M2BPGi for assessing liver fibrosis in patients with hepatitis C treated with direct-acting antivirals
Shereen A Saleh, Mohamed M Salama, Marwan M Alhusseini, Ghada A Mohamed
Abstract
Key words: Hepatitis C virus; Liver fibrosis; Human Mac-2 binding protein; Antiviral agents; Sustained virologic response; Elastography
INTRODUCTION
Hepatitis C virus (HCV) is considered a public health problem, as approximately 3%of the global population is infected with HCV[1]. It is imperative to assess the degree of liver fibrosis in patients with chronic hepatitis C (CHC) because fibrogenesis causes all the clinical events, including decompensated liver disease and hepatocellular carcinoma (HCC), affecting the prognosis of and treatment strategies used in patients with CHC[2].
Although a liver biopsy is considered the gold standard for stratifying hepatic fibrosis, its clinical utility is substantially limited because of the invasiveness and the sampling variability[3]. Additionally, a liver biopsy is impractical particularly during follow-up due to its invasive nature[4,5].
Consequently, non-invasive methods have been previously proposed and validated for the assessment of hepatic fibrosis, such as ultrasound or magnetic resonance imaging[6], elastographic techniques[7], serum biomarkers including hyaluronic acid,type IV collagen, and type III procollagen-N-peptide[8], and surrogate markers,e.g.,the aspartate aminotransferase (AST)-to-platelet ratio index[9], the fibrosis-4 (FIB-4)score[10], AST to alanine aminotransferase (ALT) ratio[11]and PAPAS [platelets/age/phosphatase/alpha fetoprotein (AFP)/AST] index[12].
Mac-2 binding protein glycosylation isomer (M2BPGi) is a glycoprotein that is produced by hepatic stellate cells (HSCs). It functions as a messenger between HSCs and Kupffer cells to promote fibrogenesis[13]. The feasibility of monitoring serum M2BPGi levels to assess hepatic fibrosis was evaluated, and some studies recommended it as an accurate method for staging hepatic fibrosis[14,15].
Subsequently, several investigators validated the usefulness of M2BPGi in various aetiologies of liver diseases, such as viral hepatitis[16-19], mortality in liver cirrhosis[20],biliary atresia[21], non-alcoholic fatty liver disease[22,23], non-alcoholic steatohepatitis[24],primary biliary cirrhosis[25], autoimmune hepatitis[26]and primary sclerosing cholangitis[27]. Furthermore, it was investigated as a marker to assess the risk of HCC development[28,29]. According to recent studies[17,18,28,30-32], M2BPGi is a useful marker for monitoring the improvement of patients with liver fibrosis who have achieved a sustained virologic response (SVR) after antiviral therapy.
Recently, interferon (IFN)-based treatment has been replaced by direct-acting antivirals (DAAs). The approval of DAAs was a revolution in HCV eradication, with SVR rates exceeding 90%, good tolerability, and increased efficacy with shorter treatment durations[33]. However, a few reports have documented the improvement in liver fibrosis in patients treated with IFN-free DAAs[31,34-37].
We aimed to investigate the diagnostic accuracy of serum M2BPGi levels in assessing the grade of liver fibrosis in patients with CHC before and after DAAsbased treatment, as well as to compare its diagnostic value with the FIB-4 score and PAPAS index.
MATERIALS AND METHODS
From December 2017 to August 2018, 80 treatment-naïve adult patients with CHC who were eligible for DAAs therapy were consecutively enrolled in this observational cohort study. For 12 weeks, 65 patients were treated with sofosbuvir/daclatasvir, and 15 patients were treated with sofosbuvir/daclatasvir and a weight-based dose of ribavirin at Knowledge and Technology Association for Hepatitis C Management Clinic, Cairo, Egypt. The exclusion criteria were (1) positivity for antibodies against human immunodeficiency virus or positivity for hepatitis B surface antigen; (2) other causes of liver disease (autoimmune hepatitis, primary biliary cirrhosis,haemochromatosis, sclerosing cholangitis, Wilson’s disease, or an α1-antitrypsin deficiency); (3) clinical or biochemical evidence of hepatic decompensation (ascites,bleeding varices or encephalopathy); (4) suspected HCC or other cancers; (5) excessive alcohol consumption (> 40 g/d) or intravenous drug abuse; or (6) a previous liver transplantation.
This study was approved by the Research Ethics Committee of our institution.Written informed consent was obtained from every patient, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
We evaluated the liver stiffness measurement (LSM), serum M2BPGi levels, FIB-4 score, PAPAS index, biochemical data, haematological data, virologic data and abdominal ultrasound at baseline and 12 weeks after the end of treatment (EOT),namely, the time SVR12 was achieved, of every patient.
Measurement of HCV RNA levels
Plasma HCV RNA levels were measured using the Roche TaqMan real-time reverse transcriptase-PCR assay version 2.0, with lower limits of quantification and detection of 15 IU/mL. SVR12 was defined as a lack of detectable HCV RNA at week 12 after EOT.
Measurement of the serum M2BPGi level
Serum M2BPGi levels were measured using human M2BPGi enzyme-linked immunosorbent assay kits, with a detection range of 0.625 - 200 ng/mL, sensitivity 0.1 ng/mL, and intra-assay and inter-assay coefficients of variation less than 15%.
LSM
The LSM was performed using Fibroscan®(Echosens, 502 Touch, Paris, France). It was conducted by an experienced examiner after the patient had fasted for at least six hours, and 10 valid measurements were recorded. The median LS in kilopascals (kPa)was reported. Only examinations with a success rate > 60% and IQR < 25% were included and considered reliable. According to Tsochatziset al[38], the following fibrosis staging cut-off values were used: F0-F1 < 7 kPa; F2 7 - 9.4 kPa; F3 9.5 - 11.9 kPa; and F4 > 12 kPa.
Non-invasive liver fibrosis assessment
The PAPAS index and FIB-4 score were calculated using the following formulas:
PAPAS index[12]= Log (index + 1) = 0.0255 + 0.0031 × age (year) + 0.1483 × log [ALP(U/L)] + 0.004 × log [AST (U/L)] + 0.0908 × log [AFP (ng/L) + 1] - 0.028 × log[platelets count (109/L)].
FIB-4 score[39]= Age (yr) × AST (IU/L)]/{platelets count (109/L) × [ALT (IU/L)]½}.
A FIB-4 score < 1.45 indicates no or minimal fibrosis.
A FIB-4 score > 3.25 indicates significant fibrosis.
Statistical analysis
Statistical analyses were performed using Stata®version 13.1 software (StataCorp.2013, College Station, TX: StataCorp LP). Patients’ characteristics are presented as mean ± SD, median (IQR) or number (percentage), as appropriate. Accordingly,pairedttest, Wilcoxon matched-pairs signed rank test or chi squared test was used, as appropriate. Values were compared between different grades of liver fibrosis using one-way ANOVA test. Pearson’s correlation analysis was used to study the correlation between serum M2BPGi levels and the characteristics of the study population. A receiver operating characteristic (ROC) curve analysis was used to identify the best cut-off value for the serum M2BPGi level with maximum sensitivity and specificity for the differentiation of different grades of fibrosis. APvalue < 0.05 was considered significant.
The statistical methods of this study were performed by Hazem M. El-Hariri from Department of Community Medicine, National Research Centre, Cairo, Egypt.
RESULTS
Patients’ characteristics
The studied patients included 40 males (50%) and 40 females (50%), with a mean age of 52.3 ± 10.7 years and BMI (kg/m2) = 28 ± 5.4. Patients’ characteristics at baseline and SVR12 are shown in Table 1.
Safety and adherence to therapy
All patients completed the scheduled course of treatment with follow up until 12 wk after EOT. SVR12 was achieved in all patients (100%). Overall, the treatment was well tolerated. The most commonly reported adverse events were fatigue (5%), followed by pruritus (4.2%), rash (2.3%), headache (2%), and a loss of appetite (1%), all of which were mild in severity.
Impact of SVR12 on the serological data
Haemoglobin levels, WBC, total bilirubin levels and international normalized ratio(INR) did not change significantly after patients achieved SVR12. Platelets count and albumin levels were significantly higher at SVR12. ALT, AST, ALP and creatinine levels decreased significantly after patients achieved SVR12. AFP levels decreased after patients achieved SVR12, but the difference was not statistically significant(Table 1).
Effect of SVR12 on liver fibrosis
Serum M2BPGi levels, LSM, FIB-4 score and PAPAS index decreased significantly after patients achieved SVR12 (Table 1). The improvement in LSM was more noticeable in patients with grade F4 fibrosis (Table 2). Only serum M2BPGi levels were significantly different between patients with different grades of fibrosis at baseline and SVR12 (Table 3).
Correlations with serum M2BPGi levels
At baseline, serum M2BPGi levels correlated positively with total bilirubin levels and negatively with AST levels. At SVR12, serum M2BPGi levels correlated positively with INR and negatively with platelets count (Table 4).
Correlations between serum M2BPGi levels, LSM, FIB-4 score and PAPAS index
At baseline, LSM correlated with serum M2BPGi levels and FIB-4 score. In addition, a significant correlation was observed between FIB-4 score and PAPAS index. At SVR12, LSM correlated with serum M2BPGi levels, FIB-4 score and PAPAS index(Table 5 and Figure 1).
ROC curve analysis for the assessment and differentiation of the grades of liver fibrosis
At baseline, compared with the FIB-4 score and PAPAS index, M2BPGi was the best marker to distinguish patients with grade F4 fibrosis (AUC = 0.801,P< 0.001),patients with grade F2 from grade F0-1 fibrosis (AUC = 0.713,P= 0.012), patients with grade F3-4 from grade F0-2 fibrosis (AUC = 0.730,P< 0.001), and patients with grade F2-4 from grade F0-1 fibrosis (AUC = 0.763,P< 0.001) (Supplementary Table 1 ,Figures 1 and 3).
At SVR12, M2BPGi had the greatest AUCs for differentiating patients with grade F4 fibrosis (AUC = 0.844,P< 0.001), patients with grade F3 from grade F0-2 fibrosis(AUC = 0.893,P= 0.002), patients with grade F3-4 from grade F0-2 fibrosis (AUC =0.891,P< 0.001), and patients with grade F2-4 from grade F0-1 fibrosis (AUC = 0.750,P< 0.001) (Supplementary Table 1 , Figures 2 and 3).
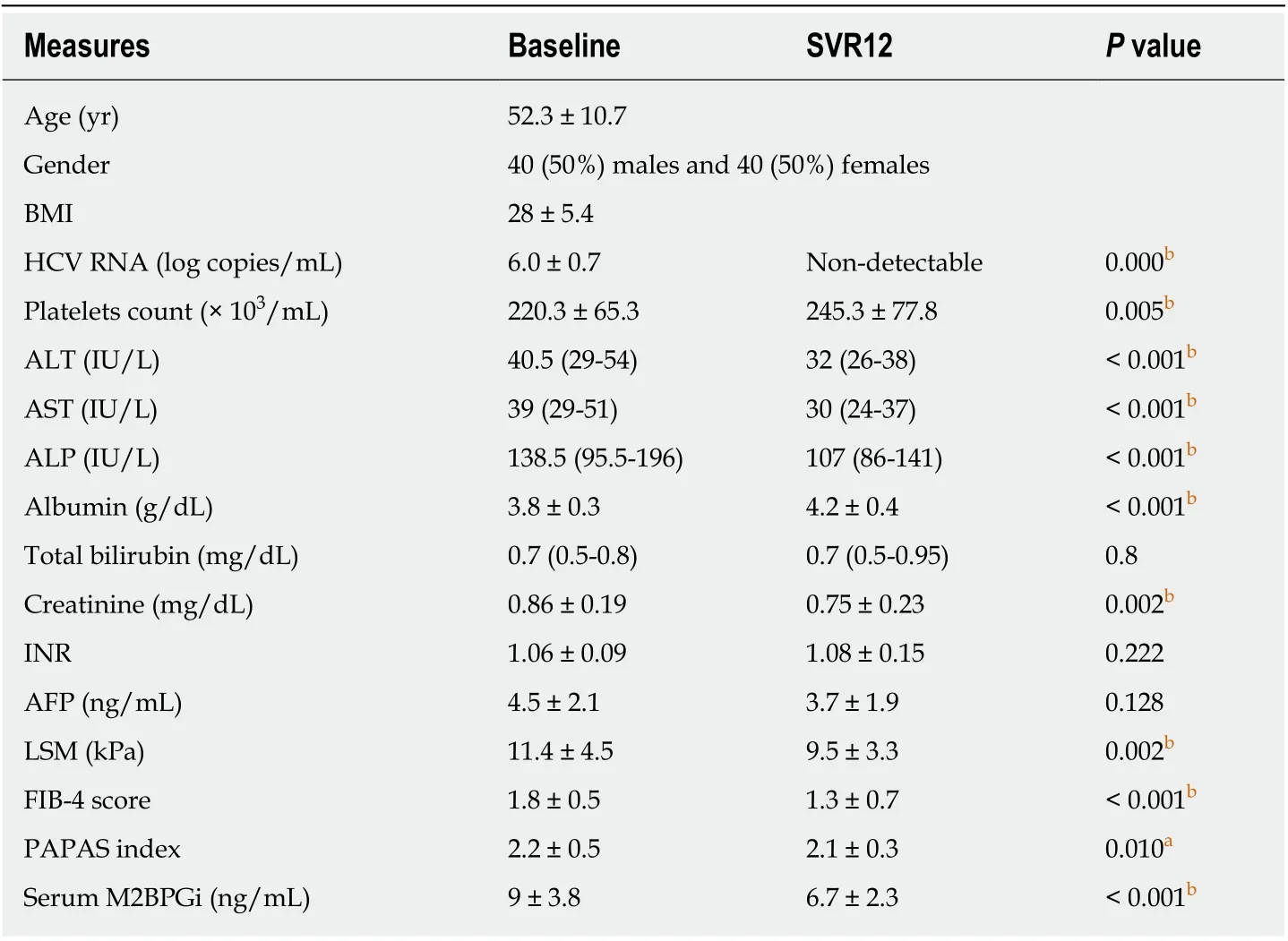
Table 1 Patients’ characteristics at baseline and sustained virologic response 12
DISCUSSION
Currently, non-invasive methods for detecting liver fibrosis are used much more frequently than liver biopsies[40]. M2BPGi has been shown to be a useful predictor of liver fibrosis[14,17,41]. Previous reports have documented a substantial improvement in liver fibrosis after patients achieve SVR12 through treatment with DAAs for HCV[42-44].Here, we aimed to investigate the diagnostic accuracy of serum M2BPGi levels for assessing the grade of liver fibrosis in patients with CHC before and after DAAsbased treatment, as well as to compare its diagnostic value with the FIB-4 score and PAPAS index.
The present study confirms the efficacy and safety of sofosbuvir + daclatasvir ±ribavirin in real-world situations. The SVR12 is 100%.
Similar to previous studies[33,45-48], we observed an improvement in liver functions after patients achieved SVR12, as indicated by a significant decrease in the levels of ALT, AST and ALP, and a significant increase in the serum albumin levels and platelets count. Additionally, in consistency with previous literature[37,45,47,49-52], serum M2BPGi levels, LSM, FIB-4 score and PAPAS index decreased significantly after patients achieved SVR12. These findings suggest the possibility of liver fibrosis regression after viral eradication was achieved.
In agreement with previous reports[3,16,31,30,53], we observed a significantly increasing trend of serum M2BPGi levels with the progression of liver fibrosis both at baseline and SVR12 (P< 0.001). Moreover, in accordance with Akahaneet al[46]and Ishikawaet al[36], serum M2BPGi levels decreased significantly at SVR12 (P< 0.001). In addition, in a study by Miyakiet al[31], serum M2BPGi levels did not change in the non-SVR group(P= 0.715), but decreased significantly in the SVR group (P< 0.0001). These results suggest that serum M2BPGi would be a good surrogate marker for predicting and differentiating liver fibrosis stages.
In the present study, pre-treatment serum M2BPGi levels correlated with bilirubin and AST levels, while serum M2BPGi levels correlated with platelets count and INR at SVR12. These findings suggest that M2BPGi reflects not only the severity of liver fibrosis but also the severity of liver inflammation in CHC patients[18]. This may beattributed to the role of M2BPGi as a messenger between HSCs and Kupffer cells and its accompanying inflammation[13].
Table 2 Liver stiffness measurements of all patients at baseline and sustained virologic response 12
Similar to our results, Uraet al[30]reported a significant negative correlation between serum M2BPGi levels and platelets count (r = -0.47,P< 0.0001). Additionally,Yamasakiet al[54]observed a significant positive correlation between serum M2BPGi and bilirubin levels (r = 0.091,P= 0.001) and a significant negative correlation with platelets count (r = -0.147,P< 0.001). However, in contrast to our results, Yasuiet al[55]observed a positive correlation between serum M2BPGi and AFP levels (r = 0.428,P<0.001), and a negative correlation with albumin levels (r = -0.471,P< 0.001).
In agreement with the present study, Tawaraet al[53]reported a correlation coefficient between serum M2BPGi levels and FIB-4 score of less than 0.4, suggesting that the correlation between serum M2BPGi levels and FIB-4 score was weak. In contrast, Uraet al[30]and Yasuiet al[55]detected a significant positive correlation between serum M2BPGi levels and FIB-4 score (r = 0.66,P< 0.0001 and r = 0.546,P<0.001, respectively). This discrepancy can be attributed to the different sample size.
In terms of differentiation of liver fibrosis grades, consistent with the present study,Xuet al[16]reported that the AUC values of M2BPGi for predicting fibrosis grade ≥ F2 and F4 were significantly superior to the values of FIB-4 score (0.774vs0.702,P<0.001 and 0.892vs0.818,P< 0.05), respectively. In contrast, Tawaraet al[53]reported that the FIB-4 score had a greater AUC value for differentiating of fibrosis grades than M2BPGi (AUC values were 0.768, 0.827 and 0.876 for fibrosis grade F ≥ 2, F ≥ 3 and F4,respectively), while the AUC values of M2BPGi were 0.747, 0.733 and 0.796 for fibrosis grade F ≥ 2, F ≥ 3 and F4, respectively.
The limitations of the present study are the absence of a paired histological evaluation due to the invasiveness of liver biopsy, and the short duration of follow up after completion of treatment. Further large-scale studies with a longer follow-up period should be performed.
In conclusion, M2BPGi is a reliable marker for the non-invasive assessment and prediction of liver fibrosis regression in patients with CHC who achieved an SVR with DAAs therapy.

Table 3 Non-invasive assessment data obtained at baseline and sustained virologic response 12 from patients stratified according to the fibrosis grade

Table 4 Correlations of serum Mac-2 binding protein glycosylation isomer levels with laboratory data

Table 5 Correlations between non-invasive assessment methods at baseline and sustained virologic response 12
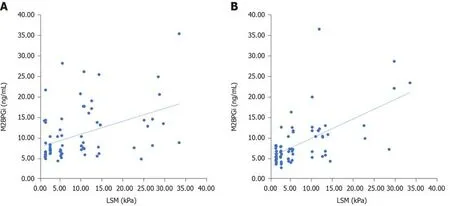
Figure 1 Correlation between serum Mac-2 binding protein glycosylation isomer levels and liver stiffness measurement at baseline and after patients achieved sustained virologic response 12. A: Correlation between serum Mac-2 binding protein glycosylation isomer (M2BPGi) levels and liver stiffness measurement (LSM) at baseline; B: Correlation between serum M2BPGi levels and LSM After patients achieved sustained virologic response 12. M2BPGi: Mac-2 binding protein glycosylation isomer; LSM: Liver stiffness measurement; SVR: Sustained virologic response.
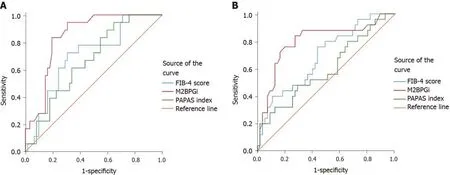
Figure 2 Diagnostic performance of non-invasive parameters in differentiating grades F4 from F0-3 at baseline and after patients achieved sustained virologic response 12. A: Diagnostic performance of non-invasive parameters in differentiating grades F4 from F0-3 at baseline; B: Diagnostic performance of noninvasive parameters in differentiating grades F4 from F0-3 after patients achieved sustained virologic response 12. FIB-4: Fibrosis-4; M2BPGi: Mac-2 binding protein glycosylation isomer.

Figure 3 Diagnostic performance of non-invasive parameters in differentiating grades F3-4 from F0-2 at baseline and after patients achieved sustained virologic response 12. A: Diagnostic performance of non-invasive parameters in differentiating grades F3-4 from F0-2 at baseline; B: Diagnostic performance of noninvasive parameters in differentiating grades F3-4 from F0-2 After patients achieved sustained virologic response 12. FIB-4: Fibrosis-4; M2BPGi: Mac-2 binding protein glycosylation isomer.
ARTICLE HIGHLIGHTS
Research background
Assessing liver fibrosis is important for predicting the efficacy of direct-acting antivirals (DAAs)and patient prognosis. Non-invasive techniques to assess liver fibrosis are becoming important.Recently, serum Mac-2 binding protein glycosylation isomer (M2BPGi) was identified as a noninvasive marker of liver fibrosis.
Research motivation
The approval of DAAs was a revolution in hepatitis C virus eradication, with sustained virologic response (SVR) rates exceeding 90%. However, a few reports have documented the improvement in liver fibrosis in patients treated with DAAs. Although liver biopsy is considered the gold standard for stratifying hepatic fibrosis, its clinical utility is substantially limited because of the invasiveness and the sampling variability. Accordingly, serum M2BPGi was evaluated as a noninvasive marker for assessing the grade of hepatic fibrosis in patients who have achieved SVR after antiviral therapy.
Research objectives
We aimed to investigate the diagnostic accuracy of serum M2BPGi levels in assessing the grade of liver fibrosis in patients with chronic hepatitis C (CHC) before and after DAAs-based treatment, as well as to compare its diagnostic value with the FIB-4 score and PAPAS index.
Research methods
Eighty treatment-naïve adult patients with CHC who were eligible for DAAs therapy were consecutively enrolled in this observational cohort study. For 12 weeks, 65 patients were treated with sofosbuvir/daclatasvir, and 15 patients were treated with sofosbuvir/daclatasvir and a weight-based dose of ribavirin. We measured serum M2BPGi levels, PAPAS index, FIB-4 score and liver stiffness measurements (LSM) at baseline and 12 weeks after the end of treatment.Serum M2BPGi levels were measured using enzyme-linked immunosorbent assay.
Research results
All patients achieved SVR12 (100%). Serum M2BPGi levels, LSM, FIB-4 score and PAPAS index decreased significantly at SVR12 (P< 0.05). Serum M2BPGi levels correlated positively with LSM at baseline and SVR12 (P< 0.001). At baseline, compared with the FIB-4 score and PAPAS index,M2BPGi was the best marker to distinguish patients with grade F4 fibrosis (AUC = 0.801,P<0.001), patients with grade F2 from grade F0-1 fibrosis (AUC = 0.713,P= 0.012), patients with grade F3-4 from grade F0-2 fibrosis (AUC = 0.730,P< 0.001), and patients with grade F2-4 from grade F0-1 fibrosis (AUC = 0.763,P< 0.001). At SVR12, M2BPGi had the greatest AUCs for differentiating patients with grade F4 fibrosis (AUC = 0.844,P< 0.001), patients with grade F3 from grade F0-2 fibrosis (AUC = 0.893,P= 0.002), patients with grade F3-4 from grade F0-2 fibrosis (AUC = 0.891,P< 0.001), and patients with grade F2-4 from grade F0-1 fibrosis (AUC =0.750,P< 0.001).
Research conclusions
M2BPGi is a reliable marker for the non-invasive assessment and prediction of liver fibrosis regression in patients with CHC who achieved an SVR with DAAs therapy.
Research perspectives
Non-invasive methods have been previously proposed and validated for the assessment of hepatic fibrosis. In this study, we confirm that serum M2BPGi is a reliable marker for liver fibrosis. Further studies are needed to investigate its therapeutic potential and ultimate clinical utility.
 World Journal of Gastroenterology2020年21期
World Journal of Gastroenterology2020年21期
- World Journal of Gastroenterology的其它文章
- Tailored classification of portal vein thrombosis for liver transplantation: Focus on strategies for portal vein inflow reconstruction
- Alternative uses of lumen apposing metal stents
- lnnate immune recognition and modulation in hepatitis D virus infection
- Use of zebrafish embryos as avatar of patients with pancreatic cancer: A new xenotransplantation model towards personalized medicine
- Gan Shen Fu Fang ameliorates liver fibrosis in vitro and in vivo by inhibiting the inflammatory response and extracellular signalregulated kinase phosphorylation
- Periportal thickening on magnetic resonance imaging for hepatic fibrosis in infantile cholestasis
