Current updates in management of relapsed/refractory small cell lung cancer
Omar Abughanimeh, Vinicius Ernani, Alissa Marr, Apar Kishor Ganti,2
1Division of Oncology and Hematology, Department of Internal Medicine, University of Nebraska Medical Center-Fred & Pamela Buffett Cancer Center, Omaha, NE 68198-6840, USA.
2Division of Oncology and Hematology, VA Nebraska Western Iowa Health Care System, Omaha, NE 68198-6840,USA.
Abstract Small cell lung cancer (SCLC) is an aggressive subtype of neuroendocrine tumor. It is characterized by a rapid doubling time and early development of metastatic disease. Despite being responsive to initial chemotherapy, most of the patients will have relapse of the disease within a few months. The prognosis of SCLC is dismal with a 5-year survival rate of less than 5%. For that reason, management of SCLC has been an active area of research. The utilization of immunotherapy has provided promising results in treatment of SCLC in the front-line setting. Therefore, utilization of immunotherapy and targeted therapy is being studied in the setting of relapsed/refractory disease, and currently, different clinical trials are exploring new drugs and further options. In this review, we will explore the latest updates in management of relapsed/refractory SCLC.
Keywords: Small cell lung cancer, relapsed small cell lung cancer, chemotherapy, immunotherapy, targeted therapy
INTRODUCTION
Lung cancer is a major public health concern. In 2020, it is estimated that the United States (US) will have more than 200,000 new cases, making lung cancer the second most common malignancy and leading in cancer-related mortality in both genders[1]. Small cell lung cancer (SCLC) accounts for ≈ 20% of the total lung cancer cases globally[2]. In the US, SCLC accounts for 16% of new lung cancer cases[3]. SCLC is divided into limited-stage disease (LS-SCLC), which shows confined growth, or extensive-stage disease (ESSCLC) which is associated with metastasis. It is estimated that approximately 60% of SCLC patients present with extensive-stage disease at the time of initial diagnosis. The most common sites of spread include contralateral lung, adrenal glands, brain, liver, bones, and bone marrow[4,5].
SCLC is highly responsive to chemotherapy[3,5]. The standard first-line treatment of LS-SCLC includes concurrent chemotherapy (cisplatin-etoposide) and radiation, while ES-SCLC is treated with a combination chemotherapy (platinum-etoposide) and an immune checkpoint inhibitor. For long time, the treatment of ES-SCLC consisted of platinum agents and etoposide[5]. This was changed recently due to results from IMpower-133 and CASPIAN studies, both of which demonstrated improved survival by adding atezolizumab and durvalumab, respectively, to platinum and etoposide[6,7].
Despite being responsive to chemotherapy, most SCLC patients will experience tumor relapse within a few months, making management of these patients challenging[8,9]. If the relapse occurs within 3 months of treatment, the disease is called refractory or resistant, and the response to further treatment is < 10%[9]. If the relapse occurs after 3 months, the expected response to further treatment is 25%. Options for patients with refractory or relapsed disease are limited and patients with relapsed or refractory disease have a median survival of 8-9 months[9,10].
The treatment of refractory/relapsed SCLC has been an active area in research given the dismal prognosis and the poor outcome. Chemotherapy remains the cornerstone of treatment, but recently newer agents including immunotherapy are being studied, with promising results. In this review, we will discuss the current agents that are used in relapsed or refractory SCLC.
TREATMENT OPTIONS OF RELAPSED/REFRACTORY SMALL CELL LUNG CANCER
Chemotherapy
Topotecan
Topotecan is a semisynthetic water-soluble analog of camptothecin which acts as an inhibitor of the nuclear enzyme topoisomerase I, leading to DNA damage[11,12]. For a long time, it was the only drug that was approved by the Food and Drug Administration (FDA) for relapsed small cell lung cancer.
One of the earliest studies of topotecan in SCLC was conducted by the European Organization for Research and Treatment of Cancer[11]. This study was a phase II trial which included 92 patients (47 patients who were refractory to first-line treatment, and 45 patients had disease relapse after 3 months of stopping chemotherapy). In both arms, patients received intravenous (IV) topotecan at 1.5 mg/m2for five consecutive days every 3 weeks. Topotecan demonstrated an overall response rate (ORR) of 6.4% (95%CI: 1.3%-17.6%) in patients who failed first-line treatment, and 37.8% (95%CI: 23.8%-53.5%) in patients who had disease relapse after 3 months of finishing chemotherapy treatment. The overall response in both groups was 21.7%. The overall median duration of response was 7.6 months (95%CI: 5.1-12.2 months), the median time to progression was 2.8 months (95%CI: 2.2-3.9 months), and the overall survival (OS) was 5.4 months (95%CI: 4.8-6.3 months). This study showed that topotecan had good activity in SCLC, specifically in patients who responded to initial chemotherapy[11].
von Pawelet al.[12]conducted a randomized phase III trial comparing topotecan to Cyclophosphamide, Doxorubicin, and Vincristine (CAV). In this study a total of 211 patients were recruited (107 treated with topotecan and 104 treated with CAV). Patients in the topotecan arm received IV topotecan 1.5 mg/m2daily for five consecutive days every 3 weeks. The ORR of topotecan was 24.3% compared to 18.3% in the CAV arm. The median time to progression and median survival were similar in both arms (13.3 weeksvs.12.3 weeks, and 25 weeksvs.24.7 weeks, respectively). Despite the similarity in outcomes, this study showed that
patients who received topotecan had better improvement in symptoms including dyspnea (P= 0.002), anorexia (P= 0.042), fatigue (P= 0.032), and hoarseness (P= 0.043).
Later in the early 2000s, a phase III clinical trial was conducted by O’Brienet al.[13]to compare oral (PO) topotecan to supportive care alone. This study demonstrated prolonged survival with topotecan compared to supportive care. Moreover, it showed that patients who received topotecan had greater symptoms control and slower quality of life deterioration.
Eckardtet al.[14]performed a randomized phase III clinical trial to compare PO topotecan with IV topotecan in relapsed SCLC. In this study, 309 patients were included, 153 patients received oral topotecan 2.3 mg/m2daily for five consecutive days every three weeks whereas 151 received IV topotecan 1.5 mg/m2daily for five consecutive days every three weeks. The study showed similar ORR in both arms of 18.3%vs.21.9% respectively. There was no difference in median time to response (6.1 weeks for both), median duration of response (18.3 weeksvs.25.4 weeks), and median time to progression (11.9 weeksvs.14.6 weeks).Hematologic complications have been commonly reported with topotecan. Despite having lower risk of grade 4 neutropenia compared to CAV, topotecan has higher risk of grade 4 thrombocytopenia and anemia[12]. Non-hematologic toxicities were also reported such as fatigue, alopecia, nausea, and other gastrointestinal complications[11,12,14].
Another study from Germany showed that a lower dose of topotecan 1.25 mg/m2had a similar efficacy to the traditional dose of 1.5 mg/m2, but most importantly it was associated with reduced toxicity[15]. It is worth mentioning that there have been some studies which evaluated the usage of weekly topotecan instead of the standard regimen, however the results were not conclusive and weekly topotecan is no longer routinely used in clinical practice[9].
Lurbinectedin
Lurbinectedin is a synthetic analog of trabectedin, which acts through inhibition of the active transcription protein-coding genes. This drug binds to CG-rich regions in the DNA causing irreversible arrest of elongating RNA polymerase on the DNA template, leading to accumulation of DNA breaks and apoptosis[10,16].
This drug was first studied in humans in 2009 when Elezet al.[17]conducted a phase I clinical trial in patients with advanced solid tumors and showed both safety and anti-tumor effect. Later, lurbinectedin was studied in combination with doxorubicin in patients with relapsed SCLC. The study showed tolerability and an overall response rate of 57.7%[18].
Recently, a single arm, multicenter, phase II clinical trial was conducted on 105 patients who were treated with 3.2 mg/m2of IV lurbinectedin every 3 weeks. Among them, 45 patient were considered to have resistant disease (defined as chemotherapy-free interval < 90 days) and 60 patients with sensitive disease (defined as chemotherapy-free interval ≥ 90 days). The study showed an ORR of 35.2% (95%CI: 26.2-45.2). Patients with sensitive disease had better ORR, [45% (95%CI: 32.1-58.4)] compared to the resistant disease group [22.2% (95%CI: 11.2-37.1)]. The overall median duration of response was 5.3 months (95%CI: 4.1-6.4), which was also higher in the sensitive disease group 6.2 (95%CI: 3.5-7.3)vs.4.7 (95%CI: 2.6-5.6). In this study, the median progression free survival (PFS) was 3.5 months (95%CI: 2.6-4.3) and the median OS was 9.3 months (95%CI: 6.3-11.8), both were also better in the sensitive disease group[16]. These results led to accelerated approval by the FDA to be used in patients with metastatic SCLC after progression on platinum-based chemotherapy. The National Comprehensive Cancer Network (NCCN) guidelines included lurbinectedin as a “preferred” agent in the second line treatment options along with topotecan or clinical trials[9].
Hematologic toxicities were reported with lurbinectedin treatment. Grade 4 neutropenia was recorded in 25% of the patients, whereas grade 4 thrombocytopenia was recorded in 4%. None of the patients developed grade 4 anemia, however 9% of them developed grade 3. Other non-hematologic toxicities included fatigue, decreased appetite and different gastrointestinal symptoms[16].
Currently, a phase III clinical trial, the ATLANTIS study (NCT02566993) is being conducted to compare the activity of lurbinectedin combined with doxorubicin, with either topotecan or CAV as second line treatment for SCLC[10].
Irinotecan
Irinotecan is a water-soluble derivative of camptothecin that acts through inhibition of DNA topotisomerase I, leading to antitumor effects[19]. The use of irinotecan in SCLC has been established in the last century. In 1990, a phase II study was conducted in Japan by Masudaet al.[19]. This study enrolled 16 patients with refractory or relapsed SCLC. All patients received IV irinotecan 100 mg/m2every week. In this study, irinotecan led to an ORR of 47% (95%CI: 21.4%-71.9%). The median duration of response was 58 days (28-156 days). These findings were supported by another study that was done in Japan which demonstrated an ORR of 50% (95%CI: 25%-75%)[20]. A newer study was conducted in Japan which evaluated 30 patients with previously treated SCLC who received irinotecan 100 mg/m2on days 1, 8, every 3 weeks. The study showed an ORR of 41.3% (95%CI: 25.5-59.3) and a disease control rate of 69%. The same study showed a median PFS and OS of 4.1 months and 10.4 months, respectively[21].
The major toxicities associated with irinotecan treatment were hematologic, mostly leukopenia, followed by nausea and pulmonary toxicity[19].
Taxanes
Paclitaxel was evaluated in multiple studies in patients with relapsed/refractory SCLC. A phase II trial was performed by Smitet al.[22]in Netherlands where patients received IV paclitaxel 175mg/m2every 3 weeks. Paclitaxel led to an ORR of 29% (95%CI: 12%-51%). Furthermore, it was associated with a median duration of response of 108 days (64-243 days), median time to progression of 65 days (33-243days), and median survival of 100 days (23-262 days).
Paclitaxel was assessed in another phase II trial that was conducted by Yamamotoet al.[23]who studied 21 patients with refractory SCLC. The study showed that single agent IV paclitaxel at a dose of 80 mg/m2weekly had an ORR of 23.8% (95%CI: 5.59-42.03), with a median survival of 5.8 months. The most common toxicity associated with paclitaxel was grade 3-4 neutropenia (66.6%), other reported side effects included neuropathy, infections, and other gastrointestinal symptoms[23].
Docetaxel is another taxane that was studied in relapsed/refractory SCLC. In the mid-1990s, docetaxel was studied in a phase II trial in patients with previously treated SCLC[24]. The study showed that IV docetaxel 100 mg/m2once every 3 weeks was associated with an ORR of 25%, with a median duration of response ranging between 3.5 and 12.6 months. The main toxicities reported in this trial were neutropenia, alopecia, and fatigue[24].
It is worth to mention that Cabazitaxel was also studied in the setting of relapsed SCLC, but a study conducted by Evanset al.[25]showed inferior PFS and OS when compared to topotecan.
Temozolomide
Temozolomide (TMZ) is an oral alkylating agent, which acts through production of O6 -alkyl-guanine lesions on DNA. These lesions are removed by O6 -methylguanine-DNA methyltransferase (MGMT).
However, if these lesions remain unrepaired, they can lead to cytotoxicity and ultimately apoptosis[26,27]. At its earliest stages, temozolomide was used in treating refractory astrocytoma and glioblastoma multiforme[26].
In SCLC, several studies suggested that TMZ can be useful. Pietanzaet al.[26]performed a phase II study on 64 patients where 48 had sensitive disease (defined as having relapse or progression > = 60 days after first line chemotherapy) and 16 had refractory disease. Patients received oral TMZ 75 mg/m2daily for 21 days during a 28-day cycle. The study reported an ORR of 23% (95%CI: 12%-37%) in the sensitive group and 13% (95%CI: 2%-38%) in the refractory group. Patients who had methylated MGMT experienced a higher response compared to patients with unmethylated MGMT (38%vs.7%,P= 0.08). Interestingly, patients with brain metastasis had an ORR of 38% (95%CI: 14%-68%)[26]. Another study showed that TMZ can also be effective and tolerable using a regime of 200 mg/m2daily for 5 days in 28-day cycles for patients with relapsed SCLC[28]. The most common toxicities reported with temozolomide were fatigue, gastrointestinal symptoms, and hematologic toxicities (most commonly lymphopenia)[26].
Etoposide
Etoposide has been used in treatment of SCLC for a long time. The use of etoposide in the second line setting has also been studied in patients who had received IV etoposide. A phase II trial showed that oral etoposide 50 mg/m2daily for 21 days can lead to an ORR of 45.5% (95%CI: 27%-65%), median duration of response of 4 months (1.5-9.5 months), and median survival of 3.5 months (1-15 months)[29]. Another phase II trial showed a response rate of 23%[30]. The most common observed toxicities were myelosuppression and alopecia[29].
Vinorelbine
Vinorelbine is a semisynthetic vinca alkaloid which acts through binding to microtubular proteins, preventing tubulin polymerization[31]. There are data that suggest efficacy of vinorelbine in the setting of SCLC. A phase II study was conducted on 26 patients with history of recurrent SCLC, who received vinorelbine 30 mg/m2weekly, having shown a partial response rate of 16% (95%CI: 4%-36%) whereas 28% of the patients had stable disease[31]. Leukopenia was the major associated toxicity with vinorelbine as it occurred in 80% of the patients. Other common toxicities included anemia, gastrointestinal symptoms, and drug related fever[31]. Recently, a study in Poland showed that combining vinorelbine and cisplatin with electroporation (EP) was associated with increased anticancer activity due to the exposure of the cells to high intensity electric pulses, allowing the usage of lower doses of drugs[32].
Bendamustine
Bendamustine is an alkylating agent that has been commonly used in different lymphoproliferative disorders. The clinical benefit of bendamustine in SCLC was demonstrated initially in a phase II clinical trial that was conducted in Europe. In this study, Schmittel and his colleagues[33]enrolled 21 patients with SCLC who had a relapse ≥ 2 months after completion of first line therapy. Twenty-one patients received bendamustine at a dose of 120 mg/m2in the first two days every 3 weeks. The study showed a response rate of 29% with a median survival of 7 months.
Subsequently, another phase II study was conducted in North America for patients with relapsed SCLC where patients received 120 mg/m2on the first 2 days of a 21-day cycle. This study subdivided the population to a sensitive disease group; defined as stable or responsive disease to a platinum containing therapy for at least 90 days, or resistant disease group. A total of 50 patients participated with a response rate of 26% (95%CI: 13.3%-39.5%). The response rate was higher in the sensitive disease group compared to the resistant disease group(33%vs.17%). The overall clinical benefit (complete response + partial response + stable disease) rate was 67%. The median OS was 4.8 months (95%CI: 3.8-6.3 months) which was also better in the sensitive group (5.7 monthsvs.4.1 months)[34]. The most common toxicities were fatigue, anemia, thrombocytopenia, and different gastrointestinal symptoms[34].
Gemcitabine
A phase II study showed that treatment with gemcitabine at a dose of 1000 mg/m2on day 1, day 8 and day 15 of a four-week cycle, resulted in an overall response rate of 13% (95%CI: 6%-27%), with a median survival of 17 weeks (4-84 weeks)[35]. Interestingly, a different study tried gemcitabine 1,250 mg/m2on day 1 and 8 every 3 weeks as a second line treatment of SCLC. But the results were not encouraging, as none of the 27 patients had a response[36].
Amrubicin
Amrubicin is a 3rd generation anthracycline and a topoisomerase II inhibitor that has a chemical structure similar to doxorubicin[37,38]. The first data about amrubicin in SCLC was obtained from Japan after a phase II trial studied it in patients with untreated ES-SCLC. This study showed an overall response rate of 75.8% (95%CI: 57.7%-88.9%) and a median survival of 11.7 months (95%CI: 9.9-15.3 months)[37]. Later, several studies were conducted, but the most notable one was a phase III clinical trial which compared amrubicin to topotecan in the second line setting. This trial evaluated 637 patients who were randomized 2:1 to amrubicin 40 mg/m2daily for three days or topotecan 1.5 mg/m2for 5 days every 3 weeks. Amrubicin was associated with a better overall response rate (31.1%vs.16.9%,P= 0.001) and median PFS (4.1 monthsvs.3.5 months,P= 0.01). However, it failed to show improvement in OS which was the primary endpoint[38]. This drug is not recommended in the NCCN guidelines for treatment of SCLC[9].
Vinflunine
Vinflunine is a microtubule inhibitor that has been used in different malignancies including non-small cell lung cancer. Spigel and colleagues[39]performed a phase II study on 51 patients with relapsed SCLC. Patients received IV vinflunine at 320 mg/m2every 3 weeks. The study showed an ORR of 19.6% % (95%CI: 10-33%). The median PFS and OS were 1.6 months (95%CI: 1.3-3.9 months) and 4.9 months (95%CI: 3.2-6.5 months) respectively. Despite being well tolerated, 5% of the patients had grade 3/4 toxicities with neutropenia being the most common (32%). Other side effects included fatigue (16%), arthralgia (16%), and different gastrointestinal symptoms[39].
Combined chemotherapy
Multiagent chemotherapy have been the standard treatment for extensive-disease SCLC for long time[40,41]. Chemotherapy regimens like etoposide with platinum, CAV, and cyclophosphamide-doxorubicin-etoposide (CDE) all showed reasonable response rate when used in the first line treatment. Nevertheless, the use of combined chemotherapy is limited in the setting of recurrent/relapsed SCLC, mainly due to intolerable toxicity at that stage[40]. The NCCN guidelines suggest only CAV in the 2nd line setting[9].
One of the few studies to evaluate combined chemotherapy agents in the 2nd line setting was a phase III trial from Japan which compared a combination of cisplatin-irinotecan-etoposide (CIE) to topotecan in the setting of relapsed SCLC. This study revealed that CIE had a better OS (18.2 monthsvs. 12.5 months;P= 0.0079). However, the toxicity was significantly higher in the CIE arm and therefore it is not commonly used in practice[42].
Table 1 summarizes some of the clinical trials that evaluated different chemotherapy agents in relapsed/refractory small cell lung cancer.
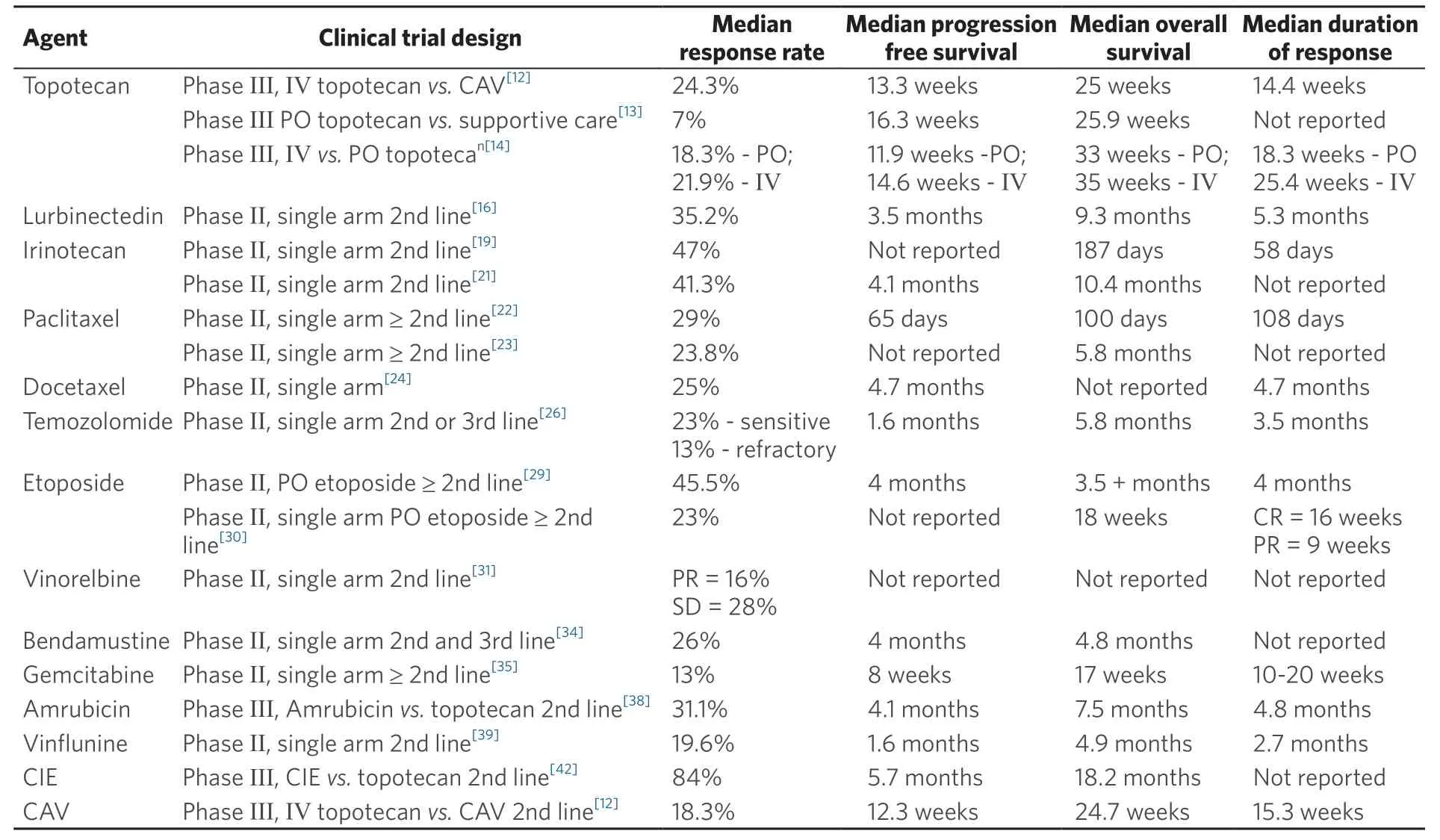
Table 1. Clinical trials of chemotherapy agents in the setting of recurrent/relapsed SCLC
Immunotherapy
The utilization of the immune system in treating cancers has been an exciting field that is being developed over the last years. The immune system recognizes cancer cells but, in most situations, it is not able to eliminate the cancer cells due to inhibitory receptors and signals (checkpoints). Programmed death-1 (PD-1) and cytotoxic lymphocyte antigen 4 (CTLA-4) are the most common checkpoints that have been studied in solid malignancies[8]. While immunotherapy is now recommended in the front-line setting, there have been trials in immunotherapy naïve patients with relapsed SCLC, that were conducted prior to availibility of IMPower 133 and CASPIAN results.
Nivolumab
The CheckMate 032 trial[43]evaluated nivolumab in the setting of recurrent SCLC. In this study, patients were randomized to three groups where they received either nivolumab 3 mg/kg alone every 2 weeks until disease progression, nivolumab 1 mg/kg + ipilimumab 3 mg/kg every 3 weeks for 4 cycles followed by maintenance nivolumab every 2 weeks, and nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for 4 cycles followed by maintenance nivolumab every 2 weeks. The number of patients in each group was 98, 61, and 54 respectively. A fourth group included only three patients who received nivolumab 1 mg/kg + ipilimumab 1 mg/kg. The study showed a response rate of 10% for nivolumab alone, 23% for the nivolumab 1 mg/kg + ipilimumab 3 mg/kg, 19% for the nivolumab 3 mg/kg + ipilimumab 1 mg/kg, and 33% for the nivolumab 1 mg/kg plus ipilimumab 1 mg/kg. Interestingly the expression of programmed death-1 ligand (PD-L1) did not correlate with the response to therapy. Grade 3-4 treatment related toxicities were most common in the nivolumab 1 mg/kg + ipilimumab 3 mg/kg group (30%) with diarrhea being the most common[43]. An updated analysis of the Checkmate 032 trial showed a higher response rate in the combination of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg compared to nivolumab alone (21.9%vs.11.6%; odds ratio 2.12; 95% CI: 1.06-4.26,P-value = 0.03)[44]. However, it demonstrated similar OS between the 2 groups. The median OS in the nivolumab group was 5.7 months (95%CI: 3.8-7.6 months) compared to 4.7 months in the combination arm (95%CI: 3.1-8.3 months). Furthermore, toxicities were higher in the combination arm. The last 2 findings led the NCCN panel to recommend nivolumab alone instead of the combination[9].
The CheckMate 331 trial (NCT02481830)[45]is an ongoing phase III clinical trial that is comparing nivolumab to topotecan and amrubicin. The trial estimated complete date is in mid-2021, however, preliminary data showed no significant difference in overall survival between nivolumab (median of 7.5 months) and chemotherapy (median of 8.4 months) with a hazard ratio of 0.86 (95%CI: 0.72-1.04).
Pembrolizumab
The KEYNOTE-028 trial (NCT02054806)[46], is a phase Ib study that evaluated the safety of pembrolizumab 10 mg/kg every 2 weeks in patients with advanced PD-L1 positive ES-SCLC. This study revealed a promising efficacy of pembrolizumab in SCLC with an ORR of 33% (95%CI: 16%-55%)[46]. The KEYNOTE-158 trial (NCT02628067)[47], is an ongoing phase II trial, to evaluate the benefit of pembrolizumab in advanced SCLC. The preliminary results showed an ORR of 18.7% (95%CI: 11.8%-27.4%). The response was higher in patients who had PD-L1 positive tumor compared to PD-L1 negative tumor (35.7%vs.6%)[47]. A recent paper was published by Chunget al.[48]who performed a combined analysis of both KEYNOTE-028 and KEYNOTE-158. The results demonstrated an ORR of 19.3% (95%CI: 11.4%-29.4%) with a median OS of 7.7 months (95%CI: 5.2-10.1 months). As revealed by the KEYNOTE-158 trial, patients who had PD-L1 positive tumor had better ORR and OS. Nevertheless, the NCCN panel added pembrolizumab as a second line therapy regardless of the PD-L1 results[9].
Durvalumab
Durvalumab is another immunotherapy agent that was approved by the FDA in 2020 to be used with combined chemotherapy in the first line setting based on the CASPIAN trial[7]. A phase I study evaluated the use of durvalumab and tremelimumab in patients who had disease progression on at least one treatment. The results of this study showed an ORR of 13.3%, PFS of 1.8 months and an OS of 7.9 months[49]. However, a phase II study did not show sufficient response of durvalumab and tremelimumab when it was used with or without radiation[50].
Atezolizumab
Atezolizumab was approved by the FDA to be used in the front line setting based on the IMpower-133 trial which demonstrated significant improvement in the PFS and OS by adding atezolizumab to chemotherapy[2]. However, the use of it in the second line setting is still under study. A recent phase I trial on 17 patients showed that atezolizumab was tolerated and it had some efficacy with a median OS of 5.9 months[51].
There are no doubts that the field of immunotherapy will continue to expand, with many different clinical trials curently ongoing. Table 2 summarizes some of the trials that investigated immunotherapy in relapsed small cell lung cancer based on reported studies in literature.
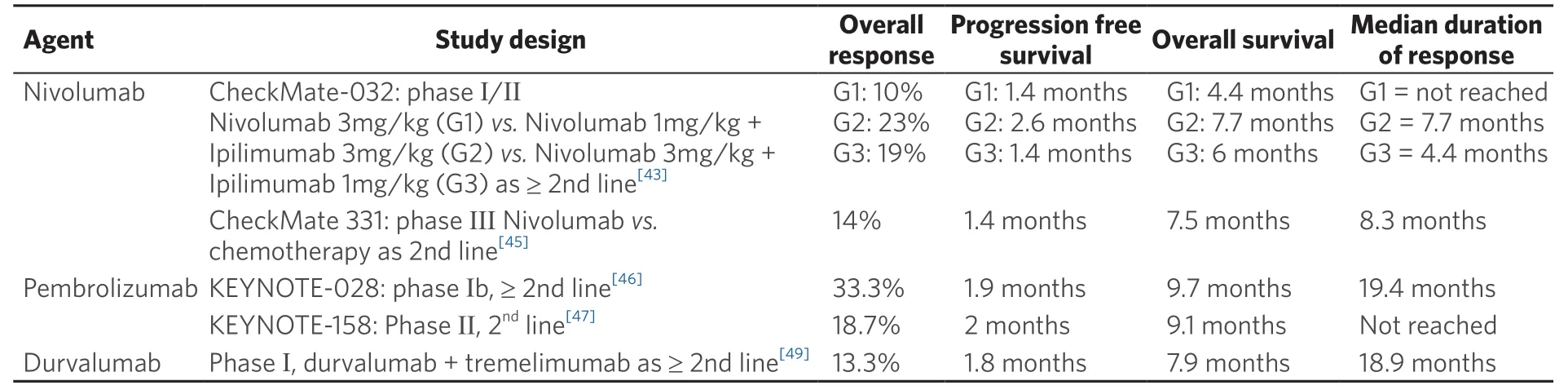
Table 2. Clinical trials of immunotherapy in the setting of recurrent/relapsed SCLC
Targeted therapy
Targeted therapy has been an exciting field for different malignancies. Until recently, its use in SCLC has not been successful[8]. Several trials were done to assess targeted therapies as a single agent or in combination with chemotherapy but many of them did not reach their primary endpoint[8]. These therapies included bevacizumab, vandetanib, aflibercept, vismodegib, cixutumumab, panobinostat, oblimersen, and obatoclax[52-58]. However, there are some targeted therapies that have shown some promising results.
Alisertib
Alisertib is an oral aurora kinase A inhibitor[8]. Melicharet al.[59]performed a study to evaluate alisertib use in different relapsed solid malignancies including SCLC. A total of 48 patients with SCLC were enrolled, with a total overall response rate of 21%. However, the time to progression was only 2.6 months.
Veliparib
Veliparib is an inhibitor of poly (ADP-ribose) polymerase (PARP)[60]. A study compared the combination of veliparib with TMZ to TMZ and placebo in patients with relapsed/refractory SCLC. The study failed to show difference in PFS or OS, but it showed that the combination of TMZ and veliparib was associated with better ORR compared to the other group (39%vs.14% respectively,P= 0.016)[27].
Pazopanib
Pazopanib is a tyrosine kinase inhibitor (TKI) of vascular endothelial growth factor receptors (VEGFR-1, VEGFR-2, and VEGFR-3), platelet-derived growth factor receptors (PDGFR), and c-kit. It showed promising results when used in the second line setting in refractory/relapsed SCLC[61]. In a phase II study, 39 patients with platinum sensitive disease and 19 patients with refractory disease received pazopanib 800 mg daily. The partial response rate was 13.8% (95%CI: 5-22.7), with 34.5% achieving stable disease. The median PFS was 2.5 months (95%CI: 1.9-3.1 months) and OS was 6 months (95%CI: 3.8-8.2 months). Interestingly, the study showed that one cycle of pazopanib resulted in significant decrease in number of patients with ≥ 5 circulating tumor cells (CTCs)/7.5ml blood. That led the authors to suggest consideration of CTCs enumeration as biomarker of response[61].
Anlotinib
Anlotinib is another TKI that targets VEGFR-2, VEGFR-3, PDGFR-b, and c-Kit. The “ALTER 1202” trial, is a phase II, double-blinded, randomized, placebo-controlled study that enrolled 120 patients with SCLC who had disease progression after at least 2 lines of treatment. Eighty-two patients received anlotinib 12 mg daily for 2 weeks on and one week off cycle while the rest got placebo. The study demonstrated a significant improvement in PFS in the anlotinib arm [4.1 months (95%CI: 2.8 to 4.2 months] compared to placebo [0.7 months (95%CI: 0.7 to 0.8 months)] (P-value < 0.0001). The disease control rate (DCR) was significantly higher in the anlotinib group compared to placebo (71.6%vs.13.2%,P-value < 0.0001)[62]. Later, an update was published which also showed an improvement in OS in the anlotinib arm (7.3 monthsvs.4.9 months)[63].
Table 3 summarizes some of the clinical trials that involved targeted therapy in recurrent/relapsed SCLC.

Table 3. Clinical trials of targeted therapies in the setting of recurrent/relapsed SCLC
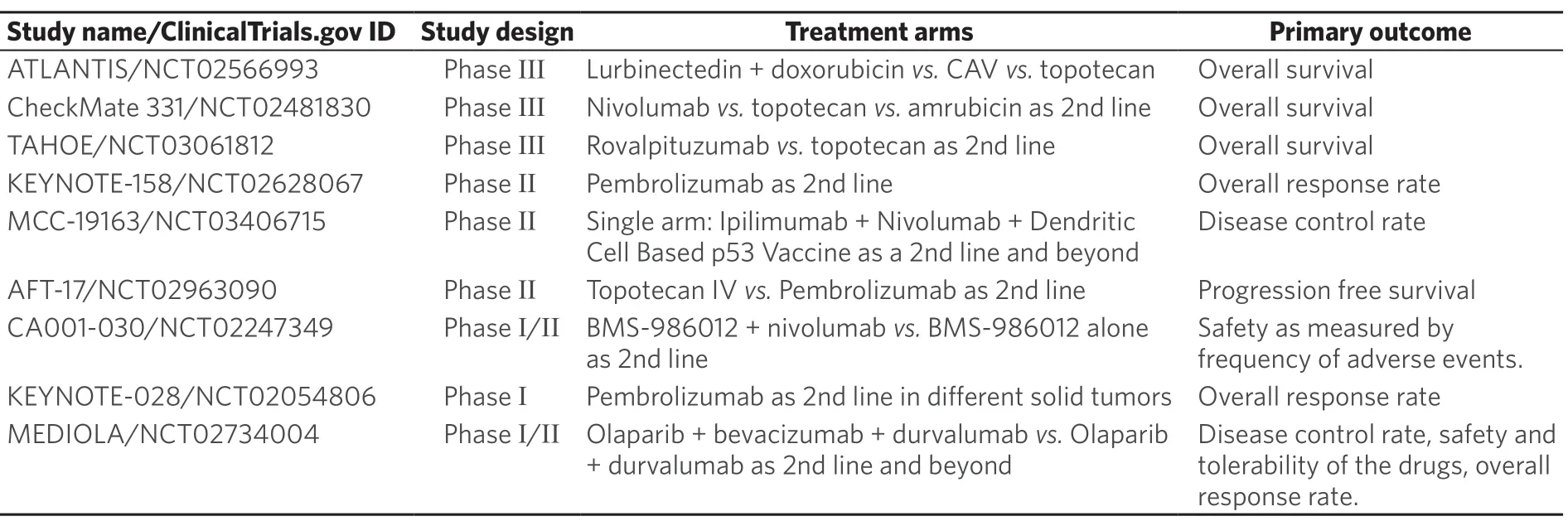
Table 4. Active clinical trials evaluating new treatments for recurrent/relapsed SCLC
FUTURE CONSIDERATIONS AND CHALLENGES
The treatment of relapsed/refractory SCLC has been challenging over the last several years given the lack of effective therapies. Till 2020, topotecan was the only FDA approved drug for relapsed SCLC before the FDA granted accelerated approval for lurbinectedin. There is no doubt that the management of SCLC is actively developing. Currently, there has been a focus on immunotherapy and targeted therapies in the relapsed/refractory disease setting especially after the results of the IMpower133 and CASPIAN trials.SCLC: small cell lung cancer; CAV: Cyclophosphamide, Doxorubicin, and Vincristine
Table 4 summarizes some of the ongoing clinical trials to investigate new approaches for relapsed/refractory SCLC.
EFFECT OF COVID-19 ON SCLC TREATMENT
In late 2019, multiple cases of atypical pneumonia had been reported in Wuhan, China, caused by a novel type of coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that led to the COVID-19 disease[64]. So far, its impact on lung cancer diagnosis and treatment is not well reported. However, a recent study from Spain did show that patients with lung cancer and COVID-19 infection have a higher mortality rate compared to the general population with COIVD-19 alone[61]. While there is a concern that treating these patients may increase the risk of complications associated with the SRA-CoV-2, relapsed SCLC has a very aggressive course. We recommend continuing treatment of these patients while monitoring for the development of COVID-19 disease.
CONCLUSION
In conclusion, relapsed SCLC remains a difficult disease with a dismal prognosis. Most of the patients will have disease relapse after a few months of first-line treatment. Till date, there are only 2 drugs approved by the FDA, topotecan and lurbinectedin both with modest efficacy. However, the recent advances in immunotherapy and targeted therapy are exciting, and the results of ongoing trials may help find a strategy that will improve outcomes for these patients.
DECLARATIONS
Authors’ contributions
Wrote the manuscript: Abughanimeh O Reviewed, edited, and approved the final manuscript: Ernani V, Marr A, Ganti AK Contributed to the manuscript: Abughanimeh O, Ernani V, Marr A, Ganti AK
Availability of data and materials
Not applicable.
Financial support and sponsorship
All authors have declared that they have no financial relationships at present that is related to this paper.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
 Journal of Cancer Metastasis and Treatment2020年12期
Journal of Cancer Metastasis and Treatment2020年12期
- Journal of Cancer Metastasis and Treatment的其它文章
- ROR2 regulates the survival of murine osteosarcoma cells in lung capillaries
- lsoflavone research towards healthcare applications
- Primary malignant tumors of bone surface: a review with emphasis in differential diagnosis
- Glycogen synthase kinase 3b biology in bone and soft tissue sarcomas
- Negative effects of tumor cell nitric oxide on antiglioblastoma photodynamic therapy
- Angiogenesis in acute myeloid leukemia
