Can Yin-Chai-Xiao-Du decoction be useful of COVID-19? the mechanism research based on network pharmacology
Lu Yang, Ning Li, Hai-Bo Hu, Bin Yin, Guo-Jing Zhao, Feng-Chan Wang, Xu-Hui Wang, Hong-Wu Wang, Xue-Chao Lu*, Huan-Tian Cui
Can Yin-Chai-Xiao-Du decoction be useful of COVID-19? the mechanism research based on network pharmacology
Lu Yang1#, Ning Li2#, Hai-Bo Hu3, Bin Yin3, Guo-Jing Zhao3, Feng-Chan Wang3, Xu-Hui Wang4, Hong-Wu Wang5, Xue-Chao Lu3*, Huan-Tian Cui6*
1Graduate School, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China;2First Clinical Hospital, Shandong University of Traditional Chinese Medicine, Jinan 250000, China;3Department of Pulmonary Disease in Qingdao Hospital of Traditional Chinese Medicine, Qingdao 266700, China;4College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan 250000, China;5College of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China;6Shandong Provincial Key Laboratory of Animal Cell and Developmental Biology, School of Life Sciences, Shandong University, Qingdao 250100, China.
: In this study, we preliminarily investigated the mechanism of Yin-Chai-Xiao-Du decoction for the treatment of COVID-19 by the method of network pharmacology.: The potential targets and pathways of Yin-Chai-Xiao-Du decoction for the treatment of COVID-19 were examined using network pharmacology; the ingredient and active targets of Yin-Chai-Xiao-Du decoction were collected from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform and PharmMapper databases; the COVID-19-related targets were obtained from the online Mendelian inheritance in man, GeneCards, and GeneMANIA databases; the STRING database and Cytoscape were used to build a protein-protein interaction network, and a Network Analyzer tool was used to perform topology analysis to screen for the key ingredients and targets; the ClueGO and KOBAS 3.0 databases were for the enrichment analysis of gene function (Gene Oncology) and gene pathway (Kyoto Encyclopedia of Genes and Genomes); the herb-ingredient-target-pathway network diagram was constructed by Cytoscape.: The core herbs screened by the network pharmacological analysis wereJinyinhua (), Lianqiao (), Chaihu (), Huangqin (), Yinchen (), Guanghuoxiang (), Roudoukou () and Qinghao (). A total of 293 active ingredients were screened by Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform, and the key ingredients were quercetin, kaempferol, isorhamnetin, stigmasterol, beta-sitosterol, and luteolin. Yin-Chai-Xiao-Du decoction has 138 COVID-19-related targets, and the key targets were mitogen-activated protein kinase 3, interleukin-6, tumor necrosis factor, vascular endothelial growth factor A, and CC motif ligand 2. Kyoto Encyclopedia of Genes and Genomes analysis revealed 120 enriched gene pathways, and the key pathways were signaling by interleukins, immune system, cytokine signaling in the immune system, and the signaling pathways of interleukin-17, tumor necrosis factor, and relaxin.: The core herbs of Yin-Chai-Xiao-Du decoction are Jinyinhua (), Lianqiao (), Chaihu (), Huangqin (), Yinchen (), Guanghuoxiang (), Roudoukou () and Qinghao ()The key ingredients are quercetin, kaempferol, isorhamnetin, stigmasterol, and beta-sitosterol; the critical targets are luteolin, interleukin-6, mitogen-activated protein kinase 3, tumor necrosis factor, and CC motif ligand 2; and the core signaling pathways are those mediated by interleukin-17, tumor necrosis factor, and relaxin.
Coronavirus disease 2019,,,, Cytokine Storm, Tumor necrosis factor, Interleukin-6
Our current study explored the potential mechanisms of Yin-Chai-Xiao-Du decoction, an empirical formula of traditional Chinese medicine derived from the classic ancient prescription Gan-Lu-Xiao-Du decoction, on COVID-19 and found its core herbs
The empirical formula Yin-Chai-Xiao-Du decoction has evolved based on the classic ancient prescription of Gan-Lu-Xiao-Du decoction, which was first mentioned in Wei Zhixiu’s() in the Qing Dynasty of China that was released in approximately 1770 C.E. Gan-Lu-Xiao-Du decoction could alleviate fever, cough and fatigue, which were the major clinical outcomes of COVID-19. However, the mechanisms of action of Yin-Chai-Xiao-Du decoction in the treatment of COVID-19 remain unclear.

Background
In February 2020, the World Health Organization announced the coronavirus disease 2019 (COVID-19) as a public health emergency of international concern [1], and its main manifestations are fever, dry cough, fatigue, and pharyngalgia. More so, pneumonia, acute respiratory distress syndrome, and septic shock characterized some of the severe cases [2]. According to the latest real-time statistics of the World Health Organization on April 28, 2020, there were 2,924,722 confirmed COVID-19 cases in total globally, with more than 200,000 cumulative death cases. Sadly, symptomatic and supportive treatments are the main methods for the disease as there are no specific drugs currently and the vaccines are under trials [3]. Many scholars have pinned their hopes on antiviral drugs such as redoxivir and chloroquine. Wang et al. showed that redoxivir and chloroquine have considerable antiviral effects in the COVID-19-infected African green monkey kidney cell line VeroE6 in vitro[4]. However, in a randomized clinical trial published on April 20, 2020, in Lancet, redoxivir did not improve the recovery of the COVID-19 patients or decrease mortality compared with the placebo control group [5]. In addition, there is no sufficient clinical evidence for the anti-COVID-19 effect of chloroquine. Moreover, both medications have some adverse effects.
Multi-ingredient, multi-target, and multi-path characterized the traditional Chinese medicine compound. Network pharmacology is a regular method for investigating the mechanisms of traditional Chinese medicine for treating COVID-19. We can find the relationship between the traditional Chinese medicine decoction and diseases by constructing the ingredient-target-pathway-disease network, identifying the key targets and submodules, and collectively analyzing the biological functions of the key targets and submodules in the network. He [7] showed that the empirical formula Xuebijing injection can treat COVID-19 by regulating the key targets of tumor necrosis factor (TNF), PRKCB, and RELA, and controlling the signaling pathways of NF-κB and hypoxia inducible factor 1. The main active ingredients of the Xiaochaihu decoction can also regulate mitogen-activated protein kinase 3 (MAPK3), Th17, and other pathways; inhibit inflammation; modulate immune function; and reduce lung injury [8].
Yin-Chai-Xiao-Du decoction is a recommended recipe for patients with severe COVID-19 by the()[11] and is derived from the ancient prescription Gan-Lu-Xiao-Du decoction. Gan-Lu-Xiao-Du decoction was first mentioned in Wei Zhixiu’s() in the Qing Dynasty that was released in approximately 1770 C.E. The prescription was composed of Huangqin (), Yinchen (), Guanghuoxiang (), Lianqiao (), Shichangpu (), Roudoukou (), Bohe (mint), Mutong (), Shegan (blackberry lily), and Chuanbeimu ().In addition, it is commonly used for treating acute infectious diseases [9]. Yin-Chai-Xiao-Du decoction has evolved based on the formula of Gan-Lu-Xiao-Du decoction. It is composed of Chaihu(), Huangqin (), Jinyinhua (), Lianqiao (), Shichangpu (), Yujin (), Guanghuoxiang (), Peilan (),Yinchen (), Roudoukou (), Danggui (), Mudanpi (), Yiyiren (), Shishangbai (), and Gancao (). In clinical application, patients with COVID-19 mainly show a low fever after the fever is reduced. Therefore,() in the original prescription is replaced by Qinghao () to subside the low fever after the fever is reduced. This study reveals the possible molecular mode of action of Yin-Chai-Xiao-Du decoction for treating COVID-19 through network pharmacology (Figure 1).

Figure 1 The composition chart of Yin-Chai-Xiao-Du decoction
Materials and methods
Virtual screening of active ingredients of Yin-Chai-Xiao-Du decoction
We searched the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (http://ibts.hkbu.edu.hk/lsp/tcmsp.php) using the keywords of,,,,,,,,,,,,,,, andto get the candidate ingredients of Yin-Chai-Xiao-Du decoction. To obtain the active ingredients of Yin-Chai-Xiao-Du decoction, these ingredients were further screened by the criteria of oral bioavailability (OB) ≥ 30% and drug-like (DL) ≥ 0.18 [10].
Potential target prediction and gene name annotation
The chemical ingredients of Yin-Chai-Xiao-Du decoction were entered into the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) by name, and the 3D structures were identified and stored in .sdf format. These data were then uploaded to the PharmMapper platform server (http://www.lilab-ecust.cn/pharmmapper/) [11] to complete the target prediction of the chemical composition and obtain the relevant targets of the active ingredients. The UniProt database (https://www.uniprot.org/) was used to standardize the target proteins as per the active ingredients and obtain the gene names as per the targets.
Screening of disease targets
The online Mendelian inheritance in man database [12] (http://omim.org/) and GeneCards database [13] (https://www.genecards.org) were searched for relevant targets using the keyword “COVID-19”. Concurrently, the coexpressed gene of severe acute respiratory syndrome coronavirus 2 for the human receptor angiotensin I converting enzyme 2 (ACE2) was identified from GeneMANIA [14] (http://genemania.org/) to obtain the disease-related targets. The Venny platform (version 2.1, http://bioinfogp.cnb.csic.es/tools/venny/) was used to intersect the targets of Yin-Chai-Xiao-Du decoction and the disease and further obtain the targets for the treatment of COVID-19.
Protein-protein interaction (PPI) network
Via the STRING database, the PPI was formed by the intersecting targets from drug and disease, and the PPI network was constructed with Cytoscape 3.7.2 (http://www.cytoscape.org/). Then we used the MCODE plugin to perform cluster analysis of the PPI network. Proteins can hardly achieve their biological and physiological functions alone, so the similarly related groups, called protein complexes or functional modules, accomplish specific cellular tasks. MCODE [16]is a clustering algorithm that can quickly detect densely connected areas in a large-scale target network and score the correlation of targets in the module. To select highly related clusters,the parameter K-Core was set at 2, i.e., the identified module contains at least three edges.
Gene Oncology (GO) genetic biological process analysis
Cytoscape’s ClueGO plugin was used to perform gene function GO enrichment analysis on the clusters obtained from common targets. The ClueGO parameter: “Homo Sapiens” in the control panel and “GO biological process” in “Ontologies/Pathways” in “ClueGO Settings” were selected, and only GO entries with≤ 0.01 were shown.
Kyoto Encyclopedia of Genes and Genomes (KEGG) gene pathway enrichment analysis
The intersecting targets of the herb and disease were imported into the KOBAS 3.0 database (http://kobas.cbi.pku.edu.cn) for pathway analysis. Settings: “Gene Symbol” in “Type” and “Homo Sapiens” in “Species” were selected, “KEGG” was checked,≤ 0.05, and further pathway enrichment analysis was performed on the genes identified in the screen.
Construction of “herb-ingredient-target-pathway” network
Cytoscape 3.7.2 was used to construct a network diagram. We first developed the network and property tables of “herb-active-ingredient-key-target-path”, and then imported them into the Cytoscape for drawing. In the output result, the herb, active ingredients, targets, and pathways were shown as colored and shaped nodes. The ingredients and potential targets were connected by edges and so were the target proteins and pathways.
Results
Search results of the chemical constituents of Yin-Chai-Xiao-Du decoction
A total of 1,992 active ingredients were obtained from the screening, including Chaihu () 288, Huangqin () 58, Jinyinhua () 236, Lianqiao () 150, Shichangpu () 105, Yujin () 222, Guanghuoxiang () 94, Peilan () 60, Yinchen () 53, Roudoukou () 71, Danggui () 125, Mudanpi () 55, Yiyiren () 38, Shishangbai () 30, Gancao () 280 and Qinghao () 127. Further, all the chemical constituents found were screened with OB ≥ 30% and DL ≥ 0.18, which revealed 293 active ingredients that met the screening criteria, as shown in Figure 2 and Table 1.
Search results of the targets of the active ingredients
The names of the active ingredients identified by the screen were entered into the PubChem database, and the obtained structures of the ingredients were imported into the PharmMapper to predict the targets. A total of 3,758 targets were obtained, whereas 168 targets remained after deduplication. Table 1 shows the ingredients, active ingredients, and targets of herbs identified in the search and screen.
Searching for disease genes and screening of key targets
We obtained 1,196 COVID-19 targets from the online Mendelian inheritance in man database, 251 COVID-19 targets from the GeneCards database, and 5,556 ACE2 coexpressed genes from the GeneMANIA. Then, by intersecting the targets of the active ingredients of the herbs with the disease targets, we finally obtained 138 possible targets as per the treatment of fever by Yin-Chai-Xiao-Du decoction. Cytoscape was used to construct the target interaction network of Yin-Chai-Xiao-Du decoction, including 138 nodes and 2,640 edges in the model. The target network model is characterized as the average degree of node connection of 23, the clustering coefficient of 0.554, and the network density of the topological structure of 0.355. As shown in Figure 3 the darker the color and the larger the size, the denser the targets are. Accordingly, interleukin (IL)-6, MAPK3, JUN, TNF, VEGFA, and CC motif ligand 2 (CCL2) had higher degrees and stronger interactions with other proteins (Figure 3).
Further cluster analysis of 138 nodes was conducted using the MCODE plugin, and six clusters were obtained (Figure 4). An analysis of the biological functions (GO) of the clusters using ClueGO plugin showed that the mechanisms were related to smooth muscle adaptation, positive regulation of cyclase activity, regulation of protein deacetylation, regulation of muscle cell apoptotic process, response to reactive oxygen species, cellular response to chemical stress, and cellular response to oxidative stress, etc.
Results of KEGG gene pathway enrichment analysis
Using the KOBAS 3.0 database, 120 enriched pathways were detected and the top 20 were chosen as per the-value. The results showed that the targets were remarkably enriched pathways in terms of multiple signaling pathways of ILs, immune system, cytokine in the immune system, neuroactive ligand-receptor interaction, IL-17, TNF, and relaxin (Figure 5).
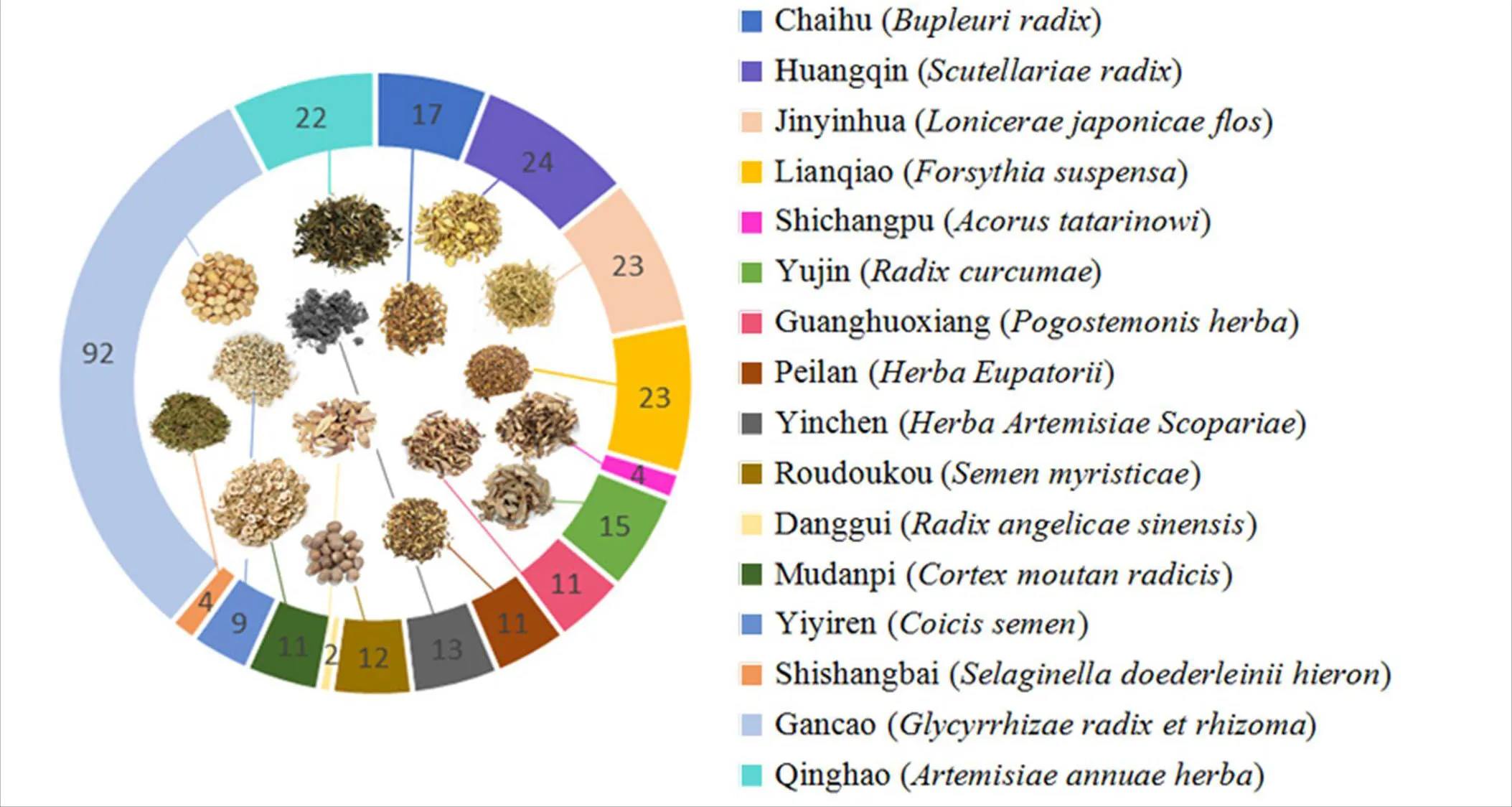
Figure 2 The number of main chemical constituents of each herb in Yin-Chai-Xiao-Du decoction
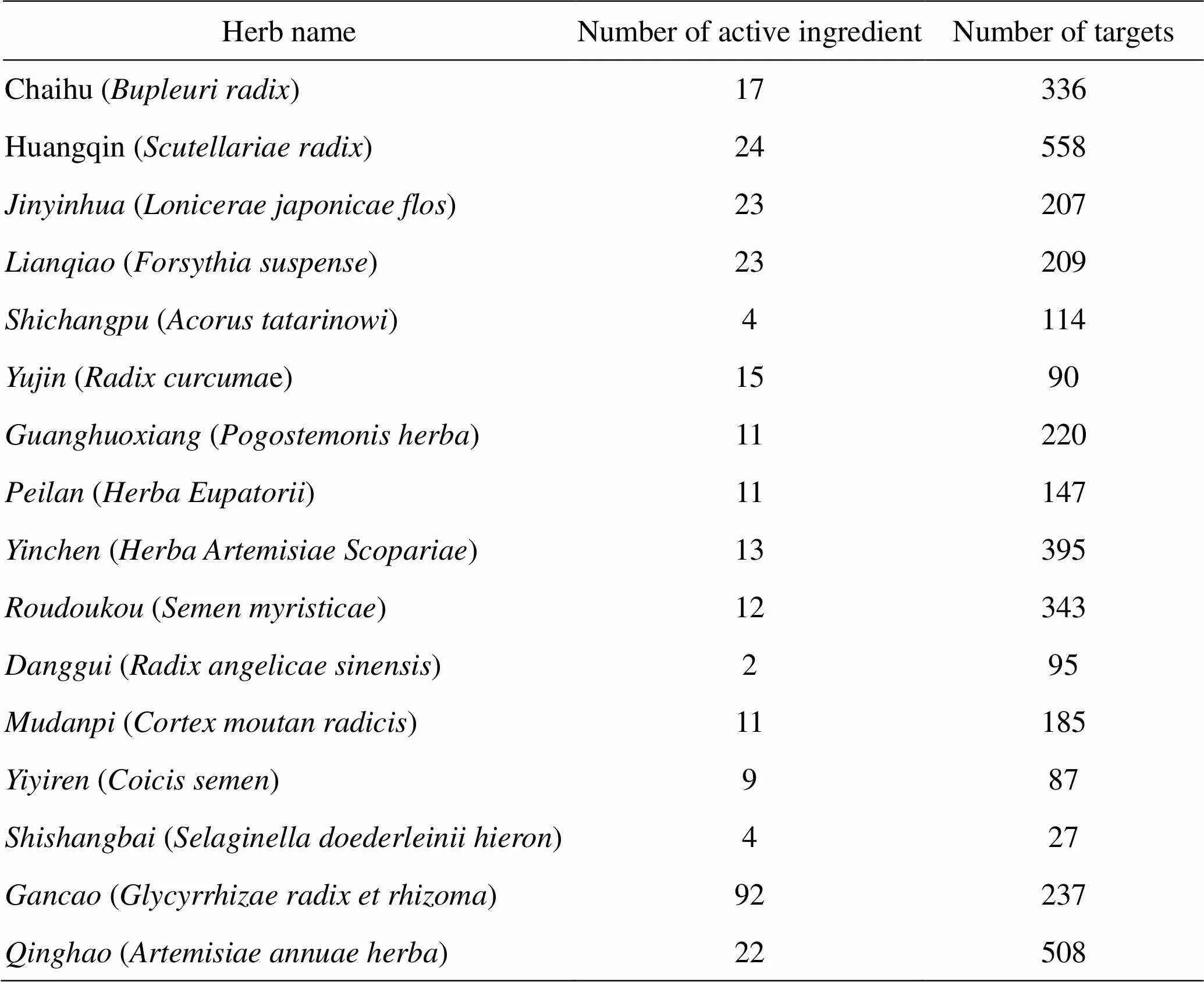
Table 1 The number of targets of chemical constituents in Yin-Chai-Xiao-Du decoction
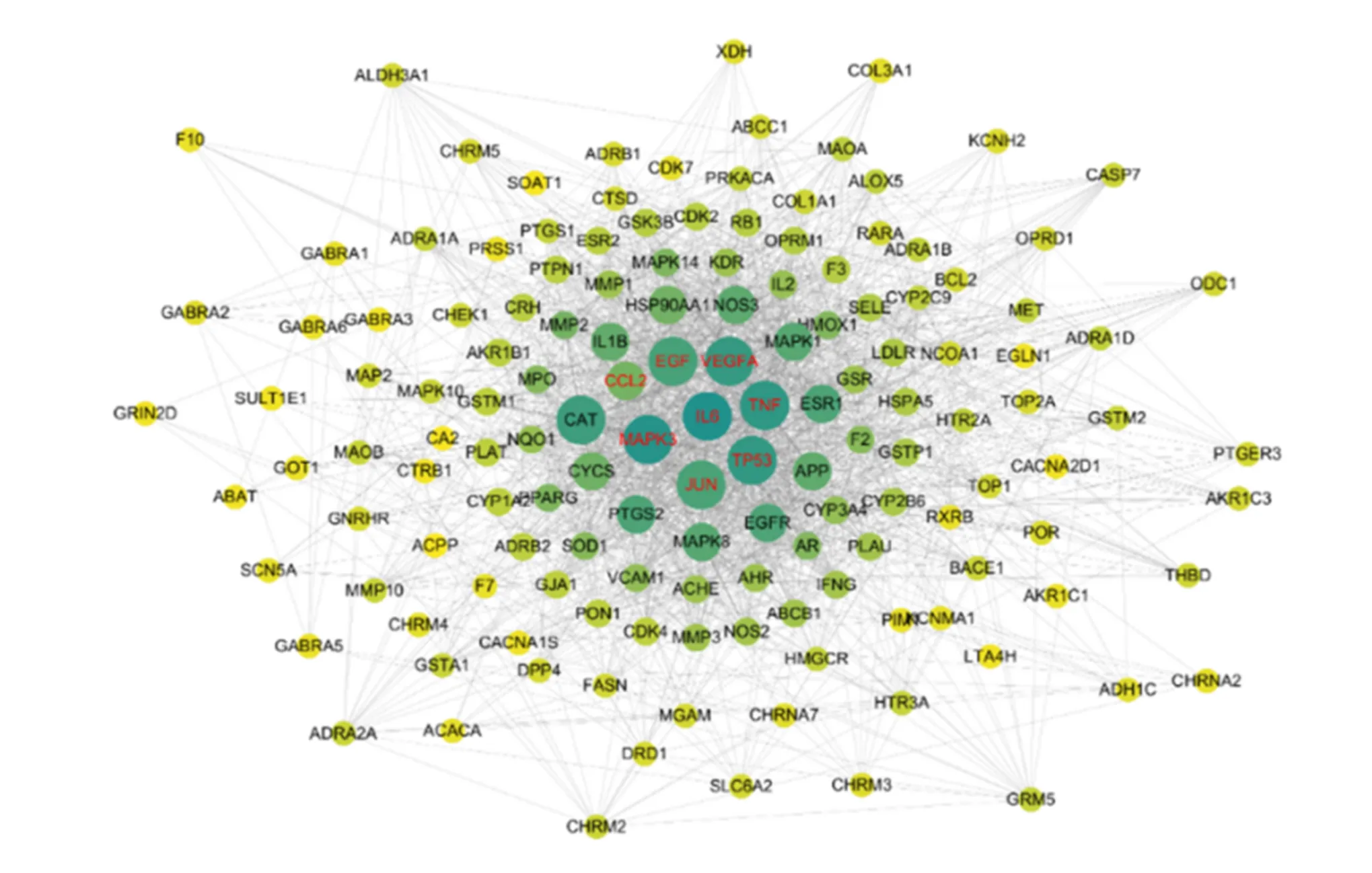
Figure 3 PPI map of common targets. Each node represents a target. The darker color and larger size indicate a higher degree of nodes, which are more possible to be key targets. The proteins with higher degrees include MAPK3, JUN, TP53, IL-6, TNF, VEGFA, CCL2, and EGF, which all have stronger interactions with other proteins.
Figure 4 The six clusters in the target network of Yin-Chai-Xiao-Du decoction. Each node represents a target and each edge represents an interaction relationship. The highly correlated nodes are enriched into a subcluster. The darker the color and the larger the size of the node, the higher its degree value is. Each cluster contains at least three edges.
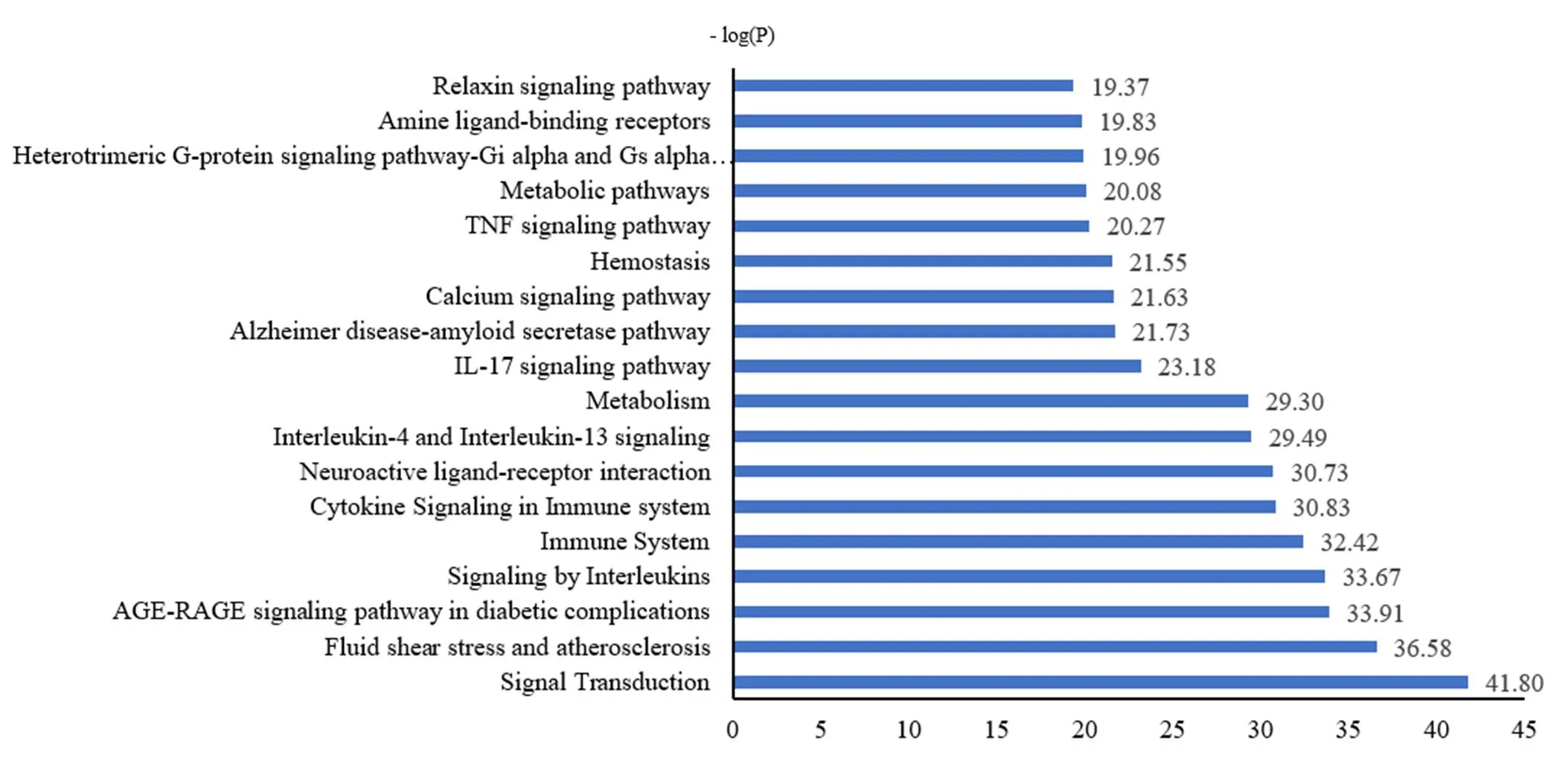
Figure 5 KEGG enrichment analysis of the targets of Yin-Chai-Xiao-Du decoction. The abscissa is the negative logarithm of the-value, and a longer band means a higher correlation; the ordinate is the name of the KEGG pathway.
Construction of the herb-ingredient-target-pathway network
A table of “herb-ingredient-target-pathway” network was developed and imported into Cytoscape 3.7.2 software. The red triangle nodes represent drugs, blue rectangle nodes represent pathways, and green ellipse nodes represent active ingredients. The herb-ingredient-target-pathway of Yin-Chai-Xiao-Du decoction for the treatment of COVID-19 was constructed by the targets with orange rectangle nodes. As shown in the figure, each active ingredient acted on multiple targets. The more connected nodes and the higher correlations, the more likely it is a key ingredient, and it is then at a core position in the mechanisms of Yin-Chai-Xiao-Du decoction for the treatment of COVID-19. After the network analysis, we found that quercetin, kaempferol, isorhamnetin, stigmasterol, beta-sitosterol, and luteolin were the top-ranked core ingredients (Figure 6).
We selected the ingredients of the top-ranked 10 targets, and the herb-ingredient network constructed by Cytoscape showed that the ingredients with the highest degrees were quercetin, kaempferol, isorhamnetin, stigmasterol, beta-sitosterol, and luteolin. Among them, quercetin and kaempferol were shared by Jinyinhua (), Lianqiao (), Chaihu (), and Qinghao (); stigmasterol and isorhamnetin were shared by Chaihu (), Huangqin () and Qinghao (); luteolin and beta-sitosterol were shared by Jinyinhua () and Lianqiao (); quercetin and isorhamnetin were shared by Yinchen () and Guanghuoxiang (); luteolin and quercetin were shared by Roudoukou () and Lianqiao (). These data suggest that Jinyinhua (), Lianqiao (), Chaihu (), Huangqin (), Guanghuoxiang (), Yinchen (), Roudoukou (), and Qinghao () are the core herbs of Yin-Chai-Xiao-Du decoction for the treatment of COVID-19 (Figure 7).

Figure 6 Herb-compound-target-pathway network. The red triangle nodes represent herbs, blue rectangle nodes represent pathways, green ellipse nodes represent active ingredients, and orange rectangle nodes represent targets.
Figure 7 Herb-top 10 ingredients network of Yin-Chai-Xiao-Du decoction. The green nodes represent the herbs, and the blue nodes represent the core ingredients.
Discussion
Yin-Chai-Xiao-Du decoction consists of Chaihu (), Huangqin (), Jinyinhua (),Lianqiao (), Shichangpu (), Yujin (), Guanghuoxiang (), Peilan (), Yinchen (), Roudoukou (), Danggui (), Mudanpi (), Yiyiren (), Shishangbai (), Gancao () and Qinghao (). The network pharmacology analysis showed that the key ingredients of Yin-Chai-Xiao-Du decoction for treating COVID-19 are shared by many herbs. For example, stigmasterol and isorhamnetin are shared by Chaihu () and Huangqin (). Stigmasterol shows good anti-inflammatory activity by considerably reducing the expression of IL-1β, IL-6, MCP-1, and COX-2 [17], and synergistic isorhamnetin can inhibit oxidative stress caused by inflammation [18]. Kaempferol is shared by Jinyinhua () andLianqiao (). Kaempferol can scavenge oxygen-free radicals and reduce tissue damage caused by hypoxia [19], and inhibit the expression of the TNF-α and IL-6 inflammatory factors by attenuating the activity of MAPK and AKT pathways, thereby exerting an anti-inflammatory effect [20]. Luteolin and beta-sitosterol are shared by Roudoukou () and Qinghao (). Luteolin can reduce the activity of catalase and superoxide dismutase in lung tissue, thereby reducing the level of oxidative damage and lipid peroxidation and further alleviating acute lung injury in mice [21]. Besides, it can also enhance acute lung injury by inhibiting the MEK/ERK and PI3K/Akt pathways and weakening the activation of neutrophils [22]. Beta-sitosterol has a certain protective effect on acute lung injury caused by lipopolysaccharide. It can improve the oxidative stress response and inhibit the accumulation and release of TNF-α inflammatory factors [23]. In addition, it can also inhibit the infiltration of neutrophils into tissues, reduce the production of β-glucuronidase and superoxide, regulate the complement system, and further regulate immunity system [24]. Furthermore, quercetin is shared by Yinchen () andGuanghuoxiang (). It has the effects of reducing lung inflammation and having antioxidant and antiviral effects. Other studies have shown that it can reduce the expression of TGF-β1, α-SMA, and TNF-α, inhibit the apoptosis of rat alveolar cells, and reduce the inflammation and fibrosis injury of rat lung tissue. Meanwhile, it can enhance pulmonary fibrosis by inhibiting the signal pathway of SphK1/S1P [25–27]. These two effects are synergistic. Jinyinhua (),Lianqiao (), Chaihu (),Huangqin (), Guanghuoxiang (), Yinchen (), Shishangbai (), and Qinghao () are the core herbs for the Yin-Chai-Xiao-Du decoction that have anti-inflammatory, antioxidant, immunoregulation, and lung injury improvement effects.
Using network pharmacology to screen ingredients, predict targets, and analyze the protein network, we found that the active ingredients of Yin-Chai-Xiao-Du decoction could act on MAPK3, IL6, VEGFA, TNF, and CCL2, which are at the center of the network for treating COVID-19. The network topology analysis showed that the treatment of COVID-19 by Yin-Chai-Xiao-Du decoction involved multiple gene functions and was very complex.
After the coronavirus invades the lung cells and infects the human body by binding with the S protein on the envelope to the ACE2 receptor [28], it is recognized by the host cell. Subsequently, the body regulates different molecular signaling pathways such as the NF-κB pathway and the MAPK/JNK pathway to trigger inflammation, resulting in the expression of proinflammatory factors and antiviral genes [29–30]. More so, activated immune cells release a large number of cytokines and chemokines, including TNF-α, interleukins (IL-1, IL-6, and IL-12), and chemokines (CCLs), leading to the initiation of inflammatory cytokine storms, which promote the transfer of immune cells to the primary infection site [31]. Then, leukocytes and lymphocytes are recruited and attracted by the boosted cytokines and chemokines to the lesion sites. They are continuously activated and proliferated in attempting to eliminate the virus by releasing inflammatory mediators [32]. Several immune cells and tissue fluids gather in the lungs to regulate the key genes, such as vascular endothelial growth factor, which changes the permeability of the blood vessel and leads to airway obstruction, lung inflammation, and edema. The patient will experience critical conditions such as extreme difficulty in breathing, blood oxygen desaturation, unconsciousness that further leads to multiple organ failure and even death [33]. Therefore, it is speculated that COVID-19 may activate the IL receptor and the MAPK pathway, increase the expression of inflammatory cytokines such as TNF-α and IL-6, and incur a local inflammatory response, therefore resulting in cytokine storms and immune disorders. Yin-Chai-Xiao-Du decoction may play an anti-inflammatory role by inhibiting these proinflammatory cytokines. It can modulate immune functions by influencing the number of immune cells and prevent multiple organ failures caused by endogenous inflammatory mediators to reduce lung damage.
Meanwhile, COVID-19 patients have congestion and edema of the respiratory tract mucosa caused by a large number of inflammatory cell infiltration, angiectasis, and increased exudation. The large quantity of mucus secretion can be retained in the bronchus and block the normal function of tracheal smooth muscle, producing symptoms of cough, expectoration, and asthma [34]. GO enrichment analysis showed that the targets of Yin-Chai-Xiao-Du decoction were remarkably enriched in the biological processes of smooth muscle adaptation, cell response to reactive oxygen species, and cellular response to chemical stress.
KEGG enrichment showed that the treatment of COVID-19 by Yin-Chai-Xiao-Du decoction involved multiple pathways such as the anti-inflammatory and immune regulation pathways of IL-17 and TNF. Via corresponding receptors, the IL-17 family transmits signals to activate downstream pathways such as NF-κB and MAPKs. The IL-17 family can induce the expression of antibacterial peptides, cytokines, and chemokines, which plays a vital role in acute and chronic inflammatory reactions [35]. The TNF signaling pathway mainly acts on inhibiting the cytokines in the immune response and inflammatory storm activated by COVID-19, alleviating the immune response, and eliminating the inflammation. TNF can bind to the recombinant human type I TNFR1 and TNFR2, which further mediate inflammation and immune regulation via the cascade reaction of the NF-κB pathway and MAPK [36].
Furthermore, the KEGG results showed that the core targets were enriched in the relaxin signaling pathway, which acts on COVID-19 mainly through the following four aspects. First, severe COVID-19 cases in the recovery stage can have pulmonary fibrosis for a long time. Specifically, cases with acute respiratory distress syndrome have a poorer prognosis [37]. Mou et al. [38] examined the lung CT of 24 patients with COVID-19. They found that the lesions began to be absorbed and showed fibrosis on the lung CT of the severe and critical patients. Relaxin has anti-fibrotic effects, which have been validated in non-germinal tissue, such as the lung, heart, kidney, and liver tissue [39]. Secondly, relaxin can promote the regeneration of muscle fibers by inhibiting inflammation and fibrosis and participate in the healing of injured ligaments and skeletal muscles [40]. Most patients with COVID-19 are complicated with a concurrent condition of myalgia, which can be relieved by relaxin. More so, clinical studies have shown that the levels of serum myocardial necrosis markers increased regardless of the patient’s condition [41], indicating that COVID-19 may damage cardiac muscle cells. Experiments showed that relaxin could effectively prevent and reverse many adverse reactions in cardiovascular animal models (ischemic/reperfusion injury, myocardial infarction, hypertensive heart disease, and cardiomyopathy), and serelaxin, a recombinant form of relaxin, is in the extended phase III clinical trial for acute heart failure [42]. Finally, Jordan et al. [43] have confirmed that relaxin can prevent the death of brain cells under hypoxia conditions and may have a direct protective effect on nerves, which help to protect the brain tissue of patients with COVID-19 under hypoxia.
Interestingly, in this network pharmacology study, chlorogenic acid and saikoside were not selected for subsequent analysis because of the low OB value (chlorogenic acid: 11.93% vs. screen criteria: OB ≥ 30) and low DL value (saikoside: 0.09 vs. screen criteria: DL ≥ 0.18). Besides, baicalein and artemisinin were not selected for subsequent analysis because their numbers of targets were 33 and 28, respectively, and ranked 17 and 44, respectively, among all ingredients. Nevertheless, more pharmacological evidence has shown that these ingredients also have anti-inflammatory, antiviral, and immunity regulation effects [44–51]; however, their values for COVID-19 treatment need to be further studied.
Conclusion
In summary, the core herbs of Yin-Chai-Xiao-Du decoction for the treatment of COVID-19 are Jinyinhua (), Lianqiao (), Chaihu (), Huangqin (), Yinchen (), Guanghuoxiang (), Roudoukou (), and Qinghao (). The key ingredients include quercetin, kaempferol, isorhamnetin, stigmasterol, beta-sitosterol, and luteolin. The core targets are IL-6, MAPK3, TNF, CCL2, and the core signaling pathways are those of IL-17, TNF, and relaxin.
1. The World Health Organization has called the 2019-ncov outbreak a public health emergency of international concern. China Health Law 2020, 2802: 34. (Chinese)
2. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323: 1061–1069.
3. Li XS, Zhang JX, Zhou XB, et al. Literature review of existing evidence for corona virus disease-19. Eval Anal Drug Use Hosp China 2020, 03: 262–267. (Chinese)
4. Wang ML, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020, 30: 269–271.
5. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395: 1569–1578.
6. Chen YT, Sun JK, Han QY, et al. Advances of studies on remdesivir against coronavirus including SARS-CoV-2. Clin Res Prac 2020, 5: 6–11. (Chinese)
7. He TM, Duan CC, Li XF, et al. Potential mechanism of Xuebijing injection in treatment of coronavirus pneumonia based on network pharmacology and molecular docking. Chin J Mod Appl Pharm 2020, 37: 398–405. (Chinese)
8. Yang L, Cui HT, Liu XG, et al. Feasibility of Xiaochaihu decoction on fever induced by coronavirus disease 2019 (COVID-19) based on network pharmacology. Chin Tradit Herb Drugs 2020, 51: 1761–1775. (Chinese)
9. Li QM. Examples of clinical application of Gan-Lu-Xiao-Du-Dan. Zhejiang J Integr Tradit Chin Western Med 2018, 09: 790–791. (Chinese)
10. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 2014, 6: 13.
11. Xue J, Shi Y, Li C, et al. Network pharmacology-based prediction of the active ingredients, potential targets, and signaling pathways in compound Lian-Ge granules for treatment of diabetes. J Cell Biochem 2019, 120: 6431–6440.
12. Obi LG, Malachi G. OMIM (online Mendelian inheritance in man). Dictionary of Bioinformatics and Computational Biology. John Wiley & Sons Ltd., 2014.
13. Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics 2016, 54.
14. David WF, Donaldson SL, Ovi C, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 2010, 38: W214–220.
15. Szklarcayk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019, 47: D607.
16. Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics 2003, 4: 2.
17. Feng SM, Ning K, Shao P, et al. Study on the treatment of acute colitis in mice by pitt-sitosterol and pitosterol. J Chin Cereal Oil Assoc 2018, 33: 80–86, 94. (Chinese)
18. Wang K, Pang H, Liang CM, et al. Effects of different doses of isorhametin on proliferation of macrophage RAW264.7 in mice with inflammatory injury. Shandong Med J 2018, 39: 48–51. (Chinese)
19. Lei XQ, Chen A, Liu Y, et al. Research progress on the pharmacological action of kaempferol. Stud Trace Elem Health 2017, 34: 61–62. (Chinese)
20. Zhou Y, Du BY, Tan YH, et al. Inhibitory effect of kaempferol on proliferation and apoptosis of rat hepatocellular carcinoma cell CBRH7919. J Guangzhou Univ Tradit Chin Med 2010, 27: 250–253. (Chinese)
21. Kuo MY, Liao MF, Chen FL, et al. Luteolin attenuates the pulmonary inflammatory response involves abilities of antioxidation and inhibition of MAPK and NF-kappaB pathways in mice with endotoxin-induced acute lung injury. Food Chem Toxicol 2011, 49: 2660–2666.
22. Lee JP, Li YC, Chen HY, et al. Protective effects of luteolin against lipopolysaccharide-induced acute lung injury involves inhibition of MEK/ERK and PI3K/Akt pathways in neutrophils. Acta Pharmacol Sin 2010, 31: 831–838.
23. Shang ZB, Liu XL, Cheng RQ, et al. Influence of β-sitosterol on gastric mucosal side effect induced by aspirin and its pharmacological functions. Chin J Exp Tradit Med Formul 2016, 22: 148–152. (Chinese)
24. Navarro A, De LH, Villar A. Anti-inflammatory and immunomodulating properties of a sterol fraction fromfoetens clem. Biol Pharm Bull 2001, 24: 470–473.
25. Ma N, Li YJ, Fan JP, et al. Research progress on pharmacological action of quercetin. J Liaoning Univ Tradit Chin Med 2018, 20: 221–224. (Chinese)
26. Wei P, Chen ZB, Wang CE, et al. Protective function and mechanism of quercetin in chronic obstructive pulmonary disease. Chin J Immunol 2019, 35: 2570–2575. (Chinese)
27. Zhang X, Cai Y, Zhang W, et al. Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. Biochem Cell Biol 2018, 96: 742–751.
28. Zhou P, Yang XL, Wang XG, et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. Bio Rxiv 2020, 1.
29. Wang JF, Shao JJ, Chang HY, et al. Advances in research on innate immune recognition receptors of viruses. Chin J Cell Mol Immunol 2009, 25: 1217–1220. (Chinese)
30. Chow KT, Gale M, Loo YM. RIG-I and other RNAsensors in antiviral immunity. Annual Rev Immunol 2018, 36: 667–694.
31. Mc NF, Mayer-Barber KD, Sher A, et al. Type I interferons in infectious disease. Nat Rev Immunol 2015, 15: 87–103.
32. Teijaro JR. Cytokine storms in infectious diseases. Semin Immunopathol 2017, 39: 501–503.
33. Clark IA,Vissel B. The meteorology of cytokine storms, and the clinical usefulness of this knowledge. Semin Immunopathol 2017, 39: 505–516.
34. Mejza F, Gnatiuc L, Buist AS, et al. Prevalence and burden of chronic bronchitis symptoms: results from the BOLD study. Eur Respir J 2017, 50: 1700621.
35. Li XX, Bechara R, Zhao JJ, et al. IL-17 receptor-based signaling and implications for disease. Nat Immunol 2019, 20: 1594–1602.
36. Zhang Y, Zhou DM, Li W, et al. The expression level of TNF-α in patients with painful diabetic neuropathy. Chin J Lab Diagn 2020, 24: 36–40. (Chinese)
37. Respiratory Disease Committee of Shanghai Association of Integrative Medicine. Suggestions of integrative medicine treatment on some hot issues about COVID-19. Shanghai J Tradit Chin Med 2020, 54: 6–9. (Chinese)
38. Mu J, Wang RP, Liu XF, et al. Preliminary discussion for chest CT dynamic findings of COVID-19 patients. Chin Imaging J Integr Tradit Western Med 2020, 18: 116–119. (Chinese)
39. Samuel CS, Royce SG, Hewitson TD, et al. Anti-fibrotic actions of relaxin. Br J Pharmacol 2017, 174: 962–976.
40. Dehghan F, Haerian BS, Muniandy S, et al. The effect of relaxin on the musculoskeletal system. Scand J Med Sci Sports 2014, 24: e220–e229.
41. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323: 1061–1069.
42. Sarwar M, Du XJ, Dschietzig TB, et al. The actions of relaxin on the human cardiovascular system. Br J Pharmacol 2017, 174: 933–949.
43. Willcox JM, Summerlee AJ. Relaxin protects astrocytes from hypoxia in vitro. PLoS One 2014, 9: e90864.
44. Huang L, Tang JL, Huang J, et al. Advances in extraction and application of chlorogenic acid. Res Lab Work Coll Univ 2012, 9: 114–117. (Chinese)
45. Wu HZ, Luo J, Yin YX, et al. Effects of chlorogeni acid, an active compound activating calcineurin, purified fromon macrophage. Acta Pharmacol Sin 2004, 25: 1685–1689.
46. Zhu J, Luo C, Wang P, et al. Saikosaponin a mediates the inflammatory response by inhibiting the MAPK and NF-κB pathways in LPS-stimulated RAW 264.7 cells. Exp Ther Med 2013, 5: 1345–1350.
47. Wan QF, Gu LG, Yin SJ, et al. Protection effect of baicalin on lung injury of mice infected with influenza FM1. China J Tradit Chin Med Pharm 2011, 26: 2818–2851. (Chinese)
48. Wan QF, Wang H, Han XB, et al. Baicalin inhibits TLR7/MYD88 signaling pathway activation to suppress lung inflammation in mice infected with influenza a virus. Biomed Rep 2014, 2: 437–441.
49. Wang JX, Tang W, Yang ZS, et al. Suppressive effect of a novel water-soluble artemisinin derivative SM905 on T cell activation and proliferation in vitro and in vivo. Eur J Pharmacol 2007, 564: 211–218.
50. Khakzad MR, Ganji A, Ariabod V, et al. Artemisinin therapeutic efficacy in the experimental model of multiple sclerosis. Immunopharmacol Immunotoxicol 2017, 39: 348–353.
51. Wen Y, Pan MM, Lv LL, et al. Artemisinin attenuates tubulointerstitial inflammation and fibrosis via the NF-κB/NLRP3 pathway in rats with 5/6 subtotal nephrectomy. J Cell Biochem 2019, 120: 4291–4300.
:
Lu Yang and Ning Li contributed to study concept; Hai-Bo Hu and Bin Yin contributed to study design and performance; Guo-Jing Zhao, Feng-Chan Wang and Xu-Hui Wang contributed to analysis of data; Hong-Wu Wang, Lu Yang and Ning Li contributed to drafting of the paper; Xue-Chao Lu and Huan-Tian Cui contributed to study supervision.
:
The authors declare no conflicts of interest.
:
This study was supported bythe National Key Research and Development Program (No. 2018YFC1704800) and the Chinese Medicine Standardization Project of State Administration of Traditional Chinese Medicine (No. SATCM-2015-BZ125).
:
COVID-19, coronavirus disease 2019; TNF, tumor necrosis factor; OB, oral bioavailability; DL, drug-like; GO, Gene Oncology; KEGG, Kyoto Encyclopedia of Genes and Genomes; MAPK3, mitogen-activated protein kinase 3; ACE2, angiotensin I converting enzyme 2; IL, interleukin; PPI, protein-protein interaction; CCL2, CC motif ligand 2.
:
Lu Yang, Ning Li, Hai-Bo Hu, et al. Can Yin-Chai-Xiao-Du decoction be useful of COVID-19? the mechanism research based on network pharmacology. Traditional Medicine Research 2020, 5 (4): 188–200.
:Rui-Wang Zhao.
:13 May 2020,
28 May 2020,
:09 June 2020.
#These authors are co-first authors on this work.
Xue-Chao Lu. Department of Pulmonary Disease in Qingdao Hospital of Traditional Chinese Medicine, No.4 Renmin Road, North District, Qingdao 266700, China. E-mail: hospitalbreathing@163.com; Huan-Tian Cui. Shandong Provincial Key Laboratory of Animal Cell and Developmental Biology, School of Life Sciences, Shandong University, No.72 Binhai Road, Jimo District, Qingdao 250100, China. E-mail: 1762316411@qq.com.
10.12032/TMR20200601185
 Traditional Medicine Research2020年4期
Traditional Medicine Research2020年4期
- Traditional Medicine Research的其它文章
- Marine natural products with anti-inflammation effects
- Dissecting the underlying pharmaceutical mechanism of Danggui Buxue decoction acting on idiopathic pulmonary fibrosis with network pharmacology
- Acupuncture and/or moxibustion for the treatment of lumbar disc herniation: quality assessment of systematic reviews
- Efficacy of Xuebijing injection for the treatment of coronavirus disease 2019 via network pharmacology
- The selection rules of acupoints and meridians of traditional acupuncture for postoperative nausea and vomiting: a data mining-based literature study
- The advances of traditional Chinese medicine in the treatment of liver diseases in 2019
