Discussion on Preparation and Application of BiOIO3-based Composite Materials
Chengdong XU Yushan WAN

Abstract BiOIO3 is a layered semiconductor photocatalyst, which has good chemical properties and has attracted wide attention from researchers because of its unique structure. However, pure BiOIO3 has defects such as insufficient response to visible light and easy recombination of photogenerated electrons. Therefore, in recent years, scholars have tried to modify BiOIO3 to expand its light absorption range, reduce the recombination of photogenerated electron-hole pairs and reduce its limitations, thereby improving its visible light catalytic performance. Current researches focus on the improvement of the catalytic activity of photocatalytic materials from morphology control, precious metal deposition, ion doping and construction of heterojunctions.
Key words BiOIO3; Photocatalysis; Composite material; Degradation
Received: June 29, 2020 Accepted: August 30, 2020
Supported by Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX20_0935).
Chengdong XU (1997-), male, P. R. China, master, devoted to research about water pollution control.
*Corresponding author. E-mail: wangyushan@126.com.
As a stumbling block on the road to sustainable development, environmental pollution and energy crisis have always been two major issues that need to be resolved urgently. It is difficult for traditional methods to deal with current problems due to their own defects. Since the 1970s, semiconductor photocatalytic materials have been widely used in the fields of degradation of organic matter, degradation of organic acids to produce hydrogen, adsorption of heavy metal ions, and catalytic degradation of dye wastewater.
BiOIO3 is an excellent broad-band gap photocatalyst. Due to its unique heterogeneous layer structure and internal polar field, it has a high photo-induced charge separation efficiency, which is very helpful for the separation of photogenerated electrons and holes, and further enhances its photocatalytic activity. However, BiOIO3 has a wide band gap and can only be excited by ultraviolet light, but ultraviolet light accounts for less than 5% of the solar spectrum, so the practical application of BiOIO3 has been greatly restricted. Therefore, it is necessary to modify the chemical composition and structure of BiOIO3.
The paper expounded the preparation methods, modification research and practical application of BiOIO3 composite materials at home and abroad in recent years, and prospected the future development direction of BiOIO3.
Preparation Method of BiOIO3
Most scholars use one-step hydrothermal method to synthesize BiOIO3 nanosheets, in which bismuth nitrate and potassium iodate are used as bismuth and iodine sources. Wang and Huang et al.[1] successfully prepared BiOIO3 by hydrothermal method. The method was as follows: First, under vigorous stirring, 1 mmol of bismuth nitrate was dissolved in 80 ml of deionized water. After stirring for 30 min, 1 mmol of potassium iodate was added to the solution, and after 10 min of continue stirring, the suspension was transferred to a 100 ml reactor at 150 ℃ and heated for 5 h. After cooling to room temperature, the product was filtered and washed with deionized water and absolute ethanol 4 times, and then dried at 60 ℃ for 12 h.
Modification Method of BiOIO3
Precious metal deposition
Precious metal deposition refers to the deposition of precious metals on the surface of semiconductor materials, in which electrons are obtained from the semiconductor depending on the surface properties of the semiconductor, which can reduce the recombination probability of photo-generated carrier-hole pairs in the semiconductor material, thereby improving its photocatalytic performance. Commonly used precious metals are Au, Ag, Pt, Pd and so on.
Huang et al.[2] used bismuth nitrate, diiodine pentoxide and tetrachloroauric acid as raw materials to deposit metal Au particles on BiOIO3 by in-situ photosynthesis, and prepared Au/BiOIO3 composite photocatalyst. Rhodamine B was degraded under visible light irradiation to study the performance of the catalyst. After 60 min of xenon lamp (500 W) irradiation, the composite sample 2% Au/BiOIO3 showed the highest photocatalytic activity, and rhodamine B was almost completely degraded. For 2% Au/BiOIO3, the apparent rate constant K was 4.3 times that of the original BiOIO3.
Su et al.[3] used bismuth nitrate, sodium iodate and chloroplatinic acid as raw materials, and prepared a series of Pt/BiOIO3 nanoplate photocatalysts with different thicknesses (25, 50, 150 nm). When the loading amount of Pt nanoparticles was 1.5wt%, the 1.5wt%Pt@150nm sample showed the highest hydrogen production rate.
Element doping
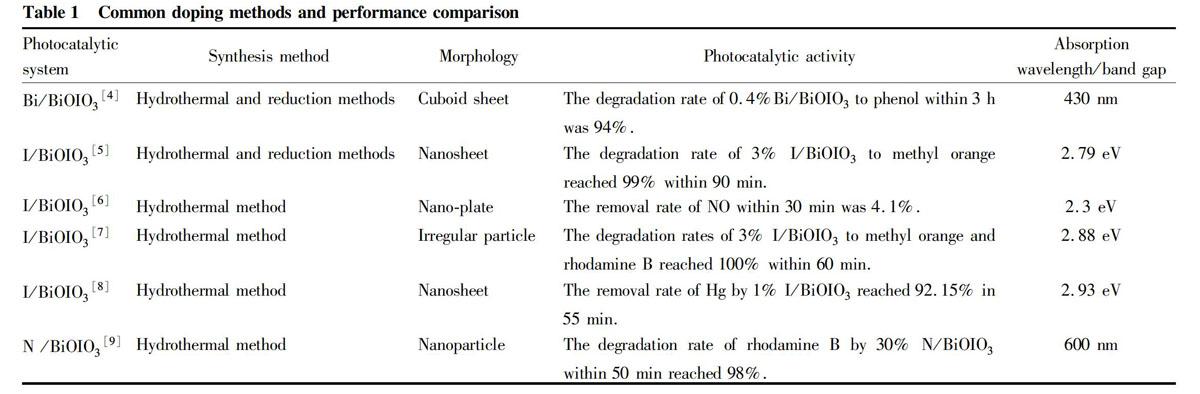
Element doping refers to the introduction of doping elements into the semiconductor structure through physical or chemical methods to adjust its energy band structure, change the trajectory of photo-generated electrons and holes, and improve its photocatalytic performance. In terms of doping, it can be divided into metal cation doping, non-metal anion doping and co-doping.
Heterojunction construction
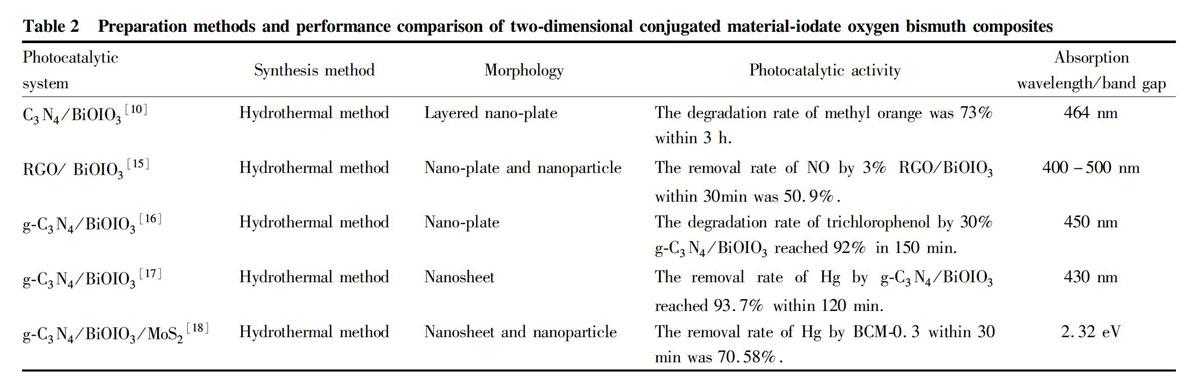
The construction of a heterojunction refers to the combination of other materials and semiconductor materials, by which the energy level difference between semiconductors promotes the transition of photogenerated carriers from the energy level of one material to the energy level of another material, which promotes the effective separation of photogenerated electrons and holes and improves the photoresponse ability of the material, thereby achieving the purpose of improving the performance of the catalyst. For bismuth oxyiodate, there have been many literatures reporting the improvement of visible light catalytic performance of catalysts by constructing C3N4/BiOIO3[10], GR/BiOIO3[11], BiOI/BiOIO3[12], Ag/AgCl/BiOIO3[13] and BiOBr/BiOIO3[14] and other heterojunctions.
Two-dimensional conjugated material-BiOIO3 composite material
Two-dimensional conjugated materials have lower dimensions, larger specific surface area and more active sites, can quickly conduct electrons into lamellae, and can effectively promote the separation of photogenerated carriers. Graphene and graphitic carbon nitride (g-C3N4) are both two-dimensional conjugated semiconductor materials. Researchers have conducted research on g-C3N4 in many fields, including sterilization, anti-fouling coatings, and carbon dioxide reduction.
Inorganic semiconductor-BiOIO3 composite material
Inorganic semiconductors can form heterojunctions with iodate oxygen bismuth, which can expand the absorption spectrum of materials, enhance the visible light photocatalytic effects of the composite materials, promote the separation and transfer of electrons and holes, and inhibit their recombination. Compared with the absorption of BiOIO3 in the ultraviolet region, this type of composite material can more effectively use sunlight.
Other BiOIO3-based composite materials
Zeng et al.[26] used the hydrothermal method to adjust the mass ratio of Fe2O3 and BiOIO3, and prepared different ratios of Fe2O3/BiOIO3 composite nanosheet materials, which improved the efficiency of charge separation and greatly improved the performance of photocatalytic materials. The photocatalytic activity of the materials was evaluated by degrading p-nitrophenol. After 80 min of visible light irradiation, the 15% Fe2O3/BiOIO3 sample had the highest oxidation ability to PNP, and the degradation efficiency reached 99.9%.
Lu et al.[27] synthesized MoS2/BiOIO3 nanocomposites using bismuth nitrate, thiourea, ammonium molybdate and potassium iodate as raw materials, and evaluated the photocatalytic performance of the samples by removing rhodamine B in the water. The experimental results showed that after 90 min of visible light irradiation, the degradation rate of BM-1.5 to rhodamine B was 98.7%.
Application of Photocatalytic Degradation of BiOIO3
Denitrification application
As one of the main pollutants in the atmosphere, NO will cause great harm to the environment and the human body. The g-C3N4/BiOIO3 heterostructure material prepared by Wang et al.[28] was used for catalytic degradation of NO under visible light, and the final removal rate of NO reached 57% within 60 min.
Matejka[29] used a two-step method to prepare a g-C3N4/BiOIO3 nanocomposite. The nanocomposite was irradiated under visible light, and the N2O concentration was measured at regular intervals. When the weight ratio of g-C3N4 and BiOIO3 was 1∶1, N2O had the highest conversion rate.
Catalytic degradation of heavy metals
At present, the content of heavy metals in the environment far exceeds the normal range. Heavy metals are widely distributed and accumulated, which directly threatens human health, and the environmental degradation is quite serious. Tao et al.[30] prepared BiOBr/BiOIO3 nanosphere photocatalytic materials by ethylene glycol solvothermal method, and removed heavy metals in a nitrogen and oxygen simulated flue gas system. The experimental results showed that 10% BiOBr/BiOIO3 showed the highest photocatalytic activity, and the removal rate of heavy metals was as high as 90.25%.
Catalytic degradation of propyl p-hydroxybenzoate
As an emerging pollutant, propyl paraben is harmful to the body, and it is irritating to the eyes, mucous membranes and upper respiratory tract. Once inhaled, symptoms such as breathing difficulties will occur. Therefore, it is necessary to reduce the propyl paraben in the environment. Yang et al.[31] used PrP as a model pollutant and prepared a series of BiOIO3 samples heat-treated at different temperatures. After 60 min of visible light irradiation, the degradation efficiency of BiOIO3-125 on PrP was the highest at 95.8%.
Degradation of antibiotics
Tetracycline is the most basic compound in the tetracycline family of antibiotics, and tetracycline drugs are also the most widely used class of drugs. Such compounds cannot be degraded easily and remain in the environment for a long time, and have huge ecotoxicological effect on the environment. Huang et al.[9] synthesized N-doped BiOIO3 photocatalyst by calcination, and evaluated the photocatalytic performance under LED light. For 30% N-BiOIO3, 80% of tetracycline were degraded within 70 min, and 90% of ciprofloxacin were degraded within 120 min. Zhang et al.[25] synthesized Ag3VO4/BiOIO3 heterojunction photocatalyst by hydrothermal method. In the visible light degradation experiment of TC, the degradation rate of TC reached 80% after 15 min of irradiation.
Catalytic degradation of mercury
Mercury is a highly toxic element that can accumulate in organisms, and its traces can be found all over the world. The impact of mercury pollution has attracted peoples attention and has also attracted the attention of countries all over the world. Ling et al.[11] prepared GR/BiOIO3 nanocomposites by hydrothermal method, and removed gaseous mercury through photocatalysis. The experiments showed that 3% GR/BiOIO3 removed 88.5% of mercury within 60 min, achieving the highest mercury removal efficiency. The g-C3N4/BiOIO3 composite catalysts prepared by Zhang et al.[32] were used to degrade mercury under visible light, and the removal efficiency of Hg by BC-5 reached 93.5% within 60 min.
Jia et al.[33] synthesized a MoS2/C500/BiOIO3 ternary composite catalyst and performed mercury removal experiments under visible light. The mercury removal efficiency was 78.32% within 40 min, which was significantly higher than C500, MoS2, BiOIO3, and MoS2/BiOIO3. Wang et al.[34] found that the catalytic degradation of mercury by TiO2-BiOIO3 composite catalyst reached 95% after 60 min. Guan et al.[18] synthesized a MoS2/g-C3N4/BiOIO3 ternary composite catalyst. Compared with BiOIO3/g-C3N4, the prepared nanocomposite exhibited better photocatalytic activity, and its highest efficiency achieved within 30 min was degrading 70.58% of elemental mercury.
Conclusions
However, as a new type of semiconductor photocatalytic material, BiOIO3 has both advantages and disadvantages, such as insufficient response to light and low utilization of sunlight. However, if the BiOIO3 composite materials can be developed to improve the photocatalytic performance, improve the use efficiency of materials and reduce the cost, it will be an important direction of the future photocatalysis research.
References
[1] WANG W, HUANG B, MA X, et al. Efficient separation of photogenerated electron-hole pairs by the combination of a heterolayered structure and internal polar field in pyroelectric BiOIO3 nanoplates[J]. Chemistry-a European Journal, 2013, 19(44): 14777-14780.
[2] HUANG H, XIAO K, TIAN N, et al. Plasmon induced Au particle and surface oxidation co-decorated BiOIO3 heteronanostructures with highly promoted photocatalysis and photoelectrochemical properties[J]. Rsc Advances, 2015, 5(99): 81078-81086.
[3] SU Y, ZHANG L, WANG W. Internal polar field enhanced H-2 evolution of BiOIO3 nanoplates[J]. International Journal of Hydrogen Energy, 2016, 41(24): 10170-10177.
[4] YU S, HUANG H, DONG F, et al. Synchronously achieving plasmonic Bi metal deposition and I-doping by utilizing BiOIO3 as the self-sacrificing template for high-performance multifunctional applications[J]. Acs Applied Materials & Interfaces, 2015, 7(50): 27925-27933.
[5] FENG J, HUANG H, YU S, et al. A self-sacrifice template route to iodine modified BiOIO3: band gap engineering and highly boosted visible-light active photoreactivity[J]. Physical Chemistry Chemical Physics, 2016, 18(11): 7851-7859.
[6] SUN Y, XIONG T, DONG F, et al. Interlayer-I-doped BiOIO3 nanoplates with an optimized electronic structure for efficient visible light photocatalysis[J]. Chemical Communications, 2016, 52(53): 8243-8246.
[7] HUANG H, OU H, FENG J, et al. Achieving highly promoted visible-light sensitive photocatalytic activity on BiOIO3 via facile iodine doping[J]. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 2017, 518(5): 158-165.
[8] WU J, XU K, LIU Q, et al. Controlling dominantly reactive (010) facets and impurity level by in-situ reduction of BiOIO3 for enhancing photocatalytic activity[J]. Applied Catalysis B-Environmental, 2018, 232(15): 135-145.
[9] HUANG L, WANG Y, LI Y, et al. Calcination synthesis of N-doped BiOIO3 with high LED-light-driven photocatalytic activity[J]. Materials Letters, 2019, 246(1): 219-222.
[10] WANG W, CHENG H, HUANG B, et al. Hydrothermal synthesis of C3N4/BiOIO3 heterostructures with enhanced photocatalytic properties[J]. Journal of Colloid and Interface Science, 2015, 442(15): 97-102.
[11] LING Y, WU J, MAN X, et al. BiOIO3/graphene interfacial heterojunction for enhancing gaseous heavy metal removal[J]. Materials Research Bulletin, 2020(122): 110620.
[12] ZHEN W, LIU Y, JIA X, et al. Reductive surfactant-assisted one-step fabrication of a BiOI/BiOIO3 heterojunction biophotocatalyst for enhanced photodynamic theranostics overcoming tumor hypoxia[J]. Nanoscale Horizons, 2019, 4(3): 720-726.
[13] XIONG T, ZHANG H, ZHANG Y, et al. Ternary Ag/AgCl/BiOIO3 composites for enhanced visible-light-driven photocatalysis[J]. Chinese Journal of Catalysis, 2015, 36(12): 2155-2163.
[14] ZHANG H, NIU CG, YANG SF, et al. Facile fabrication of BiOIO3/BiOBr composites with enhanced visible light photocatalytic activity[J]. Rsc Advances, 2016, 6(69): 64617-64625.
[15] XIONG T, DONG F, ZHOU Y, et al. New insights into how RGO influences the photocatalytic performance of BiOIO3/RGO nanocomposites under visible and UV irradiation[J]. Journal of Colloid and Interface Science, 2015, 447(1): 16-24.
[16] GONG Y, QUAN X, YU H, et al. Enhanced photocatalytic performance of a two-dimensional BiOIO3/g-C3N4 heterostructured composite with a Z-scheme configuration[J]. Applied Catalysis B-Environmental, 2018(237): 947-956.
[17] WU J, SHENG P, XU W, et al. Constructing interfacial contact for enhanced photocatalytic activity through BiOIO3/g-C3N4 nanoflake heterostructure[J]. Catalysis Communications, 2018(109): 55-59.
[18] GUAN Y, WU J, LIN Y, et al. Solvent-exfoliation of transition-metal dichalcogenide MoS2 to provide more active sites for enhancing photocatalytic performance of BiOIO3/g-C3N4 photocatalyst[J]. Applied Surface Science, 2019(481): 838-851.
[19] DONG F, XIONG T, SUN Y, et al. Controlling interfacial contact and exposed facets for enhancing photocatalysis via 2D-2D heterostructures[J]. Chemical Communications, 2015, 51(39): 8249-8252.
[20] CUI D H, SONG X C, ZHENG YF. A novel AgI/BiOIO3 nanohybrid with improved visible-light photocatalytic activity[J]. Rsc Advances, 2016, 6(76): 71983-71988.
[21] HUANG H, XIAO K, LIU K, et al. In situ composition-transforming fabrication of BiOI/BiOIO3 heterostructure: Semiconductor p-n junction and dominantly exposed reactive facets[J]. Crystal Growth & Design, 2016, 16(1): 221-228.
[22] WU J, CHEN X, LI C, et al. Hydrothermal synthesis of carbon spheres-BiOI/BiOIO3 heterojunctions for photocatalytic removal of gaseous Hg degrees under visible light[J]. Chemical Engineering Journal, 2016, 304(15): 533-543.
[23] CHEN F, HUANG H, ZHANG Y, et al. Achieving UV and visible-light photocatalytic activity enhancement of AgI/BiOIO3 heterostructure: Decomposition for diverse industrial contaminants and high mineralization ability[J]. Chinese Chemical Letters, 2017, 28(12): 2244-2250.
[24] ZHOU R, WU J, ZHANG J, et al. Photocatalytic oxidation of gas-phase Hg-0 on the exposed reactive facets of BiOI/BiOIO3 heterostructures[J]. Applied Catalysis B-Environmental, 2017, 204(5): 465-474.
[25] ZHANG J, MA Z. Ag3VO4/BiOIO3 heterojunction with enhanced visible-light-driven catalytic activity[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018(88): 177-185.
[26] ZENG H, LIU X, WEI T, et al. Boosting visible light photo-/Fenton-catalytic synergetic activity of BiOIO3 by coupling with Fe2O3[J]. Rsc Advances, 2017, 7(38): 23787-23792.
[27] LU M, XIAO X, WANG Y, et al. Construction of novel BiOIO3/MoS2 2D/2D heterostructures with enhanced photocatalytic activity[J]. Journal of Alloys and Compounds, 2020, 831(5): 154789.
[28] WANG B, CHEN D, LI N, et al. Z-scheme photocatalytic NO removal on a 2D/2D iodine doped BiOIO3/g-C3N4 under visible-light irradiation[J]. Journal of colloid and interface science, 2020, 576(15): 426-434.
[29] MATEJKA V, SIHOR M, RELI M, et al. Composites g-C3N4 and BiOIO3 for photocatalytic decomposition of N2O[J]. Materials Science in Semiconductor Processing, 2019(100): 113-122.
[30] TAO J, JIANG W, JIE S, et al. In situ self-growing 3D hierarchical BiOBr/BiOIO3 Z-scheme heterojunction with rich oxygen vacancies and iodine ions as carriers transfer dual-channels for enhanced photocatalytic activity[J]. Chemical Engineering Journal, 2020, 396(15): 125258
[31] YANG J, ZHENG D, XIAO X, et al. Iodine self-doping and oxygen vacancies doubly surface-modified BiOIO3: Facile in situ synthesis, band gap modulation, and excellent visible-light photocatalytic activity[J]. Chemical Engineering Journal, 2019, 373(1): 935-945.
[32] ZHANG X, WANG D, MAN X, et al. Influence of BiOIO3 morphology on the photocatalytic efficiency of Z-scheme BiOIO3/g-C3N4 heterojunctioned composite for Hg-0 removal[J]. Journal of Colloid and Interface Science, 2020, 558(15): 123-136.
[33] JIA T, XU K, WU J, et al. Constructing 2D BiOIO3/MoS2 Z-scheme heterojunction wrapped by C500 as charge carriers transfer channel: Enhanced photocatalytic activity on gas-phase heavy metal oxidation[J]. Journal of Colloid and Interface Science, 2020, 562(7): 429-443.
[34] WANG T, HE S, ZHANG Y, et al. Photocatalytic removal of elemental mercury on TiO2-BiOIO3 heterostructures: Mercury transformation, sulfur tolerance and SO2/SO3 conversion[J]. Chemical Engineering Journal, 2020, 388(15): 124390.
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Effects of LPS on the Gene Expression of NMB and Its Receptor in the Hypothalamic-pituitary-testicul
- Comparative Nutritional Analysis on Fish Meal and Meat and Bone Meal of Harmless Treatment of Dead Pig Carcass
- A Monophyletic Status of Axis Genus in Subfamily Cervinae Supported by the Complete Mitochondrial Genome of Chinese Hog Deer (Axis porcinus)
- Prevention and Control Measures of the Occurrence of Ceracris kiangsu Tsai in Sugarcane Areas of Yunnan Province
- Study on the Accuracy of Different CASA Systems in the Quality Detection of Fresh Boar Semen
- Evaluation and Selection of Appropriate Tobacco Varieties for Badong Hubei Province

