Nivolumab-induced severe bullous pemphigoid in a patient with renal cancer: a case report and literature review
Xiaorong Wu, Sreekanth Palvai, Awais Jalil
Department of Medical Oncology, Southend University Hospital NHS Foundation Trust, Prittlewell Chase, Westcliff-on-Sea,Essex SS0 0RY, United Kingdom.
Abstract With the widespread use of immunotherapy in numerous solid tumours, immunotherapy-related adverse events(irAEs) have started to emerge and bring new challenges for clinicians to manage.Among established irAEs,dermatologic toxicity is one of the most common toxicities; it is often mild but can be severe and potentially life-threatening, such as bullous pemphigoid.Here, we report a case of nivolumab-mediated severe, extensive,refractory bullous pemphigoid involving both skin and oral mucosa in a patient with metastatic renal cancer.We also summarise a list of selected case reports of immunotherapy-induced bullous pemphigoid by literature review.We highlight various presentations, investigations and managements of this type of skin irAEs.Meantime, we would like to discuss the correlation of skin irAEs incidence rate with immunotherapy drug benefit and resistance.
Keywords: Bullous pemphigoid, mucous membrane pemphigoid, immunotherapy, dermatologic toxicity, nivolumab
INTRODUCTION
Immunotherapy, such as anti-programmeddeath-1 (PD-1)/programmed death ligand 1 (PD-L1) antibody or anti-CTLA 4 antibody or combination, has significantly improved survival of patients with various malignancies.However, immunotherapy-related adverse events (irAEs) have started to emerge and bring new challenges for oncologists to manage.Among the known irAEs, dermatologic toxicity is one of themost common toxicities[1].Most immunotherapy-induced cutaneous toxicities are mild, such as nonspecific rashes or pruritus.However, some manifestations of skin irAEs can progress to high-grade and potentially life-threatening situations, such as bullous pemphigoid (BP)[2].Since 2015, more than 40 cases of immunotherapy-induced BP have been reported with the majority involving either the skin or mucous membrane only.Presentations of both cutaneous and oral mucous BP are rare (less than seven cases).
Here, we report a severe, extensive (involving both skin and oral mucosa), refractory case of BP that occurred 9 months after initiation of nivolumab in a patient with metastatic renal clear cell cancer.We also summarise a list of selected case reports of checkpoint inhibitor-induced BP by literature review.We highlight various presentations, investigations and management approaches of immunotherapy-induced BP.Meantime, we would like to discuss the correlation of skin irAE incidence rate with immunotherapy drug benefit and resistance.
CASE REPORT
A 66-year-old gentleman with no known history of autoimmune disease was diagnosed with right clear renal cancer in 2014.He was initially treated with radical nephrectomy with excision of tumour thrombus in the renal vein and retro-hepatic inferior vena cava.In March 2017, his cancer relapsed with a single spinal metastasis, which was treated with excision of the intradural tumour and laminectomy and radiotherapy as well, which resulted in complete neurological recovery.Post-radiotherapy, he opted for active tyrosine kinase inhibitor, sunitinib, in light of relapsed renal cancer in his spine.In October 2017,sunitinib was discontinued due to spontaneous haematoma in his right calf and thigh, which was likely due to combination of sunitinib and low-molecular-weight heparin (LMWH) for his atrial fibrillation.He then had a long period of surveillance, during which he gradually developed asymptomatic relapsed metastatic renal cancer in his left adrenal gland, followed by relapsed cancer in his left kidney.In March 2019, he commenced immunotherapy infusion with 2 weekly nivolumab, considering further disease progression with multiple lung metastatic disease.His other medical history was only atrial fibrillation, for which he was on LMWH and bisoprolol.
From August 2019, five months post-initiation of nivolumab, he developed a mild pruritic erythematous skin rash scattered mainly over his chest and upper limbs.At that time, there were no blisters reported.He continued nivolumab with the rash controlled mainly by topical steroids or short courses of oral steroids.In November 2019, he developed large, tense, haemorrhagic blisters, which were exacerbated following a 3-week holiday in Australia, leading to an emergency hospital admission.On clinical examination, he had a severe widespread bullous eruption affecting his chest, abdomen, inner thighs and upper arms.Active blisters and erosions were seen on the roof of his mouth and scrotal skin as well.Some blisters had burst leaving severely eroded skin with signs of superimposed infection.Bullae at various stages were seen,including intact ones, burst ones with an eroded base, and a few areas of early re-epithelialisation as Figure 1.He had no involvement of the scalp region and external eye examination was normal.
An incisional skin biopsy from an active blister with haematoxylin and eosin examination revealed epidermal hyperplasia and subepidermal splitting.There were few eosinophils, scattered plasma cells and neutrophils with a subjacent chronic inflammation.Direct immunofluorescence (DIF) showed clearly positive staining for IgG in the basement membrane zone, and a faint positive IgA staining.Subsequent serum antibody analysis confirmed positive pemphigoid antibodies consistent with BP see Figures 2 and 3.
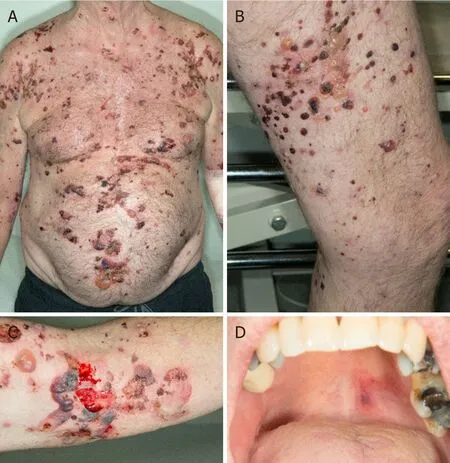
Figure 1.Clinical presentations.Multiple, grouped, large, tense haemorrhagic blisters were seen on the first clinical presentation, which affected the chest, abdomen, inner thighs and upper arms (A, B and C); active blisters and erosions were seen on the roof of the mouth(D)
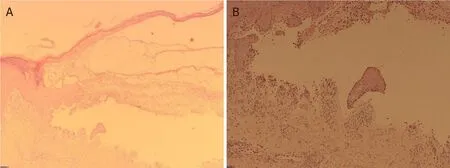
Figure 2.Haematoxylin and eosin staining of edge (A) and base (B) of blisters.Epidermal hyperplasia and subepidermal splitting were seen with few eosinophils, scattered plasma cells, neutrophils and a subjacent chronic inflammation
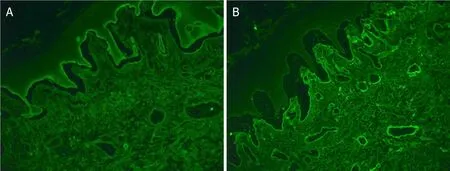
Figure 3.Direct immunofluorescence showed clearly positive staining for IgG in the basement membrane zone (A), and a faint positive IgA staining (B)
The patient was started immediately on intravenous methylprednisolone 1 mg/kg as well as flucloxacillin for 4 days and then switched to oral prednisolone and flucloxacillin when no new blisters developed.Most of the blisters gradually re-epithelialised within 4-6 weeks of oral steroids.Two months later, the corticosteroid treatment was tapered.However, the blisters reappeared when prednisolone was steppeddown to 10 mg per day, requiring re-introduction of high-dose prednisolone.Consequently, nivolumab had been permanently discontinued in view of refractory BP and further disease progression in his adrenal metastatic disease.
DISCUSSIO N
BP is an autoimmune blistering disorder that is caused by autoantibodies against hemidesmosomal protein BP180 and BP230 at the skin basement membrane[1].The causes of BP in oncologic patients could be idiopathic, paraneoplastic[2,3], secondary to cancer therapy or related to another medication.With the widespread use of immunotherapy in cancer management, BP has become a well-established cutaneous toxicity associated with immunotherapy.The cause of BP in our case is most likely nivolumab, as the patient denied a history of BP, and as his other medications were generally tolerated for years.The timing of his renal cancer diagnosis (5 years prior) and nivolumab initiation (9 months prior) makes the paraneoplastic phenomenon highly unlikely.
Here, we summarise a list of selected case reports of immunotherapy induced BP by literature review in Table 1.Most reported immunotherapy-induced BP involving either skin or mucous membrane only Table 1;combination of cutaneous BP and mucous membrane pemphigoid (MMP) is rare[4].Cutaneous BP typically presents with pruritic blisters that arise on erythematous or urticarial plaques.Gradually, the bullous lesion can erode and leave haemorrhagic crust[5].MMP usually presents with oral erosions, blistering or desquamative gingivitis, which are associated with significant morbidity and severe complications, such as tissue destruction, fibrosis and loss of function (e.g., laryngeal stenosis).In addition, MMP could be initially indistinguishable from a gingivitis due to poor oral hygiene practices[6].Thus, oncologists should be aware of cutaneous BP and MMP in all patients who have been treated with immunotherapy.
Onset of BP post-initiation of immunotherapy varies from weeks to several months [Table 1].Previous evidence showed that the median number of weeks of immunotherapy prior to onset of BP was 17 (range of 3 to 91)[7].However, delayed cases have been reported, even several months after discontinuation of immunotherapy[8,9].

Table 1.Selected reported cases of immunotherapy-induced bullous pemphigoid
Diagnostic workup usually includes skin biopsy for histology and immunofluorescence staining.Histopathology normally shows subepidermal clefting with an eosinophilic inflammatory infiltration[10].DIF characteristically shows linear deposits of IgG and/or C3 along the epidermal basement membrane zone.Indirect immunofluorescence (IIF) or enzyme-linked immunosorbent assays will confirm the deposit of autoantibodies specific for BP230 (also known as BPAG1) and/or BP180 (also known as BPAG2; collagen XVII)[6,11].Several cases have been reported with raised serum anti-BP180 associated with immunotherapyinduced BP as well[12].Interestingly, in one case, it was demonstrated that immunotherapy-induced BPmay develop in the presence of anti-LAD-1 IgG antibodies alone but absence of anti-BP180 or anti-BP230 autoantibodies[13].
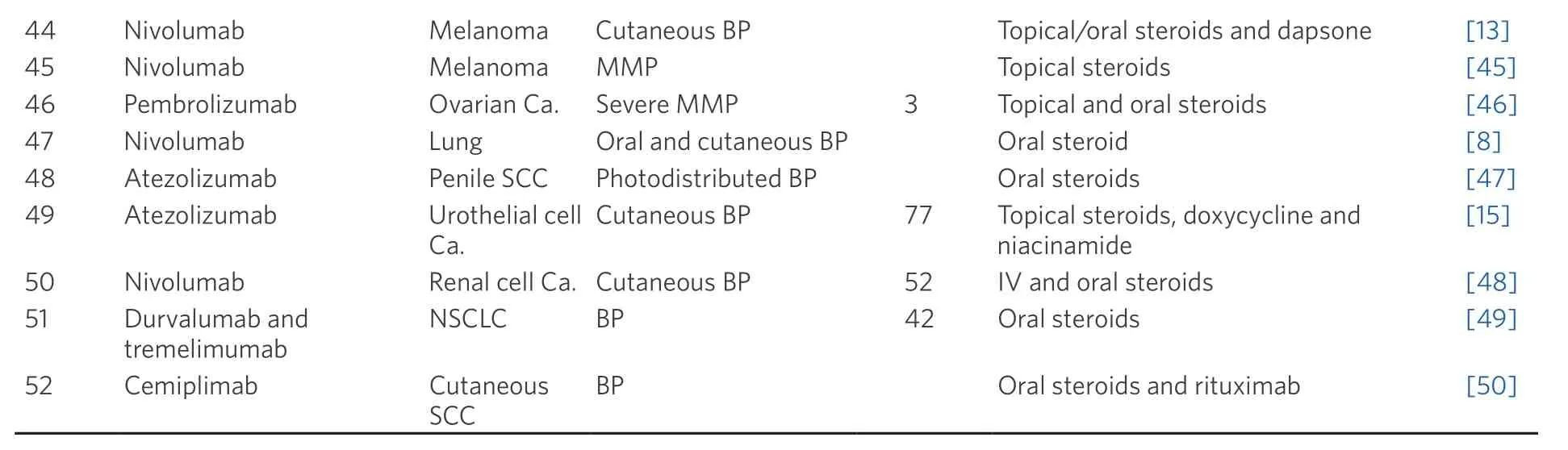
SCC: squamous cell cancer; Ca.: carcinoma; NSCLC: non-small cell lung cancer; MMP: mucous membrane pemphigoid; BP: bullous pemphigoid
Treatment regimens generally include steroids (oral or topical or combination), often in conjunction with discontinuation of immunotherapy.As steroids can potentially diminish immunomodulatory actions,topical steroids are first-line treatment for BP and are largely safe due to their limited systemic absorption.Meanwhile, oral nicotinamide and tetracycline (doxycycline or minocycline) have demonstrated a good effect in mild or moderate cases whilst completely avoiding systemic steroids[14,15].The role of nicotinamide in treating BP is modulating inflammatory cytokines and acting as poly adenosine diphosphate ribose polymerase inhibitor[16].However, severe cases have frequently required intravenous methylprednisolone(1-2 mg/kg).Refractory immunotherapy-induced BP shows response to rituximab or omalizumab[12,17],which are started after immunotherapy discontinuation and lead to resolution of BP.Future studies are warranted to explore targeted immunosuppressant agents for severe steroid-refractory BP.
The question of resuming immunotherapy after resolution of BP remains under discussion.In the situations where immunotherapy is ineffective, discontinuation is clearly warranted.While in the context of a clinical response to immunotherapy, the discontinuation of immunotherapy is challenging, and the risks of continued treatment must be carefully weighed against its benefits.As such, the decision of resuming immunotherapy should be made as a part of multidisciplinary team and on an individual basis.In one case report BP developed after the patient was transitioned to a higher dose of nivolumab.Another case report showed improvement in BP after switching from nivolumab to pembrolizumab[18].Consequently,immunotherapy should not be permanently discontinued without the trial of the previously tolerated dose or frequency or different immunotherapy.
Understanding the correlation between the incidence rate of skin irAEs and oncology benefit or immunotherapy resistance is critical for clinical practice.There is some evidence showing potential positive association between development of cutaneous irAEs and improved survival in patients on immunotherapy, particularly vitiligo[19,20].In addition, a systematic review and meta-analysis suggested that the skin, gastrointestinal and endocrine irAEs might be positively associated with clinical benefit, while the pulmonary irAEs might be associated with immunotherapy resistance[21].However, the development of severe BP in our case is not a marker of good response to immunotherapy, as the patient failed to respond to nivolumab despite the extensive, refractory, severe BP.Immunotherapy resistance can be classified as primary, such as in never-responders, or acquired, which emerges after a period of response[22].Our patient had continuously slow disease progression in his metastatic left adrenal and left kidney mass since nivolumab, which could be primary immunotherapy resistance.However, we could not totally exclude the possibility of pseudo-progression, as his other metastatic diseases were largely stable.In a later phase, theextra use of steroids and cessation of nivolumab certainly further contributed to his disease progression.Consequently, understanding the correlation between skin irAEs and clinical outcome could provide valuable information in distinguishing between pseudo-progression and immunotherapy resistance, which will assist oncologists in the decision of resuming immunotherapy after the recovery from manageable skin irAEs.
The mechanism of immunotherapy induced BP is currently unclear.While BP is associated with both a humoral and a cellular response, it is widely believed that immunotherapy induced BP could be provoked by an autoantibody targeted to a sharedantigen and the dysfunction in T-regulatory cells, which is inhibited by immunotherapy[23].The shared target antigen is located both at the dermoepidermal junction and on the surface of tumour cells, such as BP 180, whichcan be expressed on the surface of melanoma cells, nonsmall cell lung cancer cells and basement membrane skin cancer[24,25].Meanwhile, dysregulation of PD-1/PD-L1 and CTLA-4 signal pathway induced by immunotherapy can impair the balance within the immune system, resulting in the development of off-target effects and autoimmunity[26].Further studies are required to determine the underlying mechanisms of immunotherapy induced BP.
Conclusion
Here, we report a rare case of severe, extensive and refractory nivolumab-induced BP in a patient with advanced renal cancer.With the widespread use of immunotherapy, it has become increasingly important to document cases of immunotherapy-induced BP to provide more information in diagnosing and treating these cutaneous adverse events.Moreover, the close collaboration between dermatologists and oncologists is essential to allow the patients with cutaneous irAEs to continue antitumor therapy, avoiding unnecessary discontinuation of immunotherapy.
DECLARATIONS
Acknowledgments
All authors would like to acknowledge Dr.Dalila Malek, Dr.Paul Gatt, Dr.Ravi Suchak and Dr.Marice Sudaerson (Southend Hospital) for support and advice.
Authors’ contributions
Designed the study and wrote the manuscript: Wu X, Palvai S Reviewed and approved the manuscript: Jalil A
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study support by National Health Service Funding.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Written informed consent was obtained from the patient.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any companying images.
Copyright
© The Author(s) 2020.

