Study on the Quality Standard of Shema Mixture
Lilong YANG, Min HUANG,2*
1. The First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530023, China; 2. Guangxi Zhuang and Yao Medicine Engineering Technology Research Center, Nanning 530200, China
Abstract [Objectives] To establish the quality standard of Shema mixture. [Methods] Thin-Layer Chromatography(TLC) was used to qualitatively identify Ephedra sinica Stapf, Belamcanda chinensis (L.) Redouté and Citrus maxima (Burm.) Merr. cv. Tomentosa in the mixture, and High Performance Liquid Chromatography(HPLC) was established for the determination of the content of ephedrine hydrochloride, which was an effective component of principle drug E. sinica Stapf in the mixture. [Results] TLC was used to identify E. sinica Stapf, B. chinensis (L.) Redouté and C. maxima (Burm.) Merr. cv. Tomentosa in Shema mixture, and the characteristic spots were stable and clear. Ephedrine hydrochloride had a good linear relationship in the range of 2.01-14.06 μg, the average recovery of samples was 98.77%, RSD=1.58%. [Conclusions] TLC and HPLC, as the quality control methods of Shema mixture, have high accuracy and good repeatability, which laid a foundation for the quality control of Shema mixture.
Key words Shema mixture, Quality standard
1 Introduction
Shema mixture is the preparation of the First Affiliated Hospital of Guangxi University of Chinese Medicine, which was originally created by Professor Li Weiwei, a famous expert of traditional Chinese medicine and pediatrics in Guangxi, it has been used for many years in clinical practice and has a significant effect in the treatment of infantile asthma. The whole prescription of Shema mixture consists of 17 kinds of medicinal materials, such asBelamcandachinensis(L.) Redouté,EphedrasinicaStapf,Michaelmasdaisy, dried ginger,AsarumsieboldiiMiq., Common Coltsfoot Flower, RHIZOMA PINELLIAE PREPARATUM,Poriacocos(Schw.) Wolf,Citrusmaxima(Burm.) Merr. cv. Tomentosa,Schisandrachinensis(Turcz.)Baill, FRUCTUS PERILLAE, BOMBYX BATRYTICATUS,Semensinapis,DioscoreaoppositaThunb.,CrataeguspinnatifidaBunge, GLYCYRRHIZAE RADIX ET RHIZOMA andSteviarebaudiana(Bertoni) Hemsl. Among them,B.chinensis(L.) Redouté can lower lung and relieve asthma,E.sinicaStapf can diffuse the lung to calm panting, Michaelmas daisy can moisten lung for arresting cough, Asarum sieboldii Miq. can warm the lung and resolve fluid retention, Common Coltsfoot Flower can diffuse the lung to suppress cough and to calm panting,Schisandrachinensis(Turcz.)Baill can astringe lung qi, RHIZOMA PINELLIAE PREPARATUM can descend adverse qi, eliminate dampness and resolve phlegm. In the formula,E.sinicaStapf,AsarumsieboldiiMiq., dried ginger and Common Coltsfoot Flower can disperse lung qi and resolve fluid retention, in whichE.sinicaStapf is more inclined to diffuse,AsarumsieboldiiMiq. is inclined to resolve fluid retention, Common Coltsfoot Flower is inclined to disperse; RHIZOMA PINELLIAE PREPARATUM,B.chinensis(L.) Redouté andMichaelmasdaisycan suppress the lung and resolve fluid retention, in which RHIZOMA PINELLIAE PREPARATUM is inclined to enliven the spleen and eliminate dampness,B.chinensis(L.) Redouté is inclined to benefit lung and dissolve phlegm,Michaelmasdaisyis inclined to direct qi downward to resolve phlegm;Schisandrachinensis(Turcz.)Baill can astringe lung and nourish qi;Poriacocos(Schw.) Wolf is sweet and dilute, and the prescriptions and drugs of seeping dampness reinforce each other, which are mainly for warming the lung and resolving fluid retention, directing qi downward to resolve phlegm. In order to better ensure the quality of hospital preparations and ensure the clinical efficacy, TLC and HPLC will be used to carry out the qualitative and quantitative study of the mixture, so as to provide higher quality assurance for hospital preparations.
2 Instruments and reagents
2.1 InstrumentsShimadzu High-Performance Liquid Chromatography (LC-20AT, Japan); KQ3200DE ultrasonic cleaner(Kun Shan Ultrasonic Instruments Co., Ltd.); AND electronic balance (GH-252, Japan); Xiangyi high-speed table centrifuge (H1650); Phenomenex phenyl-bonded silica column bonded by polar ether (4.6 mm×250 mm).
2.2 ReagentsThe reference material ofE.sinicaStapf(batch No.121240-201106), the reference material ofB.chinensis(L.) Redouté(batch No.121015-201507), the reference material ofC.maxima(Burm.) Merr. cv. Tomentosa (batch No.121211-201410) and the reference material of ephedrine hydrochloride(batch No.171241-201007) were all purchased from National Institutes for Food and Drug Control; Shema mixture (batch No.20191106, 20191203, 20200115, specification: 100mL/bottle) was provided by the preparation room of the First Affiliated Hospital of Guangxi University of Chinese Medicine; silica gel G plate (batch No.20190912), 1% NaOH silica gel G plate (batch No.20191016) and silica gel G254 plate (batch No.20191010) were purchased in Qingdao Marine Chemical Plant; the reagents, triethylamine, di-n-butylamine and phosphoric acid used in the thin layer were all analytical pure. Chromatographic methanol was provided by Fisher Company in the United States, and the test water was ultra pure water.
3 Methods and results
3.1 Thin-layer identification of Shema mixture[1-4]
3.1.1Thin-layer identification ofE.sinicaStapf. 10 mL of mixture (batch No.20191106) was taken out, evaporated to nearly dry, added with 20 mL methyl alcohol, treated with ultrasound for 10 minutes, evaporated the filtrate, and dissolved the residue with 1 mL methanol, finally, this was taken as the test solution. In addition, the reference substance of ephedrine hydrochloride was taken out and methanol was added to make a reference substance solution of 1 mg/mL. 5 μL of the test solution and 2 μL of the reference solution were taken out by quantitative capillary, and placed on the silica gel G thin layer plate prepared by the same 1% NaOH solution, trichloromethane-methanol-strong ammonia solution(20∶3.5∶0.5) was taken as the developing solvent, after developing, it was taken out, dried in the air, sprayed with ninhydrin test solution, heated it at 105 ℃ until the spots were clear, and inspected under sunlight. This can be seen in Fig.1.
3.1.2Thin-layer identification ofB.chinensis(L.) Redouté. 10 mL of mixture (batch No.20191106) was taken out, evaporated to nearly dry, added with 50 mL methyl alcohol, treated with ultrasound for 30 minutes, evaporated the filtrate, and dissolved the residue in 2 mL water, shaken and extracted twice with chloroform and 25 mL each time, discarded the chloroform layer, and then shaken and extracted twice with ethyl acetate and 25 mL each time, then combined with ethyl acetate solution, it was evaporated to dry, and 1 mL methanol was added to the residue to dissolve, finally this was taken as the test solution. In addition, the control medicinal material of 1 gB.chinensis(L.) Redouté was taken out, 60 mL of water was added, and decocted for 20 min, the supernatant was centrifuged and taken, extracted by shaking with chloroform, the preparation method was the same as this of the above test solution to prepare the reference medicinal material solution. 5 μL of the test solution and 2 μL of the reference drug solution were taken out by quantitative capillary, and placed on the same silica gel GF254thin layer plate, ethyl acetate-methanol-water(10∶1∶0.5) was taken as the developing solvent, after developing, it was taken out, dried in the air, and inspected under the ultraviolet lamp (245 nm). This can be seen in Fig.2.
3.1.3Thin-layer identification ofC.maxima(Burm.) Merr. cv. Tomentosa. 20 mL of mixture (batch No.20191106) was taken out, vibrated with ethyl acetate for 3 times, and 20 mL each time, combined with ethyl acetate, it was evaporated to dry, and the residue was dissolved with 1 mL of methanol, finally this was taken as the test solution. Hesperidin was taken out as the control, and methanol was added to make a 1 mg/mL control solution. 4 μL of test solution and 2 μL of control solution were drawn by quantitative capillary, and then placed on the same silica gel G thin layer plate, ethyl acetate-methanol-water- formic acid (100∶17∶13∶3.5) was used as the developing agent, after developing, it was taken out, dried in the air, sprayed with 1% AlCl3test solution, and inspected under the ultraviolet lamp (365 nm). This can be seen in Fig.3.
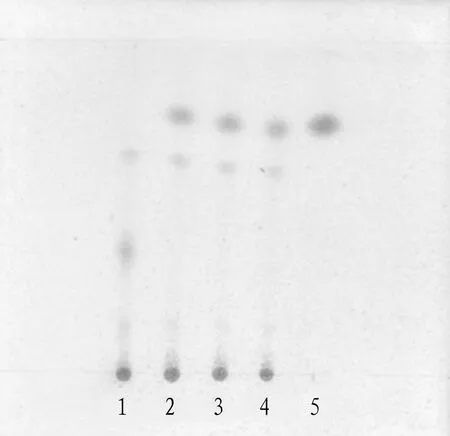
Note: 1. Negative sample; 2-4. Sample or test; 5. Ephedrine hydrochloride reference.
Fig.1 TLC identification of Ephedra sinica Stapf
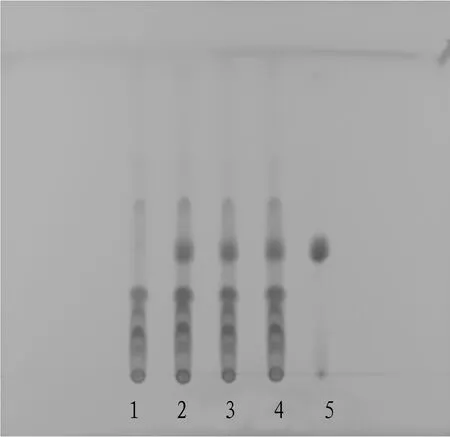
Note: 1. Negative sample; 2-4. Sample or test; 5. B. chinensis (L.) Redouté reference.
Fig.2 TLC identification of Belamcanda chinensis (L.) Redouté
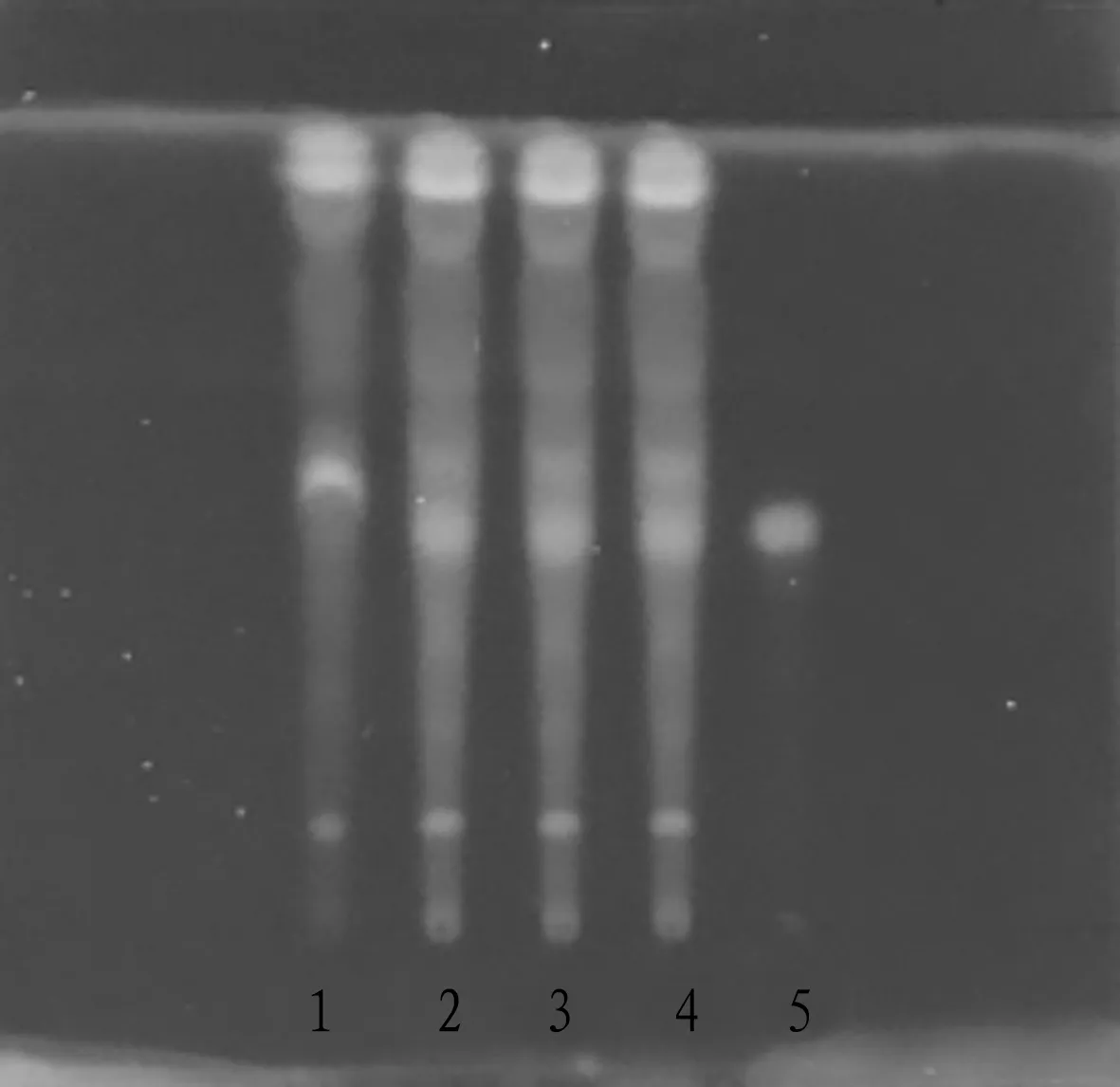
Note: 1. Negative sample; 2-4. Sample or test; 5. Hesperidin reference.
Fig.3 TLC identification of Citrus maxima (Burm.) Merr. cv. Tomentosa
3.2 Determination on the content of ephedrine hydrochloride[5-6]
3.2.1Chromatographic condition. Chromatographic column was Phenomenex phenyl-bonded silica column bonded by polar ether (4.6 mm×250 mm); the mobile phase was methanol-0.092% phosphoric acid solution (containing 0.04% triethylamine and 0.02% di-n-butylamine) (1.5∶98.5); flow rate was 1.0 mL/min, column temperature was 30 ℃; detection wavelength was 210 nm; injection volume was 10 μL.
3.2.2Preparation of solutions. (i) Reference solution. 10 mg of ephedrine hydrochloride reference was taken out, and 10.04 mg was accurately weighed, and put into a 10 mL volumetric flask, water was added to dissolve and dilute to the scale, then it was shaken well, finally a concentration of 1.004 mg/mL solution was prepared. (ii) Test solution. 5 mL of this product was taken out, and precisely weighed, and it was placed in a 500 mL round-bottom flask, 20 g of sodium chloride and 150 mL of 8% sodium hydroxide solution were added, mixed and distilled (flow rate was 1 mL/min), a 100 mL measuring flask containing 10 mL of 1 mL/L hydrochloric acid was used to catch the distillate to about 95 mL, and water was added and diluted to the scale, and shaken evenly, the solution was prepared. (iii) Negative solution.E.sinicaStapf was excluded, the sample ofE.sinicaStapf-deficient mixture according to the process of Shema mixture was prepared,E.sinicaStapf-deficient mixture was taken out to prepare negative sample solution ofE.sinicaStapf according to the preparation method of test solution under Section3.2.2above.
3.2.3Specific test. 10 μL of the control solution, the test solution and the negative sample solution under Section3.2.2were respectively absorbed, and injected into the liquid chromatograph, and determined according to the method under Section3.2.1. The results showed that there were chromatographic peaks in the same retention time of the sample solution and the control, and there were no corresponding peaks in the same retention time of the negative sample solution and the control, ephedrine hydrochloride reference was separated well without the interference of other components. This can be seen in Fig.4.
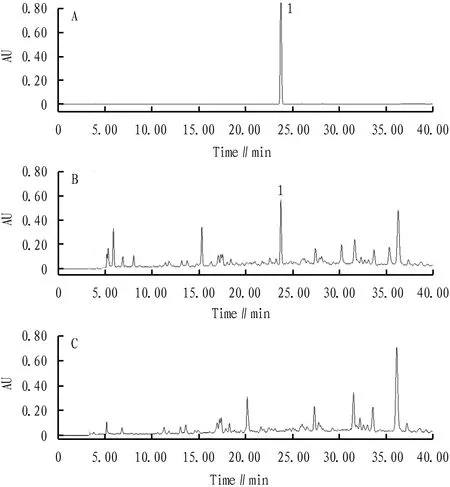
Note: A. Ephedrine hydrochloride reference; B. Shema mixture sample; C. Sample without Ephedra; 1. Ephedrine hydrochloride.
Fig.4 Liquid chromatogram of ephedrine hydrochloride in Shema mixture
3.2.4Investigation of linear relationship. 2, 4, 6, 8, 10, 12 and 14 μL of the control solution was precisely absorbed; according to the method under Section3.2.1, the peak area value (Y) was taken as the ordinate, and the sample injection amount of the reference substance(X) was taken as the abscissa, the linearity was investigated, then the linear regression equation was obtained: Ephedrine hydrochlorideY=1.2×106X+28 250 (R2=0.999 8). The results showed that ephedrine hydrochloride had a good linear relationship in the range of 2.01-14.06 μg.
3.2.5Precision test. The above-mentioned reference solution was precisely absorbed and injected 6 times continuously; the peak areas were recorded respectively according to the method under Section3.2.1, and theRSD(%) of the peak area of ephedrine hydrochloride was calculated, the results showed thatRSDwas 1.69%, indicating that the precision of the method was good.
3.2.6Stability test. The sample solution was prepared according to the preparation method of the sample solution, the peak area of the sample was respectively determined at 0, 2, 4, 8, 12 and 24 h after the preparation of the test solution according to the chromatographic conditions, the results showed thatRSDvalue of the peak area of ephedrine hydrochloride in Shema mixture was 2.20%; theRSDvalues were all less than 3.0%, indicating that the stability of the test solution was good within 24 h.
3.2.7Repeatability test. Six samples of Shema mixture of the same batch number (batch No.20191106) were weighed accurately, the sample solution was prepared according to the method under Section2.2.3, the peak area of the sample was measured according to the method under Section3.2.1, and the average concentration andRSDvalue of ephedrine hydrochloride in Shema mixture were calculated, the results showed that the average concentration of ephedrine hydrochloride was 13.88 μg/mL, the average value ofRSDwas 1.98%, which was less than 3.0%, indicating that the method had good repeatability.
3.2.8Average recovery test. Six samples of the known content of 5 mL Shema mixture (batch No.20191106) was measured accurately, and put into the conical flask with plug respectively, the corresponding ephedrine hydrochloride reference were added precisely, then prepared and filtered according to the preparation method of the reference solution, the subsequent filtrate was centrifuged at a high speed of 10 000 r/min for 10 min, the supernate was filtered with a 0.45 μm microporous membrane, the average recovery andRSDof ephedrine hydrochloride in Shema mixture were calculated according to the method under Section3.2.1. The results were shown in Table 1, indicating that the accuracy of this method was good.
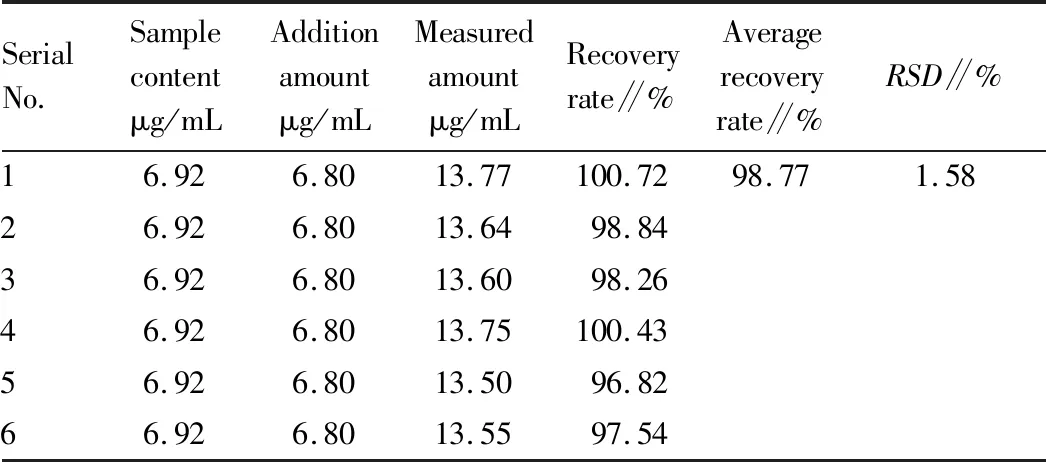
Table 1 Results of average recovery test of ephedrine hydrochloride (n=6)
3.2.9Determination on the content of three batches of samples. 5 mL of each batch of Shema mixture (batch No.20191106, 20191203, 20200115) was precisely measured, and the test solution was prepared in the same way as described in Section3.2.2above. 10 μL solution was precisely measured and injected into the high performance liquid chromatograph, the content of ephedrine hydrochloride, the effective component in ChineseEphedra, was calculated by the external standard method. The results were shown in Table 2.
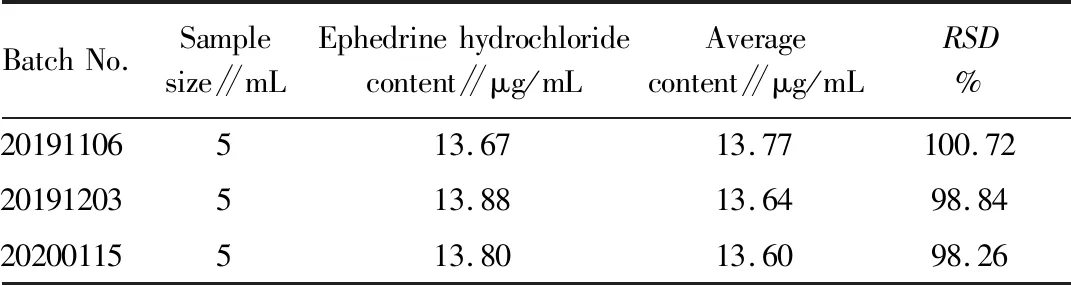
Table 2 Determination results of sample content of Shema mixture (n=3)
4 Discussions
As we all know, asthma is a kind of lung disease caused by respiratory tract inflammation, environmental factors, genetic factors and physical and chemical stimulation[7-8], it has the characteristics of recurrence, periodicity and not easy to cure in clinical practice. At present, western medicine can achieve the effect of temporarily controlling the symptoms by eliminating asthma and relieving spasm, but it is not ideal for the recovery of lung function and the control of inflammatory factors in the body, at the same time, the cost of treatment is high[9-10], resulting in heavy economic burden on patients. The Shema mixture studied in this project is a classic hospital preparation developed on the basis of the theory of traditional Chinese medicine and giving full play to the advantages of traditional Chinese medicine, it has been used in the First Affiliated Hospital of Guangxi University of Chinese Medicine for many years, and has a definite curative effect and reasonable price in the treatment of asthma. However, due to the early registration requirements of hospital preparations, this quality standard has not been systematically studied. As the basic research of this mixture, this study has established the qualitative identification of related medicinal materials and the quantitative analysis of ephedrine hydrochloride in ChineseEphedra, the sovereign drug, however, due to the limitations of conditions, a higher quality standard system has not been developed; the next step is to study the quality standard of Shema mixture based on quality markers, and establish a comprehensive evaluation system more in line with modern Chinese medicine.
- Medicinal Plant的其它文章
- Optimization of Quality Control Method and Ethanol Extraction Process of Psoralen and Bergapten in Ficus pandurata
- Optimization of Processing Technology for Roasted Licorice with Water by Orthogonal Method
- Optimization of Extraction Process and Antioxidant Activity of Polysaccharide in Embelia parviflora Wall. by RSM
- Biopharmaceutical Identification of Adventitious Buds and Root Bark of Aralia elata Seem.
- Systematic Review and Meta-analysis of Efficacy of Banxia Xiexin Decoction for Treatment of Bile Reflux Gastritis
- Effects of Dachengqi Decoctions Made from Raw Rhubarb and Vinegar-processed Rhubarb on Levels of Endotoxin, NO and TNF-α in Serum

