Marine natural products with anti-inflammation effects
Mei-Mei Zhao, Ke-Wu Zeng*
Marine natural products with anti-inflammation effects
Mei-Mei Zhao1, Ke-Wu Zeng1*
1State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing 100191 China.
The ocean possesses a complex setting with high pressure, high salinity, oligotrophy, low temperature and weak illumination conditions. To survive and evolve in such harsh surroundings, marine organisms metabolize a series of chemicals known as secondary metabolites which indicate structural and functional diversity to adapt interspecific survival competition. During recent decades, the anti-inflammatory property of marine natural products has come under scrutiny as inflammation involves in the vast majority of diseases. Correspondingly, a myriad of marine bioactive molecules including terpenes, polypeptides, polysaccharides, sterols and many others may bring a new insight into inflammation therapies with multifarious sources and minimal side effects. And a better understanding of their mechanisms of anti-inflammation may lead to better treatments for numerous diseases. Herein, the research progress of marine-derived anti-inflammation compounds and the relevant mechanisms were reviewed, to provide a basis for the research and development of anti-inflammatory marine drugs.
Marine natural products, Anti-inflammation, Mechanism of action, Drug target, Drug development
The present review reviewed the research progress of marine-derived anti-inflammation compounds including terpenes, polypeptides, polysaccharides, sterols, etc. and the relative anti-inflammatory mechanisms, to bring a new insight into inflammation therapies with multifarious sources and minimal side effects.
China has a long history of marine natural products application.The earliest Chinese pharmacopoeia,(; Eastern Han dynasty of China,200 C.E.), reported 12 kinds of marine medicines, including Haizao (seaweed;), Haige (sea clams;Gmelin), Muli (animal shell;) and Wuzeigu (cuttlefish bone;). Moreover, more than 100 traditional Chinese medicines contained in(;Ming dynasty of China, 1578 C.E.) were derived from marine. Recently, increasing numbers of marine-derived products have been reported, including 144 kinds of marine medicines collected in(1977) and more than 600 kinds of marine medical products collected in(1988). Haizao (seaweed;)is a traditional Chinese medicine. In the ancient literature(Xia and Shang dynasties of China, 1,600 B.C.E.), Haizao (seaweed;) was reported to prevent tumorigenesis like thyrophyma. With the development of modern medicine, the novel mannose oligosaccharide diacid derived from brown algae, a kind of seaweed, namely GV-971, has been conditionally approved for market in China, for the treatment of mild to moderate Alzheimer's disease.

Background
Numerous studies have showed that inflammation is a crucial risk factor driving many diseases, such as cancer, neurodegeneration, autoimmunity and infections [1, 2]. Therapeutic agents for inflammatory diseases mainly include non-steroidal and steroidal [3]. Traditional nonsteroidal anti-inflammatory drugs are restricted with adverse gastrointestinal side effects resulted from inhibition of cyclo-oxygen-ase-1 [4] while selective cyclo-oxygen-ase-2 inhibitors may raise cardiovascular toxicity as well as kidney injury [5, 6]. The toxic side effects of long-term usage of glucocorticoids involving immunodeficiency and hormonal disturbances also limit their clinical value [7], let alone the fancy price of biologicals. Therefore, alternative or additional strong agents are needed. Recently, marine-derived natural products with anti-inflammatory activities gradually raise concerns and may provide a promising new direction for novel anti-inflammatory agents with multifarious sources and minimal side effects.
The ocean represents the largest habitat of the earth covering over 70% surface and harbors an enormous number of marine organisms, whose living environments are quite different from land-based counterparts, including high pressure, high salinity, oligotrophy, low temperature and weak illumination. These differences then constitute remarkable features of marine organisms on metabolism, existence mode, message passing and adaptability. Therefore, the distinctive secondary metabolism and enzymic mechanisms make marine organisms produce massive bioactive molecules, and become one of the most promising fields for new drug exploration.
China has a long history of marine natural products application from the ancient Chinese records(Xia and Shang dynasties of China, 1,600 B.C.E.). In this ancient literature, Haizao (seaweed;) was reported to prevent tumorigenesis like thyrophyma. The earliest Chinese pharmacopoeia,(; Eastern Han dynasty of China,200 C.E.), reported 12 kinds of marine medicines, including Haizao (seaweed;), Haige (sea clams;Gmelin), Muli (animal shell;) and Wuzeigu (cuttlefish bone;). Moreover, among1,900 traditional Chinese medicines (TCMs) contained in(;Ming dynasty of China, 1578 C.E.), more than 100 are derived from marine. Recently, increasing numbers of marine-derived products have been reported, including 144 kinds of marine medicines collected in(1977) and more than 600 kinds of marine medical products collected in(1988).
Hetun (puffer fish) is a TCM firstly reported in(; Xia and Shang dynasties of China, 1,600 B.C.E.). The provenance of its medical use comes from(; Five dynasties of China, 907–979 C.E.) as a natural toxin. The venom of Hetun (puffer fish) was first discovered in the filefish byChun Tahara in 1909 and was named as tetrodotoxin (TTX). In 1998,Wexus technologies successfully developed tetrodinfrom TTX for drug addiction. It may be originated from the TCM principle of “combating poison with poison”. With the development of modern pharmacological research, TTX has been found as a potentsodium channel blockerfor neuralgia treatment, local anesthesia and antispasmodic[8].
The real marine drug development started with the discovery of cytarabine and vidarabine. As synthetic nucleosides from, a Caribbean sponge, they were successively marketed by 1970s, and nowadays are known for drugs against cancer and virus infection respectively [9]. More importantly,mannose oligosaccharide diacid (GV-971)is an oceanic oligosaccharide molecule derived from brown algae, a TCM called Haizao (seaweed;). The first recorded report of the use of Haizao (seaweed;)is from(; Song dynasty of China, 1057–1060 C.E.). With the development of modern medicine, this novel drug derived from Haizao (seaweed;), namely GV-971 has been conditionally approved for market in China, for the treatment of mild to moderate Alzheimer's disease[10–12].
During recent decades, numerous natural products from marine are exhibiting novel chemical structures and characteristic biological activities. For example, some marine derived terpenoids, polypeptides, alkaloids and many others have offered great opportunity in discovery of bioactive lead compounds that may be developed into drugs for inflammatory disorders. In addition, marine natural products also indicate promising strategies for improving the anti-inflammatory activity of clinical drugs based on structural modification of existing natural products.
Here, we introduce some novel marine natural products and their synthetic analogues with anti-inflammatory activity. The possible molecular mechanisms behind the biological effects are also presented.
Terpenoids
Since the discovery of sponge-derived manoalide (Figure 1A), which is the first identified marine bioactive compound with phospholipase A2 (PLA2) inhibitory effect[13], a variety of manoalide analogues or marine PLA2 inhibitors have been discovered and tested for their anti-inflammatory activities [14]. For example, a class of diterpenes extracted from Mediterranean spongeis named Gracilins,among which the nor-diterpene Gracilin A(Figure 1B) is reported to be an efficient inhibitor of PLA2 with a 69% inactivation efficiency [15] and have the most potent immunosuppressive and neuroprotective activities compared with other gracilins [16]. Gracilin L (Figure 1C) and its synthetic analogues also have been demonstrated for anti-inflammation properties via blocking the translocation of NF kappa-B to the nucleus and activating nuclear factor erythroid-2-related factor 2 (Nrf2) pathway [17]. Therefore, natural and synthetic gracilins hold promise in the treatment of inflammation-driven disorders.
Moreover, cembranoid diterpenes are isolated from Bornean softcoralsp. As a TCM, coral can also be found in ancient book(; Tang dynasty of China,659 C.E.). In the study, sinularolide F (Figure 1D)and denticulatolide (Figure 1E) show significant suppressive effect on NO, interleukin-1β (IL-1β), and interleukin-6 (IL-6) that are involved in down-regulating inducible nitric oxide synthase expression [18], suggesting that cembranoids could be promising candidates for anti-inflammatory drugs.
Polypeptides
Marine polypeptides are specific protein fragments that in addition to serving as sources of nitrogen and amino acids have abundant pharmacological functions. In particular, in the screening and efficacy evaluation of anti-inflammatory substances, marine host defense peptides (HDPs) havegained popularity in recent years.HDPs, also called antibacterial peptides, are key players for innate immune system. Natural HDPs with anti-inflammatory and immunoregulatory activities are alkaline polypeptides which consist of about 30 amino acids. Epinecidin-1 (Figure 2A)extracted from orange-potted grouper () has been validated to activate Th2 cell response againstinfection by enhancing significant secretion of immunoglobulin G1 [19]. Further studies have found that epinecidin-1 could disrupt the affinity between lipopolysaccharide (LPS) and LPS binding protein, and inhibit Toll-like receptor 4endocytosis, contributing to suppression of LPS-induced reactive oxygenspecies signaling and subsequent pro-inflammatory mediator secretion. Overall, these findings advocate that epinecidin-1may be a potential drug candidate for inflammation-related pathologies[20].
Moreover, hydrostatin-TL1 (H-TL1) (Figure 2B), an antibacterial peptide from the venom of, a Chinese medicine recorded in(1979), has been widely studied for its anti-inflammatory activity. It is found to inhibit the interaction between tumor necrosis factor alpha (TNF-α) and TNF receptor 1. Further, in vivo tests show that H-TL1 can attenuate dextran sodium sulfate- andLPS- induced inflammation [21]. In view of its notableanti-inflammation action both in vitro and in vivo, H-TL1 is a potential peptide for drug discovery to improve TNF-α-associated inflammatory diseases.
Lectin
Lectin is a kind of natural glycoprotein, which shows pivotal effect on immunocyte recognition and adhesion. Recent reports reveal that some marine-derived lectins show potent inhibitory effect on inflammatory response. Lectin from green seaweedvar.has been revealed to show a significant inhibitory effect on rat temporomandibular joint inflammation induced by zymosan with the reduction of IL-1β and TNF-α[22]. Moreover,var.also inhibits IL-6 and cyclo-oxygen-ase-2 expression and halts edema formation around rat paws induced by carrageenan as well as neutrophilic infiltration [23]. In addition, lectin obtained from the red seaweedhas also been reported to possess an anti-inflammatory activity. Its major mechanism of action is attributed to the suppression of TNF-α and IL-1β production and thus inhibition of neutrophilinfiltration to inflammation sites [24].
Alkaloids
Asperversiamides is a kind of prenylated indole alkaloid isolated from the oceanic fungus. Recent studies have elucidated their anti-inflammatory activities. In particular, aperversiamide G (Figure 2A)exhibited the most significant inhibitory effectagainst inducible nitric oxide synthase and NO release in LPS-induced Raw264.7 cells [25]. Given the infrequency oflinearly fused prenylated indole alkaloids, the discovery of this type not only enriches the chemical diversity of alkaloids, but also offers insights into the development of this skeleton-like agent against inflammatory disorders.
(10Z)-debromohymenialdisine (Figure 2B) is an active pyrrole alkaloid from marine spongesp. It is reported that (10Z)-debromohymenialdisine significantly facilitate thenuclear translocation of Nrf2 and subsequent up-regulation of heme oxygenase-1 (HO-1) expression, supporting the therapeutic potentialof debromohymenialdisine to treat inflammatoryconditions [26].
Sterols
Fucosterol (Figure 3A), a sterol mostly existed in brownalgaeincluding,hasbeen proven to provide beneficial effects against neuroinflammation induced by amyloid β-protein. Itcould raise a dose-dependent inhibitory effect on pro-inflammatory cytokines namely interleukins (IL-6, IL-1β), TNF-α, and prostaglandinE2in LPS- or amyloid β-protein-induced microglial cells, suggesting that fucosterol may represent a promising drug candidate for Alzheimer’s disease, a multifactorial neurodegenerative disorder [27].
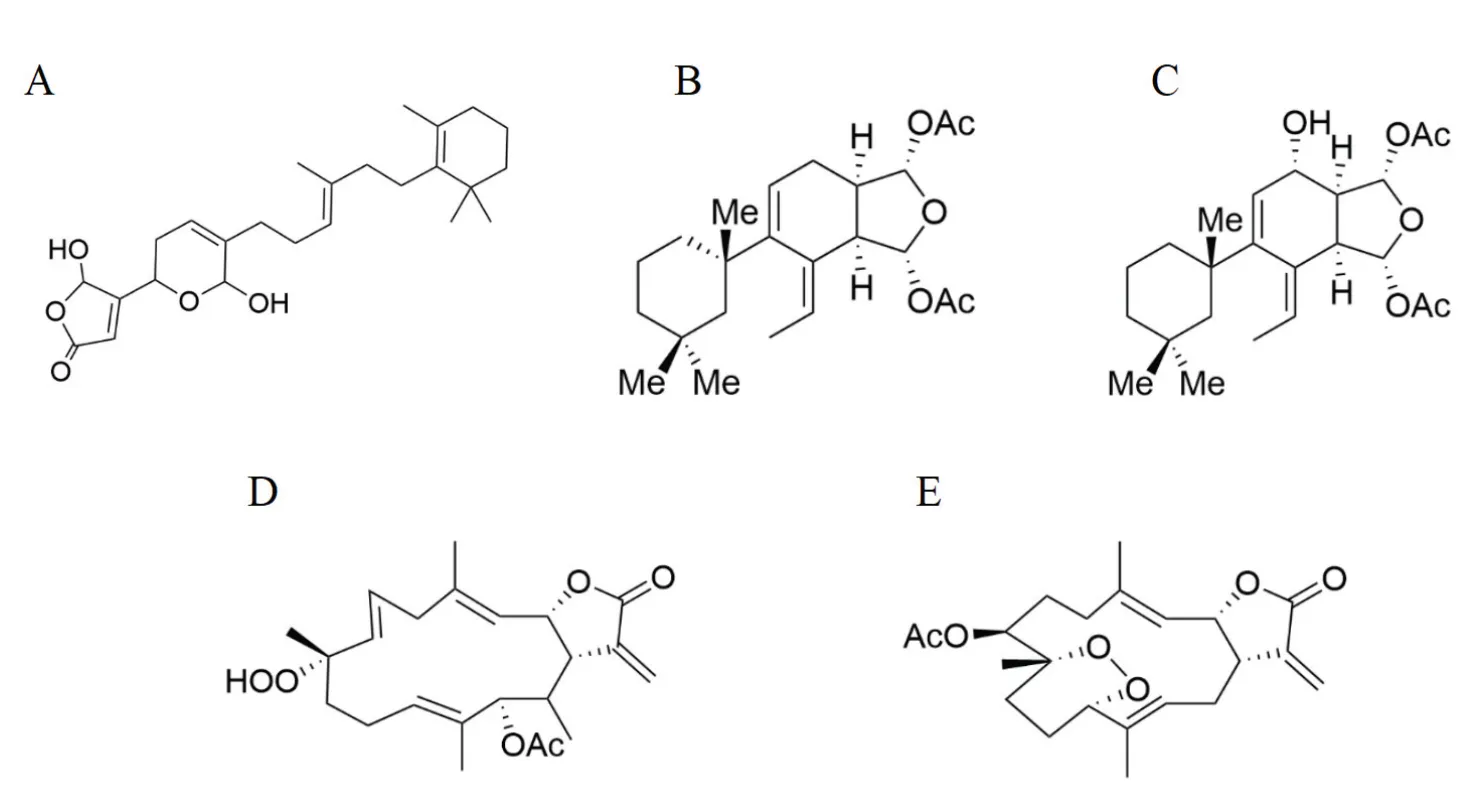
Figure 1 Structures of manoalide (A), gracilins (B: gracilin A, C: gracillin C) and cembranoid diterpenes (D: sinularolide F, E: denticulatolide)
Figure 2 Structures of aperversiamide G (A) and (10Z)-debromohymenialdisine (B)
Moreover, a kind of new polyoxygenated steroids, namely michosterols have recently been isolated from the soft coral. Of these compounds, michosterol A (Figure 3B) exerts moderate anti-inflammatory activities in the preventionof superoxide anion generation and elastase release in the N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B- stimulated neutrophils [28]. This finding highlights michosterol A as a promising therapeutic agent for inflammation.
Polysaccharides
As prolific primary metabolites of marine organisms, sulfated polysaccharides have been reported to exert anti-inflammatory activity through various mechanisms including HO-1 pathway and mitogen-activated protein kinase pathway, which were evidenced in carrageenan-induced rats paw edema [29] and LPS-induced RAW264.7 cells [30], respectively. Fucoidan (Figure 4A), a family of sulfated polysaccharides with enriched L-fucose, was primarily found in brown algae and firstly marketed for chronic renal failure treatment. Further studies revealed that fucoidan fromhad a renoprotective effect on diabetic kidney disease through repression of transforming growth factor-β1 pathway, which is against oxidative stress and inflammation [5]. Furthermore, after degradation, a highly sulfated fraction called low-molecular-weight fucoidan was also produced [31], and could effectively ameliorate inflammatory response by down-regulating IL-6 and up-regulating IL-10 levels [32, 33]. In addition, glucosamine sulfate (Figure 4B), secondary compound of chitin with clear structure has been renowned for controlling osteoarthritis symptoms. Recent study suggests that one of its anti-inflammatory targets in macrophages is NOD-like receptors-3 inflammasome [34], a central signaling hub that regulates innate immunity and subsequent caspase-1 activation [35, 36],implying apotentialforclinical strategy of glucosamine sulfate in treatment of inflammatory diseases driven by NOD-like receptors-3 inflammasome.
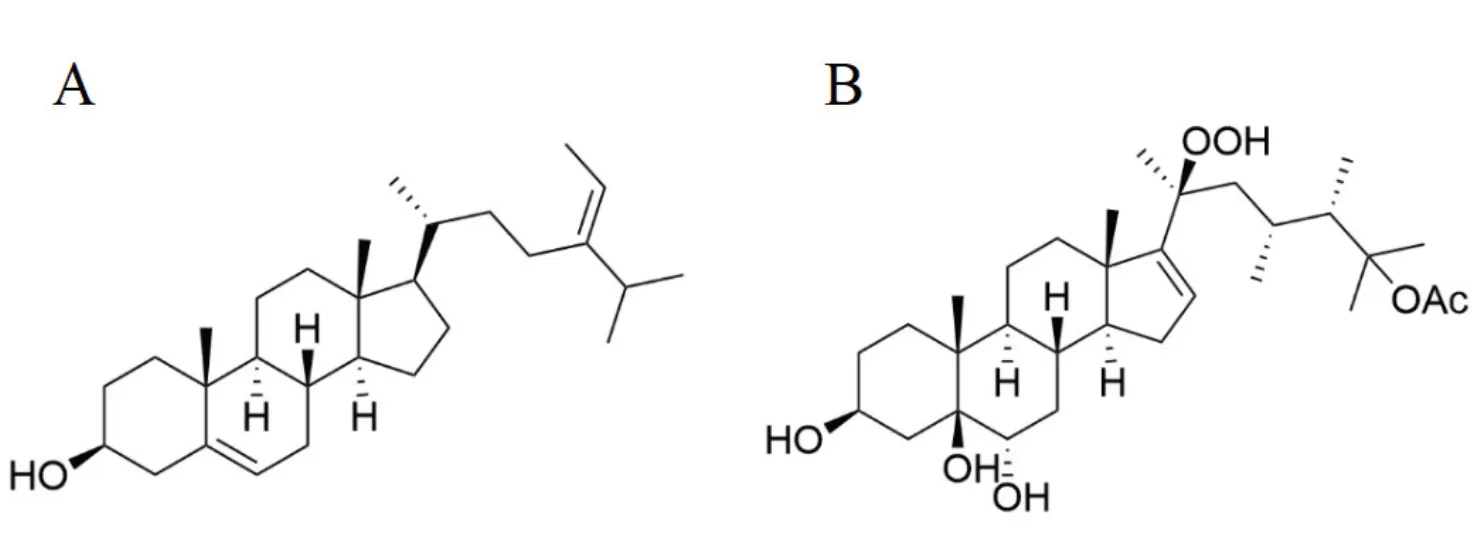
Figure 3 Structures of fucosterol (A) and michosterol A (B)
Figure 4 Structures of fucoidan (A) and glucosamine sulfate (B). (): Structure of type 1 fucoidan molecules with a backbone of (1 → 3)-linked α-l-fucopyranose residues. (): Structure of type 2 fucoidan molecules with a backbone alternating (1 → 3)-linked α-l-fucopyranose and (1 → 4)-linked α-l-fucopyranose residues. The ‘R’ can be a monosaccharide or a sulfate group.
Astaxanthin
Astaxanthin (Figure 5), a type of lipid-soluble xanthophyll carotenoid, was firstly extracted from(European lobster) in 1938 and is abundant in green microalga[37]. As a proprietary prodrug, the anti-thrombotic activity of CDX-085 has been ascribed to anti-oxidant and anti-inflammatory functions [38, 39]. Numerous reports have demonstrated that pretreatment with astaxanthin can prevent LPS-induced productions of pro-inflammatory cytokines via Toll-like receptor-4 and subsequent activation of downstream signaling pathways including NF kappa beta, mitogen-activated protein kinases, signal transducer and activator of transcription and Nrf2/HO-1 [40–43]. Further reports show that astaxanthin treatment in obese mice could alter splenic macrophages sensitivity to LPS by stimulating macrophage phenotype switching from M1 to M2 [44]. Moreover, recent studies pay more attention on peroxisome proliferator-activated receptors-γ (PPAR-γ) primarily found in adipose tissues. Intriguingly, there are conflicting views on the anti-inflammation role of astaxanthin in PPAR-γ expression [45]. Astaxanthin has been reported to activate PPAR-γ and antioxidant target gene catalase to inhibit reactive oxygenspecies-induced mitochondrial dysfunction [46]. On the contrary, other studies have indicated that astaxanthin may act as a PPAR-γ antagonist [45]. Thus, further comprehensive studies are necessary to better explore the therapeutic potential of astaxanthin and advance it further as a PPAR modulator.
Others
Other marine-derived compounds like mannose oligosaccharide diacid (GV-971) (Figure 6), an oceanic oligosaccharide isolated from brown algaehas been reported to provide significant cognitive benefits via harnessing neuroinflammation [12, 47]. Importantly, it could reshapegut microbiota, decrease the associated phenylalanine/isoleucine accumulation, and reduce Th1-related neuroinflammation in the brain [10].Furthermore, numerous other compounds or derivatives including lipids, polyketides and phenols that are distributed in marine organisms also exhibit promising anti-inflammation effects. More in-depth studies are still in need of clarifying novel anti-inflammatory mechanisms and toxicological characteristics of these compounds for their possible applications in clinical trials.
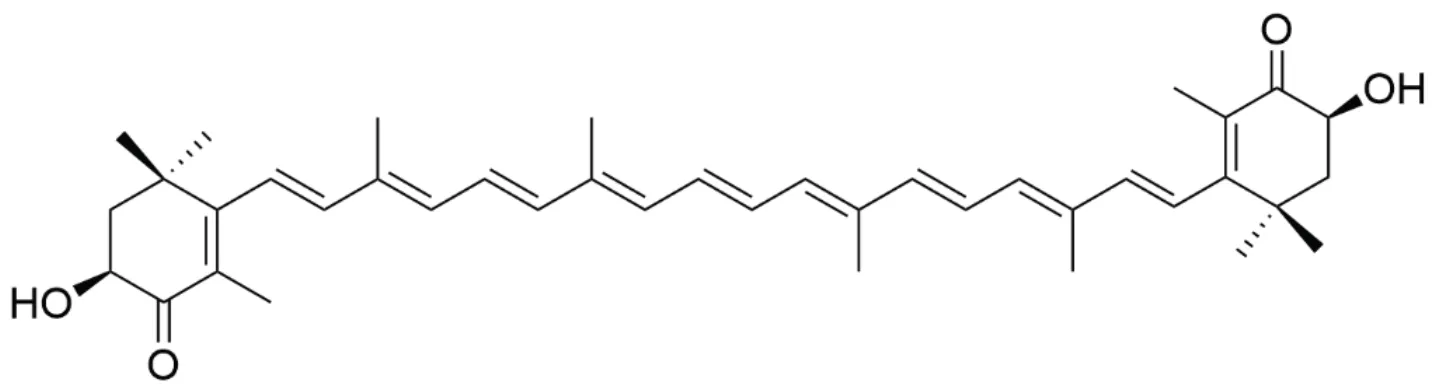
Figure 5 Structure of astaxanthin
Figure 6 Structure of mannose oligosaccharide diacid (GV-971)
Conclusion
As blue drug storage, ocean holds vast opportunities for exploitation of bioactive natural products with potential anti-inflammatory effects. So far, 14 marine innovative drugs have been approved, along with about 30,000 marine compounds with novel skeletons and diverse activities having been identified. Although cancer and cardiovascular diseases may stay the main indications, an extension of the spectrum of indications can be expected. For instance, a variety of marine compounds share anti-inflammatory function. With their broad application prospects, some anti-inflammatory agents currently used in clinical applications are expected to be partly replaced or concomitantly used with marine-derived natural products for higher safety and less toxicity[48]. Besides, when compared with their analogues from terrestrial origins, marine natural products have superiority in synergistic action of diverse substances simultaneously existing in certain marine organisms, and thus more influential [49]. For instance, marine algae could yield a rich array of anti-inflammatory compounds including terpenes, polyphenols, sulfated polysaccharides, fatty acids, proteins and several other molecules [50], indicating a potential for marine natural products as templates for the discovery and development of anti-inflammatory agents with compelling synergistic effect.
Additionally, with the ongoing advancement in marine pharmacy, new tools have been employed, like metabolomics, to examine marine natural products from different perspectives [51].
Taken together, aforementioned existing studies indicate that marine-derived bioactive compounds may function to treat inflammation by multiple pathways and on multiple targets, whereas the underlying mechanisms especially the interaction between signal transduction pathways is still unclear at present. This review summarized the latest research progress of the marine natural products with anti-inflammatory activity and related inflammatory pathways to provide a scientific basis of marine natural products research and development with respect to inflammation therapies.
1. Divella R, De Luca R, Abbate I, et al. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer 2016, 7: 2346–2359.
2. Netea MG, Balkwill F, Chonchol M, et al. A guiding map for inflammation. Nat Immunol 2017, 18: 826–831.
3. de Anda-Jáuregui G, Guo K, McGregor BA, et al. Exploration of the anti-inflammatory drug space through network pharmacology: applications for drug repurposing. Front Physiol 2018, 9: 1–12.
4. Moore N, Duong M, Gulmez SE, et al. Pharmacoepidemiology of non-steroidal anti-inflammatory drugs. Therapie 2019, 74: 271–277.
5. Hu SW, Wang JH, Wang JF, et al. Renoprotective effect of fucoidan fromin streptozotocin/high fat diet-induced type 2 diabetic mice. J Funct Foods 2017, 31: 123–130.
6. Rothschild BM. Maligned non-steroidal anti-inflammatory drugs: misunderstanding of their safety profile in patients with renal insufficiency. World J Rheumatol 2018, 8: 1–4.
7. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf 2016, 15: 457–465.
8. Makarova M, Rycek L, Hajicek J, et al. Tetrodotoxin: history, biology, and synthesis. Angew Chem Int Ed Engl 2019, 58: 18338–18387.
9. Mayer AM, Glaser KB, Cuevas C, et al. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci 2010, 31: 255–265.
10. Wang X, Sun G, Feng T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Res 2019, 29: 787–803.
11. Huang LK, Chao SP, Hu CJ. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci 2020, 27: 1–13.
12. Dong Y, Li X, Cheng J, et al. Drug development for Alzheimer's disease: microglia induced neuroinflammation as a target? Int J Mol Sci 2019, 20: 1–24.
13. Lombardo D, Dennis EA. Cobra venom phospholipase A2 inhibition by manoalide. A novel type of phospholipase inhibitor. J Biol Chem 1985, 260: 7234–7240.
14. Ong WY, Farooqui T, Kokotos G, et al. Synthetic and natural inhibitors of phospholipases A2: their importance for understanding and treatment of neurological disorders. ACS Chem Neurosci 2015, 6: 814–831.
15. Leirós M, Alonso E, Rateb ME, et al. Gracilins:-derived promising compounds for Alzheimer disease. Neuropharmacology 2015, 93: 285–293.
16. Alvariño R, Alonso E, Abbasov ME, et al. Gracilin A derivatives target early events in Alzheimer's disease: in vitro effects on neuroinflammation and oxidative stress. ACS Chem Neurosci 2019, 10: 4102–4111.
17. Gegunde S, Alfonso A, Alonso E, et al. Gracilin-derivatives as lead compounds for anti-inflammatory effects. Cell Mol Neurobiol 2020, 40: 603–615.
18. Kamada T, Kang MC, Phan CS, et al. Bioactive cembranoids from the soft coral genussp. in Borneo. Mar Drugs 2018, 16: 1–14.
19. Lee SC, Pan CY, Chen JY. The antimicrobial peptide, epinecidin-1, mediates secretion of cytokines in the immune response to bacterial infection in mice. Peptides 2012, 36: 100–108.
20. Su BC, Huang HN, Lin TW, et al. Epinecidin-1 protects mice from LPS-induced endotoxemia and cecal ligation and puncture-induced polymicrobial sepsis. Biochim Biophys Acta Mol Basis Dis 2017, 1863: 3028–3037.
21. Wang N, Huang Y, Li A, et al. Hydrostatin-TL1, an anti-inflammatory active peptide from the venom gland ofin the South China Sea. Int J Mol Sci 2016, 17: 1–15.
22. da Conceição Rivanor RL, Chaves HV, do Val DR, et al. A lectin from the green seaweedreduces mechanical hyper-nociception and inflammation in the rat temporomandibular joint during zymosan-induced arthritis. Int Immunopharmacol 2014, 21: 34–43.
23. de Queiroz IN, Quinderé AL, Rodrigues JA, et al. Dual effects of a lectin from the green seaweedvar.on inflammatory mediators in classical models of inflammation. Inflamm Res 2015, 64: 971–982.
24. Fontenelle TPC, Lima GC, Mesquita JX, et al. Lectin obtained from the red seaweed: secondary structure and anti-inflammatory activity in mice. Int J Biol Macromol 2018, 112: 1122–1130.
25. Li H, Sun W, Deng M, et al. Asperversiamides, linearly fused prenylated indole alkaloids from the marine-derived fungus. J Org Chem 2018, 83: 8483–8492.
26. Lee SM, Kim NH, Lee S, et al. (10Z)-debromohymenialdisine from marine spongesp. regulates intestinal inflammatory responses in co–culture model of epithelial Caco-2 cells and THP-1 macrophage cells. Molecules 2019, 24: 1–15.
27. Gan SY, Wong LZ, Wong JW, et al. Fucosterol exerts protection against amyloid beta-induced neurotoxicity, reduces intracellular levels of amyloid beta and enhances the mRNA expression of neuroglobin in amyloid beta-induced SH-SY5Y cells. Int J Biol Macromol 2019, 121: 207–213.
28. Huang CY, Tseng WR, Ahmed AF, et al. Anti-inflammatory polyoxygenated steroids from the soft coral. Mar Drugs 2018, 16: 1–10.
29. Ribeiro NA, Abreu TM, Chaves HV, et al. Sulfated polysaccharides isolated from the green seaweedplays antinociceptive and anti-inflammatory activities in a way dependent on HO-1 pathway activation. Inflamm Res 2014, 63: 569–580.
30. Sanjeewa KK, Fernando IP, Kim EA, et al. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweedin RAW 264.7 cells. Nutr Res Pract 2017, 11: 3–10.
31. Xu Y, Zhu W, Wang T, et al. Low molecule weight fucoidan mitigates atherosclerosis in ApoE (-/-) mouse model through activating multiple signal pathway. Carbohydr Polym 2019, 206: 110–120.
32. Hwang PA, Phan NN, Lu WJ, et al. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr Res 2016, 60: 1–9.
33. Xu Y, Xu J, Ge K, et al. Anti-inflammatory effect of low molecular weight fucoidan fromon atherosclerosis in apoE-knockout mice. Int J Biol Macromol 2018, 118: 365–374.
34. Chiu HW, Li LH, Hsieh CY, et al. Glucosamine inhibits IL-1beta expression by preserving mitochondrial integrity and disrupting assembly of the NLRP3 inflammasome. Sci Rep 2019, 9: 1–13.
35. Lamkanfi M, Dixit VM. In Retrospect: the inflammasome turns 15. Nature 2017, 548: 534–535.
36. Wen H, Ting JP, O'Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat Immunol 2012, 13: 352–357.
37. Choi CI. Astaxanthin as a peroxisome proliferator-activated receptor (PPAR) modulator: its therapeutic implications. Mar Drugs 2019, 17: 1–12.
38. Khan SK, Malinski T, Mason RP, et al. Novel astaxanthin prodrug (CDX-085) attenuates thrombosis in a mouse model. Thromb Res 2010, 126: 299–305.
39. Ryu SK, King TJ, Fujioka K, et al. Effect of an oral astaxanthin prodrug (CDX-085) on lipoprotein levels and progression of atherosclerosis in LDLR(-/-) and ApoE(-/-) mice. Atherosclerosis 2012, 222: 99–105.
40. Farruggia C, Kim MB, Bae M, et al. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J Nutr Biochem 2018, 62: 202–209.
41. Han JH, Lee YS, Im JH, et al. Astaxanthin ameliorates lipopolysaccharide-induced neuroinflammation, oxidative stress and memory dysfunction through inactivation of the signal transducer and activator of transcription 3 pathway. Mar Drugs 2019, 17: 1–18.
42. Li MY, Sun L, Niu XT, et al. Astaxanthin protects lipopolysaccharide-induced inflammatory response inthrough inhibiting NF-kappaB and MAPKs signaling pathways. Fish Shellfish Immunol 2019, 86: 280–286.
43. Niu T, Xuan R, Jiang L, et al. Astaxanthin induces the Nrf2/HO-1 antioxidant pathway in human umbilical vein endothelial cells by generating trace amounts of ROS. J Agric Food Chem 2018, 66: 1551–1559.
44. Kim B, Farruggia C, Ku CS, et al. Astaxanthin inhibits inflammation and fibrosis in the liver and adipose tissue of mouse models of diet-induced obesity and nonalcoholic steatohepatitis. J Nutr Biochem 2017, 43: 27–35.
45. Jia Y, Kim JY, Jun HJ, et al. The natural carotenoid astaxanthin, a PPAR-alpha agonist and PPAR-gamma antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Mol Nutr Food Res 2012, 56: 878–888.
46. Kim SH, Lim JW, Kim H. Astaxanthin inhibits mitochondrial dysfunction and interleukin-8 expression in-Infected gastric epithelial cells. Nutrients 2018, 10: 1–17
47. Cummings J, Lee G, Ritter A, et al. Alzheimer's disease drug development pipeline: 2019. Alzheimers Dement (N Y) 2019, 5: 272–293.
48. Shinde P, Banerjee P, Mandhare A. Marine natural products as source of new drugs: a patent review (2015-2018). Expert Opin Ther Pat 2019, 29: 283–309.
49. D'Orazio N, Gammone MA, Gemello E, et al. Marine bioactives: pharmacological properties and potential applications against inflammatory diseases. Mar Drugs 2012, 10: 812–833.
50. Fernando IPS, Nah JW, Jeon YJ. Potential anti-inflammatory natural products from marine algae. Environ Toxicol Pharmacol 2016, 48: 22–30.
51. Sogin EM, Puskás E, Dubilier N, et al. Marine metabolomics: a method for nontargeted measurement of metabolites in seawater by gas chromatography-mass spectrometry. mSystems 2019, 4: 1–14.
:
Ke-Wu Zeng wrote the initial draft of the paper. Ke-Wu Zeng and Mei-Mei Zhao wrote the manuscript. All authors contributed and approved the final version of the manuscript.
:
This work was financially supported by National Key R&D Project of China (No. 2019YFC1708902, 2019YFC1711000) and National Natural Science Foundation (No. 81973505, 81773932).
:
TTX, tetrodotoxin; TCM, traditional Chinese medicine; PLA2, phospholipase A2; Nrf2, erythroid-2-related factor 2; HDPs, host defense peptides; H-TL1, hydrostatin-TL1; TNF-α, tumor necrosis factor alpha; LPS, lipopolysaccharide; HO-1, heme oxygenase-1; PPAR-γ, peroxisome proliferator-activated receptors-γ; IL-1β, interleukin-1β, IL-6, interleukin-6.
:
The authors declare that they have no conflict of interest.
:
Mei-Mei Zhao,Ke-Wu Zeng. Marine natural products with anti-inflammation effects. Traditional Medicine Research 2020, 5 (4): 252–260.
:Xiao-Hong Sheng.
:10 March 2020,
2 April 2020,
: 23 April 2020
Ke-Wu Zeng. State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, No.5 Yiheyuan Road, Haidian District, Beijing 100191, China. E-mail: ZKW@bjmu.edu.cn.
10.12032/TMR20200417173
 Traditional Medicine Research2020年4期
Traditional Medicine Research2020年4期
- Traditional Medicine Research的其它文章
- Dissecting the underlying pharmaceutical mechanism of Danggui Buxue decoction acting on idiopathic pulmonary fibrosis with network pharmacology
- Acupuncture and/or moxibustion for the treatment of lumbar disc herniation: quality assessment of systematic reviews
- Can Yin-Chai-Xiao-Du decoction be useful of COVID-19? the mechanism research based on network pharmacology
- Efficacy of Xuebijing injection for the treatment of coronavirus disease 2019 via network pharmacology
- The selection rules of acupoints and meridians of traditional acupuncture for postoperative nausea and vomiting: a data mining-based literature study
- The advances of traditional Chinese medicine in the treatment of liver diseases in 2019
