoparticles and gold is suitable for efficient diagnosis. It has been used to identify pathogens such asMycobacterium tuberculosis[60],Helicobacter pylori[61], the dengue virus[62], and influenza A[63]. On the other hand, RNA aptamers are single-stranded RNA oligonucleotides that specifically bind to a target molecule, which makes them good alternatives for the development of HCV serological tests[59]. Nanoparticles and aptamers, can be used as biosensors for diagnosis as the fusion of both can be bound to the HCV core protein, enabling easy detection of this protein[64].
There are several reasons that can lead to diagnostic failure in early HCC and as a consequence, failure in the correct selection of therapies. First, the absence of early identification of the at-risk population; second, no application of routine surveillance (e.g., performance of ultrasound twice a year); and finally, mistakes in the interpretation of screening tests[1]. There is no evidence to suggest that HCC screening improves survival in high-risk groups, although many medical professionals do use several diagnostic strategies such as AFP and liver ultrasonography for HCC screening[65]. Nevertheless, screening tests for HCC are very important to increase survival and quality of life. For example, when detected early, 5-year survival is greater than 50% but when diagnosed late and patients are symptomatic or the cancer is late stage, 5-years survival is less than 10%[18].

Table 4. Liver transplants in Latin America and the Caribbean in 2016
THERAPY
The Latin America Association for the Study of the Liver (ALEH) published the clinical guidelines for the management of HCC in the region. The ALEH indicates the staging procedures that should be carried out. Also, one of its main objectives is to define the best therapeutic strategy for each patient. The most widely used staging system is the Barcelona Clinic Liver Cancer (BCLC) system since it relates each stage of HCC with the most appropriate treatment according to scientific evidence[5,66]. Generally, HCC can be approached by curative or systemic treatments. Curative treatment is possible if HCC is diagnosed at an early stage.
Curative treatment
According to the BCLC classification, three curative treatments are available: liver resection, liver transplantation, and local ablation[67].
Liver resection is the best therapeutic option for HCC patients with or without cirrhosis, when the liver is still functional[18]. The aim of this surgical procedure is to obtain at least 2 cm margins through anatomic resection, except when the cirrhotic patient’s healthy residual liver is compromised[70-73]. Liver resection and liver transplantation are the only curative treatments for HCC patients, but unfortunately only 5% to 10% of patients are candidates because most have advanced disease and poor liver function[68].This option has shown good results with up to 60% 5-year survival and low perioperative mortality (0.8%-3%)[69]. If liver transplantation is contraindicated, the alternative is locoregional therapy. To select the ideal candidate, CT or MR evaluation of tumor size, presence of satellite lesions and vascular involvement are very important[5]. In some Latin American regions, HCC resection is recommended in patients classified as intermediate stage, when the liver has not completely lost its function, and survival of 5 years can still be achieved (patients with Child-Pugh A)[66,74].
Liver transplantation is the best option for treatment taking into account the tumor and the concomitant disease. In Latin America, the main problem is the absence of a organ donation culture[18]. Liver resection and transplantation are curative surgical treatments for HCC by removing both the tumor and cirrhosis. It is important then, to considerer: (1) the candidate according to their tumor stage, liver function, physiological status, (2) the experience of the medical staff performing the surgery[75]. BCLC guidelines are the most widely accepted for assessing a HCC patient’s prognosis[76]. According to Mazzaferroet al.[77, a patient could be eligible for liver transplantation when its expected survival is at least 70% at 5 years; this survival also depends of lesions size. In Mexico, liver transplantation is the first choice treatment for patients with Child-Pugh C score and HCV co-infection. Currently, Latin America has transplant programs and more than 2500 liver transplants are performed in the region every year[8]. Worldwide, in 2017, an estimated total of 32,348 liver transplants were performed with 2894 in Latin America. Table 4 shows the number of liver transplants performed as well as the number of patients on the waiting list in the LAC countries[78,79].
Loco-regional therapy
Several minimally invasive treatment options for patients with unresectable HCC have been developed including (1) curative therapies such as radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), microwave ablation (MWA), cryoablation (CA), irreversible electroporation (IRE), and (2) palliative therapies such as chemoembolization transcatheter-arterial chemoembolization (TACE) or trans-arterial radioembolization (TARE).
Curative options: liver ablation
These are ablative techniques that use chemical or thermal energy. EASL clinical practice guidelines recommend the use of this ablative therapy in very early (single lesion < 2 cm) and early stage (2-3 nodules < 3 cm) HCC. Ablation is recommended when resection or transplantation are not an option for HCC patients[80]. Tumor cell destruction can be produced through chemical substance injection (PEI) or through temperature alteration (radiofrequency, microwave, laser or cryotherapy). Although there are several options, RFA is the first choice[5].
RFA is uses an alternating electric current between 460-500 kHz which is applied to the lesion via a radiofrequency electrode. It induces the electromagnetic field to produce an oscillation of tissue ions and frictional heating, leading to coagulative necrosis and cell death a temperatures of 60-100 °C[80]. RFA is more beneficial than PEI in patients with early stage HCC. It offers 5-year-survival rates up to 76% when used as the main therapy in patients with resectable HCC. Based on BCLC criteria[81], it is important to note that results of RFA are optimal in patients with tumor > 3 cm[82]. In Mexico, PEI and RFA are available treatment options with successful results[18].
On the other hand, PEI is a good option for nodular HCC. This is the most widely used method for chemical ablation but has the disadvantages of non-uniform diffusion and uncontrollability of injected alcohol in large tumors. Hence, PEI is applied for the treatment of small HCC. It produces complete necrosis in 90% of tumors < 2 cm, and in 50% of tumors measuring 3-5 cm[83,84]. The main drawback of PEI is the high local recurrence rate, which is 43% in lesions > 3 cm[85]. In Brazil, percutaneous ablation of early HCC is recommended more frequently than in other LAC countries[74].
Regarding MWA, it was initially developed to work around the heat sink and tissue impedance limitations of RFA in the liver. RFA and MWA have similar mechanism of inducing cell death through increasing tissue temperature by the continuous realignment of polar molecules within a microwave field at frequencies of 915/2450 MHz[86]. Microwaves radiate throughout all tissue without impedance, allowing a larger tissue to be heated with each application. This technique is less invasive than hepatic resection and may be considered for patients for whom resection may be contraindicated because of age or comorbidities such as portal hypertension[87].
Other modalities of ablation currently employed in clinical practice are CA and IRE. CA destroys tissue, causing necrosis of the tumor by freezing at temperatures between -35 °C to -20 °C using the Joule-Thomson theory in the thawing process. This theory describes the temperature change of a gas when it is forced through a valve while it is kept insulated so that no heat is exchanged with the environment[80]. Tissue destruction is carried out through two mechanisms, a direct cellular injury and a vascular related injury. With respect to patient selection, it is necessary to consider: (1) patients with a single HCC < 5 cm in diameter, or up to three HCCs with each < 3 cm in diameter; (2) absence of portal venous thrombosis; (3) Child-Pugh A or B, and (4) no significant coagulopathy[88].
Finally, IRE is a novel, non-thermal form of tumor ablation that produces less collateral damage. IRE relies on short pulses of high frequency energy to induce pores in the lipid bilayer of cells, leading to cell death via apoptosis[89]. Patients who are candidates for RFA or MWA, but have tumors adjacent to structures that would cause either heat sink or collateral damage, can therefore be treated with IRE.
Palliative options, chemoembolization
This treatment consists of the administration of a chemotherapeutic agent into the tumor followed by an embolizing agent. This process increases the survival rate to 41% in 2 years[90]. TACE is the gold standard in patients unsuitable for surgery and for percutaneous ablation techniques with multinodular HCC, but have preserved liver function without vascular invasion or extrahepatic spread. This technique is based on occlusion of the arterial blood supply of the target neoplastic lesion by embolizing microparticles, in combination with an injection of chemotherapeutic drugs[80]. A variety of chemotherapeutic agents have been used, including monotherapy with doxorubicin or cisplatin, or a mixture with cisplatin, doxorubiicn and mitomycin C. Contraindications to TACE include decompensated cirrhosis (Child-Pugh C), encephalopathy, active infection and uncorrectable bleeding diathesis. Other relative contraindications include serum bilirubin of 3-7 mg/dL, advanced cancer stage, portal vein thrombosis, iodinated contrast allergy, biliary obstruction and renal insufficiency[90].
TARE is an important loco-regional treatment,used in patients with intermediate or advanced stages of HCC who are not candidates for TACE or Sorafenib. TARE, also known as selective internal radiation therapy, consists of intra-arterial delivery of a radioactive material to the tumor to limit systemic irradiation and preserve healthy liver. Ytrium-90 (90Y) has suitable characteristics for the treatment of tumors. It is a pure b emitter characterized by a short half-life (64.2 h) and has limited tissue penetration. Two types of microspheres are available, TheraSphere which is made of glass, and SIR-Spheres made of resin, and both have been demonstrated effective and safe in treating primary and secondary liver cancers[91]. Emission of a b particle with decay90Y to90Zr (zirconium) enables delivery of targeted radiation to the lesion, limiting radiation exposure to normal parenchyma while reducing the risk of radiation exposure induced liver disease[92].
According to the clinical practice guidelines for the management of HCC by the Latin American Association for the Study of the Liver (LAASL), chemoembolization is an option for patients, particularly those who are not candidates for resection, liver transplantation, or percutaneous ablation[5].
Systemic therapies
These are options for patients with the diagnosis of advanced stage HCC or in patients with tumor progression after loco-regional therapy[93].
Sorafenib
This is considered and approved as first-line treatment for patients with advanced HCC[93]. It is a multikinase inhibitor that targets Raf-1, B-Raf, vascular endothelial growth factor receptor, platelet derived growth factor receptor, and c-kit receptors. It inhibits tyrosine kinase activity and serine-threonine kinase receptor, acting as an antiproliferative and anti-angiogenic agent. Its efficacy has been demonstrated in phase II and III clinical trials[93].
HCC patients with Child-Pugh category A and advanced disease and with an Eastern Cooperative Oncology Group score of 0-2 are eligible to receive Sorafenib[35]. However, the main disadvantage is its high cost that makes it non-affordable to most patients. A retrospective analysis of 127 HCC cases treated with sorafenib in 8 medical centers in 5 South American countries between January 2010 and June 2017, showed that 38% of cases was due to HCV and the median survival after initiation of treatment was 7.5 months (IQR 2-17) in all subjects[94].
According to current guidelines established by ALEH, Sorafenib is standard systemic therapy for HCC in patients categorized as Child-Pugh C, or with underlying cirrhosis and advanced tumor (stage C according to BCLC guidelines), or with tumor progression even after loco-regional therapy. However, there are no other therapeutic alternatives in the event of treatment failure with Sorafenib[5].
Levantinib
This is a tyrosine kinase inhibitor that blocks VEGFR1-3, fibroblast growth factor receptors (FGFRs) 1-4, PEGFR, RET, and KIT. The overall mean survival time after treatment is 13.6 months and the rate of objective response (according to response evaluation criteria in solid tumors) is 18.8% for patients receiving levantinib (< 1% complete response and 18% partial response). The most common adverse events for this treatment are hypertension, diarrhea, decreased appetite, and weight loss[95].
Sorafenib and levatinib are approved as first-line therapy while regorafenib and cabozantinib are indicated in patients who have progressed or are intolerant to sorafenib[96].
Regorafenib
This is a multi-target inhibitor of VEGFR1, TIE-2, RETRAF-1, BRAF, PDGFR, FGFR, and CSF1R. It improves overall survival with a hazard ratio of 0.63, and the median duration of overall survival of patients who received it is 10.6 months. Adverse events include hypertension, hand and foot skin reactions, and diarrhea[97].
Cabozantinib
This targets the mesenchymal-epithelial transition factor (c-Met) pathway, as well as the VEGF and RET receptors. Compared to placebo, cabozantinib increased the median overall survival (10.2 monthsvs.8.0 months). The hazard ratio for death by treatment is 0.76; (95%CI: 0.63-0.92). However, grade 3 or 4 adverse events occurred in 68% and 36% of patients in the cabozantinib and placebo groups respectively. The most common high-grade adverse events include palmar-plantar erythrodysesthesia, hypertension, increased aspartate aminotransferase level, fatigue and diarrhea[98].
Ramucirumab
This is a fully humanized IgG1 monoclonal antibody directed against the extracellular domain of VEGFR-2. It has shown clinical efficacy either alone or in combination with chemotherapy in the treatment of a number of malignancies. It can be given on a twice or thrice weekly schedule and binds with much higher affinity to VEGFR-2 than its natural ligands, thus preventing the VEGF-VEGFR-2 interaction. Ramucirumab has also shown an advantage in delaying the worsening of disease-related symptoms and prolonging overall survival compared with placebo[99].
Nivolumab
This is a fully human immunoglobulin G4, anti-PD-1 monoclonal antibody that has been approved for the treatment of multiple advanced malignancies. In the phase I/II trial Checkmate-040, nivolumab showed response across all cohorts in 14%-20% of patients. The most common adverse events were fatigue, pruritus and rash, but manageable. Grade 3/4 serious adverse events occurred in 4%[100].
Doxorubicin
This is the most studied and widely used chemotherapeutic agent for HCC treatment and is chosen when the patient’s disease is critical. It is a DNA intercalating agent and its mechanism of action is related to inhibition of topoisomerase II[101]. Its use though is not recommended by many clinical guidelines such as AASLD or EASLD. In Latin America, patients receiving doxorubicin with arterial embolization have a 58% survival rate at 2 years[5]. In patients with advanced HCC however, doxorubicin administration failed to improve survival[5]. The combined effects of doxorubicin and sorafenib have been studied but favorable results were not obtained because of higher toxicity, mainly cardiotoxicity and neutropenia[102].
Interferon
These are agents with an immunomodulatory and antiproliferative effect on tumor cells in HCC. Adjuvant interferon (IFN) therapy has been demonstrated to reduce the recurrence of HCC, but does not improve the survival of HCV-related HCC patients. IFN is effective in intermediate and advanced HCC patients[103]. In terms of survival, tumor response and toxicity, IFN administration is superior to doxorubicin in patients with HCC.
IFN has several properties including antiviral, anti-tumor, and immunomodulatory effects[104]. Three IFN subtypes have been identified: type 1 (IFN a and b), 2 (IFN-g), and 3 (IFN-λ), but only IFN-type 1 is used in the treatment of chronic viral hepatitis to reduce the risk of HCC in patients infected with HCV[103]. Evidence was first obtained from a randomized controlled trial with 90 patients where IFN treatment was effective in decreasing the incidence of HCC[105]. In patients with HCV-related cirrhosis treated with IFN, there was a sustained virological response (SVR) after treatment that in turn, resulted in improved clinical outcomes including a lower risk of decompensation and HCC development[106].
In Latin America and according to the LAASL, the preventive effect of antiviral therapy in patients with HCV is more effective when using pegylated IFN plus ribavirin, which achieves higher SVRs. It is important to note however, that chronic administration of low dose pegylated IFN, without achieving SVR, fails to reduce the risk of HCC[5].
Direct acting antivirals
As previously mentioned, chronic HCV is the leading cause of HCC worldwide. The implementation ofin vitroreplication models using sub-genomic replicons and the cell culture system of HCV enabled the discovery and development of direct-acting antivirals (DAAs) for the treatment of chronic HCV. These antivirals have considerably improved the sustained viral response in the treatment of all HCV genotypes, with cure rates of more than 95%[107]. Treatment with DAAs in Latin America lagged behind Europe, Asia and North America but results have been really good in terms of viral eradication. Nevertheless, this must still be interpreted with caution since the number of studies in Latin America are still scarce.
In a multicenter study performed in Latin America to evaluate the association between DAAs and HCC waitlist progression or its recurrence following liver transplantation (LT) between 2012 and 2018, 503 patients without chronic HCV and 481 patients with chronic HCV were recruited (197, 19, 24, 180, 18, 5, 45 and 3 patients each from Argentina, Uruguay, Chile, Brazil, Mexico, Peru, Colombia and Ecuador respectively). From these HCV+patients, 327 were not treated with DAAs while 164 were. The most commonly used DAA regimen was sofosbuvir/daclatasvir in 68.3% (112) of patients, followed by peritaprevir/ritonavir/ombitasvir/dasabuvir in 12.2% (n= 20), and sofosbuvir/ledipasvir in 6.7% (n= 11). The overall SVR was 89.8% 95%CI (81.0-97.1), which was not statistically different between patients treated before 90.6%CI (83.9-94.1) or after transplantation 89.2%CI (80.4-94.9). While the patients were on the waiting list period, 13.4% (n= 66) of HCV patients received DAA treatment and 86.6% (n= 425) did not. The cumulative incidence of HCC progression was 24.2% (n= 222). Patients treated with DAAs before LT presented a similar cumulative incidence of tumor waitlist progression when compared with the HCV+untreated DAA group (26.2%vs.26.9%P= 0.47); both had a similar HCC drop-out rate [12.1%CI (0.4-8.1)vs.12.9 %CI (3.8-27.2)]. A non-significant but numerically higher proportion of patients with pre-LT DAAs presented with extrahepatic progression or vascular invasion when compared to those without DAAs (4/66, 6.1%vs.17/425, 4%;P= 0.12). A lower incidence of post-transplant HCC recurrence among HCV+patients treated with pre- or post-LT DAAs was observed [0.7%CI (0.2-4.9)]. Although some patients with DAA treatment developed HCC, DAA treatment was neither associated with increased HCC recurrence after LT nor with waitlist tumor progression[108].
A prospective, multicenter cohort study was performed in 23 hospitals (from Argentina, Brazil, Chile, Colombia and Uruguay) from Latin America with 1760 patients treated with DAAs, in order to evaluate disease progression during a median follow-up of 26.2 months. Results showed an overall, cumulative incidence of disease progression of 8.3 in non SVRvs.3.9 after SVR achievement. Disease progression was seen with the development of liver fibrosis (HR = 3.4; 95%CI: 1.2-9.6), clinically significant portal hypertension (HR = 2.1; 95%CI: 1.2-3.8) andde novoHCC (HR = 0.2; 95%CI: 0.1-0.8) in the overall cohort. SVR was associated with an 80% reduction in disease progression when compared with DAA failure, which supports significant reduction in the risk of new liver-related complications[109]after treatment of HCV infection with DAAs.
CONCLUSION
HCC is the second leading cause of cancer related-deaths worldwide according to the WHO in 2015. This global health problem has caused the death of about 1.34 million people. Governments worldwide have implemented strategies to reduce this mortality but critical issues such as early diagnosis and appropriate treatment in at risk populations remain to be addressed. In Latin America, it is imperative that clearer strategies to understand the extent of the problem in this region be implemented. One possible strategy could be conducting annual epidemiological studies to identify high-risk populations and the main etiological causes. The updated data might then provide health authorities with more effective preventive approaches and enable implementation of the most effective treatments on time.
DECLARATIONS
Authors’ contributions
Contributed to the planning, bibliographic revision and writing of the manuscript: Galicia-Moreno M
Contributed to bibliographic revision, figure design, and manuscript writing: Campos-Valdez M, Sanchez-Meza J
Responsible for the planning of, conducting the study and figure design: Monroy-Ramirez HC
Responsible for manuscript revision: Sanchez-Orozco L
Responsible for manuscript planning and revision: Armendariz-Borunda J
Approved the final manuscript: Galicia-Moreno M, Monroy-Ramirez HC, Campos-Valdez M, Sanchez-Meza J, Sanchez-Orozco L, Armendariz-Borunda J
Availability of data and materials
Not applicable.
Financial support and sponsorship
Campos-Valdes M and Sanchez-Meza J are members of the CONACYT Doctoral Fellowship Program. Armendariz-Borunda J is recipient of a CONACYT grant 259096.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
- Hepatoma Research的其它文章
- Recent advances regarding tumor microenvironment and immunotherapy in hepatocellular carcinoma
- The advancement of immunotherapy in hepatocellular carcinoma
- Mechanisms and immunotherapies of HBV- and NAFLD-related hepatocellular carcinoma
- Direct-acting antivirals and risk of hepatocellular carcinoma: from genetic signature to metabolic risk factors
- Treatment options for recurrence of hepatocellular carcinoma after surgical resection: review of the literature and current recommendations for management

