Role of exosome-associated adenosine in promoting angiogenesis
Nils Ludwig,Edwin K.Jackson,Theresa L.Whiteside,4
1Department of Pathology,University of Pittsburgh School of Medicine,Pittsburgh,PA 15213,USA.
2UPMC Hillman Cancer Center,Pittsburgh,PA 15213,USA.
3Department of Pharmacology and Chemical Biology,University of Pittsburgh School of Medicine,Pittsburgh,PA 15213,USA.
4Departments of Immunology and Otolaryngology,University of Pittsburgh School of Medicine,Pittsburgh,PA 15213,USA.
Abstract
Keywords:Exosomes,extracellular vesicles,angiogenesis,adenosine,adenosine receptors,endothelial cells
INTRODUCTION
Extracellular vesicles (EVs) and their functional role in health and disease are of great current interest.Especially exosomes,a virus-size subset of EVs (~30-100 nm),show great potential as disease biomarkers,drug carriers,or therapeutics.They are actively produced by parent cells and carry a complex cargo,which includes proteins,nucleic acids,and lipids[1].
ROLES OF EXOSOMES IN ANGIOGENESIS
One hallmark of exosomes is the promotion of angiogenesis.Skoget al.[2]reported in 2008 that glioblastoma-derived exosomes contain mRNA,microRNA (miRNA),and angiogenic proteins and that these exosomes reprogram endothelial cells (ECs) to an angiogenic phenotype.A variety of research groups extended the work of Skoget al.[2]addressing the pro-angiogenic functions of exosomes in different health and disease settings.Multiple pathways which are used by exosomes to stimulate blood vessel formation were uncovered in recent years and most research focuses on the direct interaction of exosomes with ECs[3].It was shown that exosomes can deliver signals to receptors on ECs,which activate the relevant molecular pathways and contribute to altered cellular responses[3].The reported ligands on the surface of exosomes which can induce an angiogenic response include vascular endothelial growth factor (VEGF),interleukin 6 (IL-6),IL-8,fibroblast growth factors (FGF),and urokinase-type plasminogen activator (uPA)[2,4].Besides surface-mediated receptor-ligand interactions,ECs internalize exosomes within 2-4 h[4].Numerous pathways are utilized by ECs for the internalization of exosomes,such as phagocytosis,micropinocytosis,or lipid raft-mediated internalization[5].However,the main uptake mechanism is endocytosis,as recently reported[4].The internalization of exosomes allows for the delivery of messages that are then translated by ECs[2].miRNAs are frequently described components of the exosome cargo which can induce proangiogenic responses in ECs[6].
ADENOSINE PATHWAY AND ANGIOGENESIS
In addition to these well-described pathways,signaling via purines may also contribute to exosome-mediated effects on angiogenesis.One pathway that has not been studied thus far and that is particularly interesting in the context of exosomes and angiogenesis is the adenosine pathway.Extracellular adenosine exhibits a broad range of effects on cell cycle control,immunoregulation,and cytokine regulation through both direct and indirect mechanisms and ultimately leads to the progression of malignant diseases[7].Additionally,adenosine has been recognized as a potent stimulator of angiogenesis and Adair estimated that adenosine can contribute up to 50%-70% of the angiogenic response in some situations[8,9].Adairet al.[10]also described that intravenous infusion of adenosine can increase plasma levels of VEGF in humans.In cultured cells,it was shown that adenosine induces EC proliferation and migration by increasing levels of VEGF and other angiogenic growth factors[9].Additionally,adenosine can stimulate EC proliferation independently of VEGF,which probably involves modulation of other pro-angiogenic and anti-angiogenic growth factors and perhaps an intracellular mechanism[9].
Initiation of the adenosine signaling cascade requires binding of adenosine to the specific adenosine receptors (ADORs),which are divided into four subtypes:A1R,A2AR,A2BR and A3R.The main difference between these receptors is the affinity for adenosine,since adenosine binds to A1R,A2AR and A3R in the nanomolar range,whereas adenosine binds to A2BR in the micromolar range[11].This indicates that physiologic concentrations are sufficient to induce A1R-,A2AR- and A3R-mediated signaling,and elevated adenosine concentrations,which are usually found in inflammatory or tumor microenvironments,can activate an A2BRsignaling cascade[11].It was shown that adenosine induces EC growth by activating A2BR and that A2BR plays a critical role in regulating vascular remodeling associated with EC proliferation in angiogenesis,collateral vessel development,and recovery after vascular injury[12,13].However,the other receptor subtypes have also been reported to be involved in angiogenesis.Although there is no direct stimulation of ECs,ADORs act in a functional cooperative fashion to promote angiogenesis by a paracrine mechanism involving the differential expression and secretion of angiogenic factors from other cell types[14,15].Ernenset al.[16]reported that adenosine upregulates thrombospondin-1 production by macrophages via A2AR and A2BR,resulting in stimulation of angiogenesis.Adenosine also stimulates the production of VEGF,IL-8,and angiopoetin-1 from mast cells via A2BR and A3R,as reported by Feoktistovet al.[15].Clarket al.[17]demonstrated that A1R activation elicits an angiogenic response and promotes VEGF-release from cultured monocytes.Thus,the literature suggests that all ADORs are involved in regulating blood vessel development,and that the underlying pathway for stimulating angiogenesis is highly context/microenvironment-dependent.
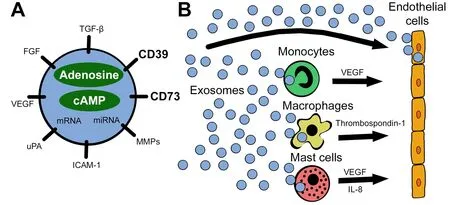
Figure1.A schematic visualizing the reprogramming of endothelial cells by exosomes.A:exosomes carry a variety of pro-angiogenic factors including the ectonucleotidases CD39 and CD73.Besides surface-bound molecules,exosomes encapsulate pro-angiogenic factors,nucleic acids,adenosine,and cAMP,as well as other purine metabolites;B:tumor-derived exosomes interact directly with endothelial cells or reprogram other cells in the tumor microenvironment to release pro-angiogenic factors.All these interactions involve signaling via adenosine receptors expressed on responder cells:specifically,A2BR on endothelial cells,A1R on monocytes,A2AR and A2BR on macrophages,and A2BR and A3R on mast cells.miRNA:microRNA;VEGF:vascular endothelial growth factor;FGF:fibroblast growth factor;TGF-b:transforming growth factor beta;MMPs:matrix metalloproteinases;ICAM-1:intercellular adhesion molecule 1;uPA:urokinase-type plasminogen activator;IL-8:interleukin 8;cAMP:cyclic adenosine monophosphate
ADENOSINE-MEDIATED STIMULATION OF ANGIOGENESIS BY EXOSOMES
Taken together,the above findings suggest the presence of a possible link between angiogenesis and exosome-associated adenosine,as presented in Figure1.Specifically,it was shown that exosomes contribute to extracellular adenosine production and hence might modulate ECs indirectly[18].Exosomes from diverse cancer cell types exhibit potent ATP- and 5’-AMP-phosphohydrolytic activity,partly attributed to activity of CD39 and CD73,respectively,on the surface of exosomes[19].This exosome-generated adenosine is functionally active and can trigger a cyclic adenosine monophosphate (cAMP) response in A2AR-positive but not A2AR-negative cells[19].
While it is well recognized that exosomes encapsulate functional proteins and nucleic acids,it is currently unclear whether purine metabolites are encapsulated within exosomes.Sayneret al.[20]recently reported that EVs encapsulate cAMP to provide an additional second messenger compartment.Our preliminary data show that exosomes not only encapsulate cAMP but also adenosine and adenosine metabolites (inosine,hypoxanthine,and xanthine).This may indicate that exosomes can induce ADOR signaling independently of the production of adenosine by exosome-associated enzymes.Adenosine in the lumen of exosomes is protected against uptake and metabolism by other cells,such as red blood cells.Thus,exosomal adenosine may represent a mechanism for adenosine to serve as a circulating,rather than strictly local,factor.
Exosome-associated adenosine emerges as a potential stimulator of angiogenesis in different settings.Studying this pathway promises to uncover important aspects of exosome functions,which are ultimately leading to the stimulation of blood vessel formation.Understanding this pathway might also help to find targets for the stimulation or inhibition of exosome-induced angiogenesis.
DECLARATIONS
Authors’ contributions
Writing of the manuscript:Ludwig N
Supervision and editing of the manuscript:Jackson EK,Whiteside TL
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by National Institutes of Health grants HL109002,DK091190,HL069846,DK068575,and DK079307 to EKJ and the NIH grant R01-CA 1686288 to TLW.NL was supported by the Leopoldina Fellowships LPDS 2017-12 and LPDR 2019-02 from German National Academy of Sciences Leopoldina.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.

