Comprehending the therapeutic effects of stereotactic body radiation therapy for small hepatocellular carcinomas based on imagings
Zhao-Chong Zeng, Jia Fan, Jian Zhou, Meng-Su Zeng, Yi-Xing Chen, Zhi-Feng Wu, Jing Sun, Jian-Ying Zhang, Yong Hu, Qian-Qian Zhao
1Department of Radiation Oncology, Zhongshan Hospital, Fudan University, Shanghai 200032, China.
2Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai 200032, China.
3Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai 200032, China.
Abstract Surgical resection or radiofrequency ablation (RFA) is considered first-choice treatment for small hepatocellular carcinomas (HCCs). When a patient has a small HCC that is inoperable or unsuitable for RFA, what are alternative treatments? Some oncologists recommend transarterial chemoembolization (TACE), chemotherapy, moleculartargeted therapy, or immunotherapy. However, these treatments have minimally beneficial effects in small HCCs.Stereotactic body radiation therapy (SBRT) is a liver-directed radical therapy for small HCCs, with treatment outcomes similar to those for surgical resection or RFA, but many oncologists do not comprehend its efficacy or accept this therapy. We herein discuss 11 typical patients who received SBRT for various indications: refusal to undergo resection or RFA; surgical resection or RFA considered difficult or unfeasible; residual cancer after surgical resection or RFA or incomplete iodized oil retention after TACE; or tumor recurrence after resection or RFA. We describe each case, including the radiation field, tumor radiation dose, and response to SBRT in both the tumor and liver parenchyma. These clinical data should help readers understand this new therapeutic technique.We also conducted a literature review and found evidence to support survival benefit with SBRT, including good three- and five-year overall survival rates. The purpose of this article is to encourage readers to accept the concept that SBRT is a low-toxicity and effective therapeutic option for patients with small HCCs, which offers substantial local control and improved overall survival, especially for patients with a tumor that is unresectable or unsuitable for RFA, residual tumor after local therapy, or intrahepatic recurrent tumor.
Keywords: Small hepatocellular carcinomas, stereotactic body radiation therapy, treatment outcomes, toxicity,imaging changes
INTRODUCTION
Recent technological advances offer precise and safe radiation delivery to tumors in various areas of the body through imaging guidance. External beam radiation therapy (EBRT) has been recommended as a therapeutic option for HCCs that are considered unresectable according to National Comprehensive Cancer Network guidelines because of the tumor’s location, inadequate hepatic reserve, or the presence of comorbidities. SBRT is a type of EBRT technique requiring special equipment for patient positioning and the delivery of high-dose radiation to tumors. There is growing evidence supporting the usefulness of SBRT for patients considered unsuitable for hepatectomy or RFA. However, most oncologists or hepatologists do not understand this treatment technique because of limited use of SBRT in their clinical practice. Herein,we discuss 11 typical patients with HCC to clarify the indications, therapeutic outcomes, treatment-related toxicities, and doses of SBRT, as well as the expected post-SBRT imaging features.
DEFINITIONS
SBRT is an advanced technique of hypofractionated EBRT with photons, which delivers large ablative doses of radiation to tumors. This complex technique relies on the following: (1) stringent control of breathing motion for liver cancer, using four-dimensional computed tomography scans to track respiration-induced hepatic movement; (2) extremely precise patient positioning; and (3) image guidance for radiation delivery.Early-stage HCC is defined as a solitary tumor with a maximum diameter ≤ 5 cm or multiple nodules(≤ 3 total), each with a maximum diameter ≤ 3 cm, without vascular invasion or extrahepatic metastasis and accompanied by Child-Pugh Class A or B hepatic function (Cases 1-3 and 5). Not all small tumors are classified as early-stage because some have decreased in size after treatment (Case 8). If intrahepatic recurrence (Cases 9 and 10) or Child-Pugh Class C (Case 11) function is present, HCC is considered later stage.
CLINICAL EFFECTIVENESS OF SBRT FOR HCC
There is a growing body of evidence indicating the usefulness of SBRT for the management of patients with HCC. We conducted a literature review and identified several retrospective studies involving the use of SBRT for HCC. These studies have primarily included patients in whom surgical resection or RFA was difficult,unfeasible, or rejected, as well as some patients with intermediate- or advanced-stage HCC. The results of these studies are summarized in Table 1. This table is restricted to studies involving the use of ≤ 10 fractions of SBRT.
Overall survival
As shown in Table 1, overall survival (OS) rates have varied between studies. For early-stage HCC, 2-year OS rates of 78%-80%[1,2], 3-year OS rates of 66%-73%[2,3], and a 5-year OS rate of 64% after SBRT have been reported[3].
Local tumor control
As shown in Table 1, local tumor control rates at one and two years were approximately 95% in most studies,especially those reported in more recent years[1-13].
Bridging before liver transplantation
SBRT is suitable bridging therapy for patients with HCC awaiting liver transplantation. Sapisochinet al.[14]compared the efficacy of SBRT, TACE, and RFA as a bridge to transplantation in a large cohort of patients with HCC and concluded that SBRT can be a safe alternative to the other, more conventional bridging therapies. However, SBRT has been safer than RFA and TACE when ascites or poor coagulation function are present, as often occurs in patients with underlying liver disease.

Table 1. Outcomes of small-sized liver cancers after SBRT
RADIATION DOSE
The SBRT dose to HCC has been significantly associated with OS (P= 0.005) in multivariate analysis. Highdose SBRT may increase local control and improve OS in patients with inoperable HCC[11]. In most studies described in the literature, a biologically-equivalent dose (BED) 10 of > 80 Gy has been delivered to the tumors [Table 1]. Of the 11 typical patients discussed in this manuscript, all received BED10 of ≥ 78 Gy and are alive and well. Although no evidence has emerged to clearly support a minimum or maximum dose of SBRT for HCC, we recommend BED10 of ≥ 80 Gy.
INDICATIONS FOR SBRT
SBRT may be an effective therapeutic option for early-stage HCC (as defined above). Patients with earlystage HCC usually undergo surgical resection or RFA attempts, unless contraindicated. However, SBRT can be a useful alternative treatment in patients who refuse surgery or RFA. Case 1 [Figure 1] is a typical example of this.

Figure 1. SBRT for HCC in a 59-year-old man who refused surgical resection and RFA. A: Axial arterial phase MRI image showed hyperenhancement of a 1.8-cm liver nodule in the right lobe of the liver; B: the patient received SBRT with a dose of 50 Gy in five fractions; C:axial arterial phase MRI image 2 months after SBRT revealed a stable-size, enhancing lesion (arrowhead). The normal liver parenchyma received 25 Gy and appeared as a low-density area, indicating acute radiation injury; D: axial arterial phase MRI image 4 months after SBRT showed decreased size of the tumor, with atrophy of the perilesional hepatic parenchyma; E: axial arterial phase MRI image 6 months after SBRT showed necrotic changes and a hypovascular target lesion. The perilesional hepatic parenchyma, which received high-dose radiation, also exhibited necrotic changes; F: axial arterial phase MRI image nine months after SBRT demonstrated complete regression of the treated lesion and atrophy of the irradiated hepatic parenchyma; G: axial arterial phase CT image 18 months after SBRT showed a scar in the radiation field. Complete repair of the radiation damage was also observed; H,I: axial arterial phase MRI images 6 years (H) and 7 years (I) after SBRT demonstrated a stable lesion size but the presence of complete necrosis and ring enhancement,indicating a complete radiologic response. Note the SBRT-related changes, which can be differentiated from tumor recurrence. SBRT:stereotactic body radiation therapy; HCC: hepatocellular carcinoma; RFA: radiofrequency ablation; MRI: magnetic resonance imaging; CT:computed tomography
Currently, SBRT is most frequently used when surgical resection or RFA would be difficult or unfeasible,as with tumors located at the center of liver, in the hepatic hilar region, or close to large blood vessels (as in Cases 2 and 3, which are shown in Figures 2 and 3). It is also commonly used in patients who are older [Case 4; Figure 4] or have significant comorbidities, such as poor liver function [Case 5; Figure 5].
SBRT may also be used for residual cancer after surgical resection, as in Case 6 [Figure 6], or after RFA, as in Case 7 [Figure 7]. In these situations, the tumor is often expected to be difficult to treat with surgery or RFA,but these treatments are tried initially. When residual tumor is found during follow-up, SBRT will generally be the most appropriate treatment.
SBRT may serve as adjuvant treatment for intrahepatic tumors with incomplete iodized oil retention, as in Case 8 [Figure 8]. TACE can be used to reduce the size of HCCs; however, it is often considered palliative therapy because of incomplete iodized oil retention by intrahepatic tumors. Adjuvant SBRT can function as consolidation treatment, converting palliative therapy to potentially curative therapy.
SBRT has also been used as salvage treatment for intrahepatic tumor recurrence after RFA or surgical resection [Cases 9 and 10; Figures 9 and 10]. Although metachronous intrahepatic recurrence is sometimes difficult to distinguish from de novo HCC, a course of SBRT can be considered. In addition, SBRT can be a suitable bridging therapy for patients with HCC awaiting liver transplantation [Case 11; Figure 11].
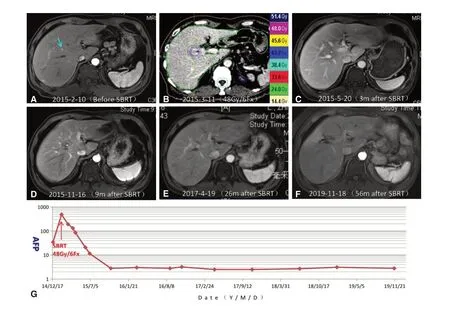
Figure 2. SBRT for very early-stage HCC in a 52-year-old man. Both surgical resection and RFA were considered difficult because the tumor was located in the center of the liver and was too small to be easily detected. A: Axial arterial phase MRI image showed hyper enhancement of an 0.8-cm liver nodule (arrowhead) located in the center of the right lobe of the liver. The patient was clinically diagnosed with very early-stage HCC based on the Barcelona Clinic Liver Cancer staging system; B: the patient received SBRT with a dose of 48 Gy in six fractions; C: axial arterial phase MRI image three months after SBRT demonstrated complete tumor response.Hypodensity in the radiation field (about 30 Gy) indicated the presence of radiation-induced focal liver injury; D: axial arterial phase MRI image 9 months after SBRT showed clear reduction in size of the area of radiation injury; E,F: axial arterial phase MRI images 26 months(E) and 56 months (F) after SBRT revealed complete regression of the tumor lesion; G: serum AFP levels are shown in relation to the treatment timeline. The elevated serum AFP level prior to SBRT dramatically declined to normal (< 20 µg/L) after SBRT, and remained within normal limits thereafter. SBRT: stereotactic body radiation therapy; HCC: hepatocellular carcinoma; RFA: radiofrequency ablation;MRI: magnetic resonance imaging; AFP: alpha-fetoprotein
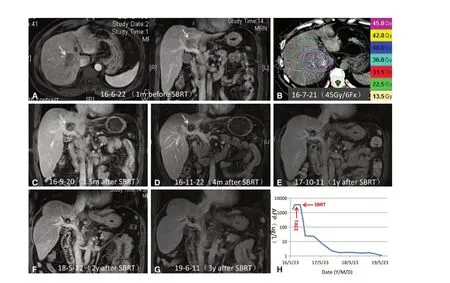
Figure 3. SBRT for unresectable HCC in a 47-year-old man. A: Axial and sagittal MRI images showed a hepatic lesion (arrowhead) near the inferior vena cava and main portal vein. The lesion enhanced in the arterial phase and washed out in the portal venous phase; B:the patient underwent SBRT with a dose of 45 Gy in six fractions; C: arterial phase MRI image 1.5 months after SBRT revealed dramatic regression of the lesion; D: arterial phase MRI image four months after SBRT demonstrated further reduction in size of the lesion, as well as necrosis of the targeted hypovascular lesion, consistent with a nonviable tumor; E-G: MRI images 1 year (E), 2 years (F), and 3 years (G)after SBRT showed progressive reduction in tumor size and complete hypovascularity of the lesion. These findings suggested a good tumor response; H: serum AFP levels are shown in relation to the treatment timeline. The elevated serum AFP level prior to SBRT (1,709 µg/L)declined to normal (< 20 µg/L) after SBRT and remained within normal limits thereafter. SBRT: stereotactic body radiation therapy; HCC:hepatocellular carcinoma; MRI: magnetic resonance imaging; AFP: alpha-fetoprotein

Figure 4. SBRT for HCC in a 99-year-old man for whom SBRT was chosen because of his age and comorbidities. A: Axial venous phase MRI image showed a 6-cm low-density lesion adjacent to the diaphragm (arrow); B: the patient underwent SBRT with a dose of 54 Gy in six fractions; C: axial arterial phase MRI image three months after SBRT demonstrated reduced size of the intrahepatic lesion and vascular enhancement (arrow); D,E: axial arterial phase MRI images 10 months (D) and 17 months (E) after SBRT showed further reduction in size of the tumor, which was hypovascular (arrows). The perilesional liver parenchyma in the enhanced phase appeared as ill-defined areas of enhancement, favoring SBRT-related changes; F: Serum AFP levels are shown in relation to the treatment timeline. AFP levels decreased to normal after SBRT. SBRT: stereotactic body radiation therapy; HCC: hepatocellular carcinoma; MRI: magnetic resonance imaging; AFP:alpha-fetoprotein

Figure 5. SBRT for HCC consisting of an extensive fat-tissue component in a 41-year-old man with Child-Pugh Class C liver function.A: Axial arterial phase CT image showed a 2-cm nodule containing fat attenuation (arrow). Mild ascites and splenomegaly were also evident; B: multiphasic CT images six months after liver protecting treatment demonstrated enlargement of the lesion to 3 cm (arrow),with enhancement in the arterial phase and washout in the portal venous phase. The patient was diagnosed with HCC with an extensive fat-tissue component. The ascites had disappeared, but splenomegaly was still present; C: the patient underwent SBRT with a dose of 54 Gy in six fractions; D: axial arterial phase CT image two months after SBRT showed the lesion (arrow) with a stable size and partial devascularization; E-G: axial arterial phase CT images 6 months (E), 9 months (F), and 13 months (G) after SBRT demonstrated progressive reduction in tumor size and devascularization of the lesion, consistent with a partial response based on mRECIST criteria; H:axial arterial phase CT image 25 months after SBRT showed complete response of the treated lesion, with marked focal atrophy of the irradiated hepatic parenchyma. SBRT: stereotactic body radiation therapy; HCC: hepatocellular carcinoma; CT: computed tomography;mRECIST: modified Response Evaluation Criteria In Solid Tumor
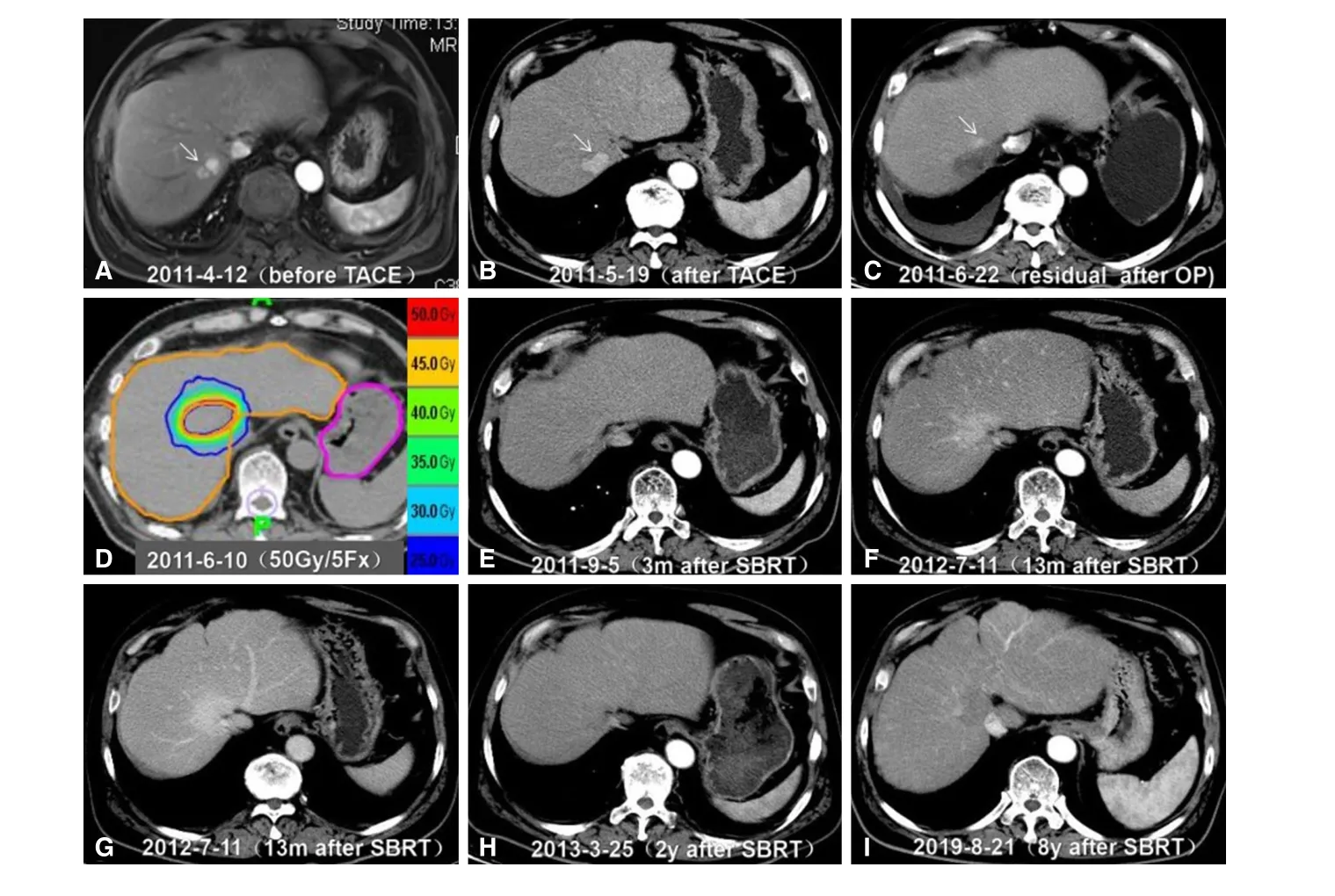
Figure 6. SBRT for residual HCC after surgical resection in a 59-year-old man. A: Axial arterial phase MRI scan 6 years after initial surgery showed a 2.1-cm recurrent hyper-enhancing nodule adjacent to the inferior vein cava, located in the second hepatic hilar segment. The patient underwent TACE for the recurrent lesion; B: axial arterial phase CT image one month after TACE showed a hyper-enhancing lesion (arrow), with no lipiodol deposition. Then, the patient underwent hepatic wedge resection in May 2011; C: axial arterial phase CT image one month after surgery revealed residual tumor with hyper enhancement (arrow); D: the patient underwent SBRT with a dose of 50 Gy in five fractions in June 2011; E: axial arterial phase CT image 3 months after SBRT showed complete response of the target lesion;F,G: Axial arterial (F) and portal venous phases (G) CT images 13 months after SBRT showed persistent enhancement of the radiation field because of post-SBRT changes representing congestion and edema; H,I: Follow-up CT images 2 years (H) and 8 years (I) after SBRT demonstrated a normal liver. SBRT: stereotactic body radiation therapy; HCC: hepatocellular carcinoma; MRI: magnetic resonance imaging; TACE: transarterial chemoembolization; CT: computed tomography
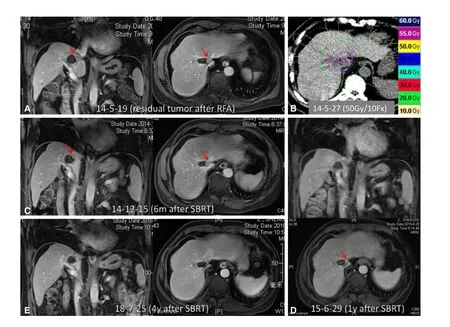
Figure 7. SBRT for residual tumor after RFA in a 66-year-old man. A: Axial and sagittal MRI images approximately 1.5 months after RFA showed a hepatic lesion with a residual cavity and viable tumor (arrowhead) close to the inferior vena cava; B: the patient underwent SBRT with a dose of 50 Gy in 10 fractions for the residual tumor; C-E: MRI images obtained 6 months (C), 1 year (D), and 4 years(E) after SBRT illustrate gradual shrinkage and eventual disappearance of the tumor. SBRT: stereotactic body radiation therapy; RFA:radiofrequency ablation; MRI: magnetic resonance imaging
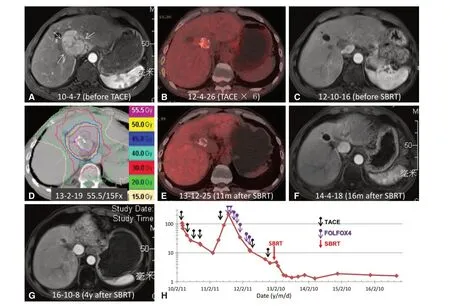
Figure 8. SBRT for residual tumor with incomplete iodized oil retention in a 54-year-old man who had undergone multiple cycles of TACE and chemotherapy. A: Original axial arterial phase MRI image showed a 7.5-cm enhancing mass in the hepatic hilar region, consistent with HCC; B: the lesion decreased substantially in size after six cycles of TACE. FDG PET/CT image demonstrated hypermetabolic activity in the region with incomplete iodized oil retention, consistent with residual viable tumor; C: arterial phase MRI image after multiple TACE cycles and chemotherapy but before SBRT showed no enhancement of the 1.8-cm liver lesion; D: the patient underwent SBRT for residual tumor with a dose of 55.5 Gy in 15 fractions; E: FDG PET/CT image 11 months after SBRT showed no metabolic activity; F: axial arterial phase MRI image 16 months after SBRT demonstrated reduced size and hypovascularity of the treated lesion; G: axial arterial phase MRI image 4 years after SBRT showed stable size and hypovascularity of the treated lesion; H: serum AFP levels are shown in relation to the treatment timeline. AFP further declined to normal after SBRT. SBRT: stereotactic body radiation therapy; HCC: hepatocellular carcinoma;MRI: magnetic resonance imaging; TACE: transarterial chemoembolization; AFP: alpha-fetoprotein; FDG: flurodeoxyglucose; PET: positron emission tomography; CT: computed tomography

Figure 9. SBRT for recurrent HCC at the surgical margin in a 63-year-old man. A: Axial arterial phase MRI image showed hyper enhancement of a 2.4 cm recurrent focus at the surgical margin (arrowhead). The patient received TACE for this recurrent lesion; B: axial arterial phase MRI image six months after TACE showed reduced size and hypovascularity of the lesion; C: axial arterial phase CT image 3 years after TACE demonstrated enlargement of the treated lesion to 2.9 cm; D: the patient received SBRT with a dose of 48 Gy in six fractions; E-G: CT images 40 days (E), 2 years (F), and 4 years (G) after SBRT showed complete tumor response; H: serum AFP levels are shown in relation to the treatment timeline. The elevated serum AFP prior to SBRT (53 µg/L) declined to normal (2.1 µg/L) after SBRT and remained within normal limits thereafter. SBRT: stereotactic body radiation therapy; HCC: hepatocellular carcinoma; MRI: magnetic resonance imaging; TACE: transarterial chemoembolization; CT: computed tomography; AFP: alpha-fetoprotein
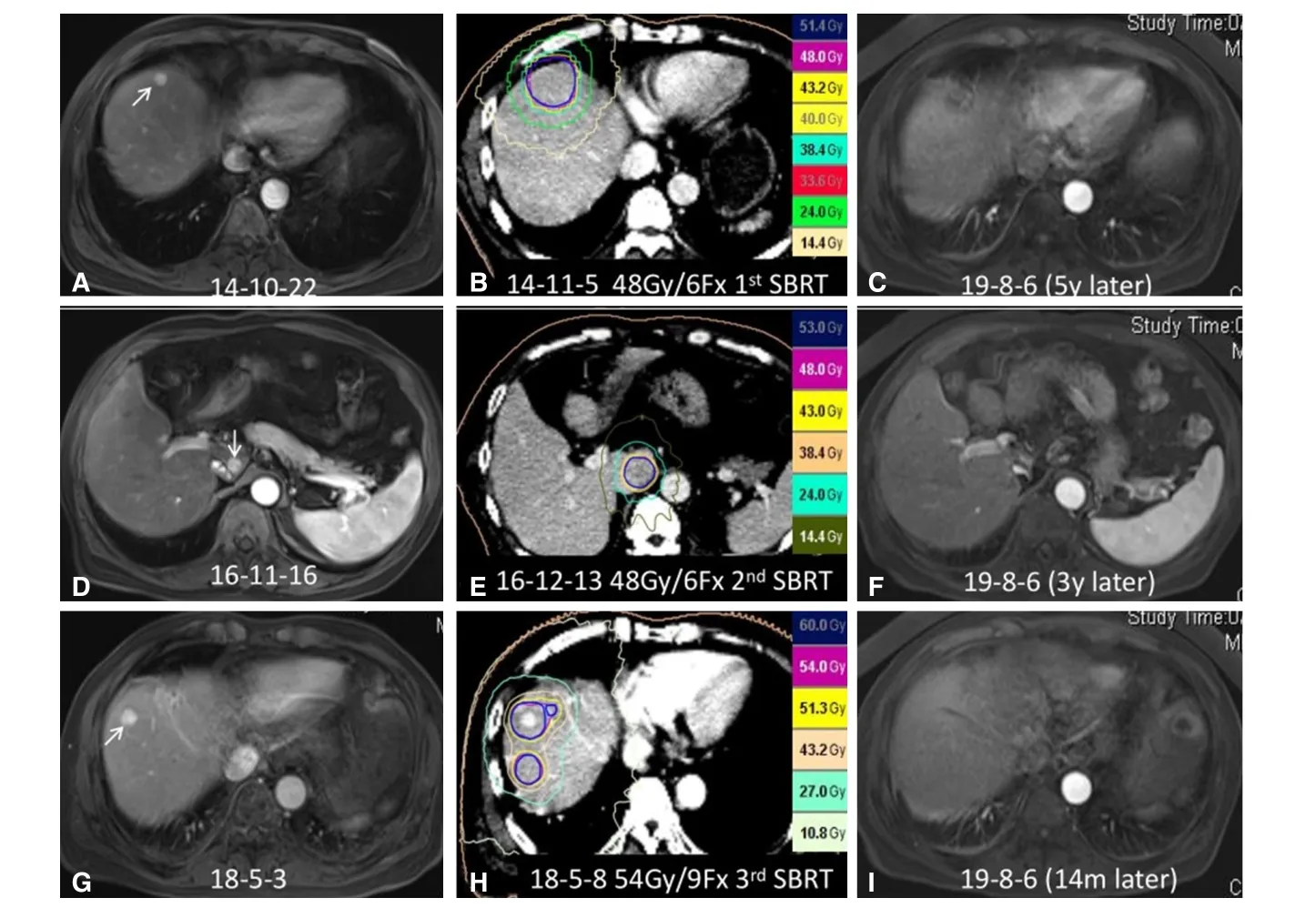
Figure 10. SBRT for repeated de novo HCC in a 63-year-old man with previous surgical resection of HCC. A: Axial arterial phase MRI image showed a 1.2-cm enhancing nodule in the right hepatic lobe, adjacent to the diaphragm; B: the patient received his first course of SBRT with a dose of 48 Gy in six fractions; C: axial arterial phase MRI image 4 years after SBRT demonstrated complete response of this right lobe lesion; D: axial arterial phase MRI image two years after the first SBRT showed a 1-cm enhancing nodule in the caudate lobe of the liver; E: the patient received a second course of SBRT with a dose of 48 Gy in six fractions; F: axial arterial phase MRI image 3 years after the second course of SBRT revealed complete response of the caudate lobe lesion; G: axial arterial phase MRI image 18 months after the second SBRT showed a 1.2-cm enhancing lesion in the right hepatic lobe, as well as an additional lesion at a lower level (not shown),suggesting de novo HCC; H: the patient received a third course of SBRT with a dose of 54 Gy in nine fractions. I: axial arterial phase MRI image one year after this SBRT revealed complete tumor response of the de novo lesion. SBRT: stereotactic body radiation therapy; HCC:hepatocellular carcinoma; MRI: magnetic resonance imaging
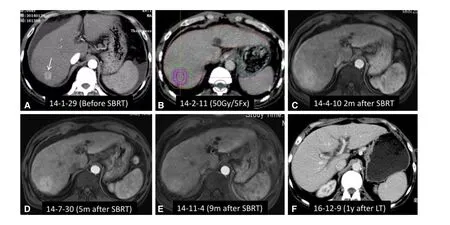
Figure 11. SBRT as a bridge to liver transplantation for HCC in a 55-year-old man with alcohol-related cirrhosis and Child-Pugh Class C liver function. A: Axial arterial phase CT image showed hyper enhancement of a 2 cm mass in the right hepatic lobe (arrowhead),accompanied by moderate ascites. Liver function was scored as Child-Pugh Class C; B: the patient underwent SBRT with a dose of 50 Gy in five fractions; C-E: Axial arterial phase MRI images 1.5 months (C), 5 months (D), and 9 months (E) after SBRT showed complete tumor response. However, focal reaction with enhancement indicated congestion of the hepatic parenchyma in the radiation field. The patient received a liver transplant 22 months after SBRT; F: axial arterial phase CT image one year after liver transplantation demonstrated a normal liver. SBRT: stereotactic body radiation therapy; HCC: hepatocellular carcinoma; MRI: magnetic resonance imaging; CT: computed tomography
CONTRAINDICATIONS FOR SBRT IN SMALL HCC
If tumors and luminal structures (esophagus, stomach, duodenum, or intestine) are closely situated at < 1 cm,SBRT is relatively contraindicated for this patient. However, hypofraction imaging-guided radiation therapy could be recommended when HCC is inoperable or unsuitable for RFA.
At least 700 mL of normal liver (Child-Pugh Class A) must receive < 15 Gy. If this condition is not met, we must be careful in choosing such patients.
The safety of liver radiation for HCC in patients with Child-Pugh Class C cirrhosis has not been established,but SBRT could be used as bridging therapy for patients with HCC awaiting liver transplantation.
RESPONSE TO SBRT
HCC
Tumor response rates increase over time after SBRT. Sanukiet al.[15]reported that HCC complete response increased from 24% at three months after SBRT to 67%, 71%, and 93% at 6, 12, and 24 months, respectively,after SBRT[15]. Using modified Response Evaluation Criteria In Solid Tumor (mRECIST) criteria, complete response occurred within three months after completing SBRT in Cases 9 and 11 and > 9 months after SBRT in Cases 1, 5, and 7. Of note, we prefer to evaluate tumor response to SBRT using European Association for the Study of the Liver (EASL) or mRECIST criteria rather than RECIST criteria[16]. Case 7, for example,exhibited a complete response according to EASL or mRECIST criteria in the fourth year after completion of SBRT, but only a stable disease and partial response using RECIST criteria, at six months and one year after completing SBRT, respectively. Similarly, Case 5 had a partial response using EASL or mRECIST criteria, but stable disease using RECIST criteria, at six months after completing SBRT.
Liver parenchymal reactions
Early focal liver reaction refers to a surrounding low-intensity area observed on both computed tomography(CT) and magnetic resonance imaging (MRI) scans within six months after completing SBRT. This focal reaction is more visible in patients who undergo initial therapy with SBRT, as shown in Cases 1, 2, and 5.
Delayed focal liver reactions are classified as areas of hyperdensity, isodensity, and hypodensity in all enhanced phases on follow-up MRI or CT > 6 months after SBRT completion[17,18]. Features of hyperdensity were found in Cases 6 [Figure 6F and G] and 11 [Figure 11D]; features of isodensity were found in Cases 3[Figure 3E], 7 [Figure 7C and D], and 11 [Figure 11E]; and features of hypodensity were found in Cases 1[Figure 1E], 2 [Figure 2D], and 5 [Figure 5E].
The incidence of hyperdensity reactions in irradiated hepatic parenchyma may gradually increase after 6 months post-SBRT completion, potentially interfering with accurate assessment of treatment response and being misinterpreted as recurrent tumor. Lack of washout in the delayed phase in hypervascular areas helps distinguish SBRT-related changes from residual or recurrent HCC[19]. Hyperdensity will typically disappear 2-3 years after treatment, as shown in Case 6 [Figure 6H]. Hypodensity represents the presence of regional liver atrophy within 1-2 years, as shown in Cases 1 [Figure 1F], and 5 [Figure 5H].
The types of focal reaction did not appear to be related to liver function in our cases. The focal liver reaction threshold dose following SBRT for HCC is 30 Gy for livers with Child-Pugh Class A function and 25 Gy for livers with Child-Pugh Class B function, when delivered in five fractions[20,21]. The doses used in our cases[Figures 1C, 2C, and 5D] were consistent with these thresholds.
TOXICITY
Hepatic damage
A consensus article summarizing the results of 15 previously published studies, including 1063 patients with HCC undergoing SBRT, reported that only eight patients (0.8%) developed Grade 5 liver failure, and most fatalities from liver failure occurred in patients with Child-Pugh Class B liver function[22]. However, we have observed no fatal radiation-induced liver disease in our clinical practice.
The safety of SBRT for HCC in patients with Child-Pugh Class C liver function has not been established.Class C function is generally considered a contraindication to all HCC treatment except liver transplantation.No deterioration in liver function occurred after SBRT in Cases 5 and 11, who had Child-Pugh Class C liver function. Furthermore, some patients undergo > 1 course of SBRT, as exemplified by Case 10, who received a total of three courses because of the development of de novo HCCs. Together, these observations suggest that SBRT produces little liver parenchymal damage.
Other toxicities
Gastrointestinal toxicity is another potential concern with SBRT, especially when luminal structures, such as the esophagus, stomach, duodenum, or intestines, are close to the tumors being treated. Grade 3 or higher gastrointestinal toxicities were reported in 1.4% of patients from previously published studies, but fatal gastrointestinal bleeding did not occur[22]. Other complications, such as rib fractures, chest or abdominal wall pain, biliary stricture, and musculoskeletal discomfort, have been noted occasionally. Overall, most toxicities from SBRT are generally infrequent and mild.
THE LIMITATION OF SBRT
Firstly, there is a lack of randomized clinical trials to compare the treatment outcome among SBRT, resection,and RFA. Thus, SBRT can only be used as an alternative treatment when operation and RFA are impossible.Secondly, vaguely defined SBRT has led to inconsistent radiation doses and fractions globally, with ≤ 5 fractions in the United States and ≤ 10 fractions in the rest of the world. A dose of 55.5 Gy in 15 fractions was delivered to Case 8, and complete response was achieved. This is not strictly part of SBRT; at this point, we are not interested in the definition of SBRT, as we care more about the local control and long-term survival.
CONCLUSION
SBRT is an effective therapeutic option based on proven studies for patients with small HCCs; is complementary to the existing treatment options, as illustrated by the typical cases; and is safe with minimal toxicities.
DECLARATIONS
Authors’ contributions
Made a substantial, direct and intellectual contribution to the work, and approved it for publication: Zeng ZC, Fan J, Zhou J, Zeng MS, Chen YX, Wu ZF, Sun J, Zhang JY, Hu Y, Zhao QQ
Availability of data and materials
Not applicable.
Financial support and sponsorship
The research was supported by a grant from the National Key R & D Program of China (2017YFC0112100).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
- Hepatoma Research的其它文章
- Risk factors of portal vein thrombosis after splenectomy in patients with liver cirrhosis
- Stereotactic body radiation therapy for the management of HCC
- Genetics of alcohol-related hepatocellular carcinoma - its role in risk prediction
- Imaging assessment after SBRT for hepatocellular carcinoma
- β-catenin in intranuclear inclusions of hepatocellular carcinoma

