Effects of LPS on the Gene Expression of NMB and Its Receptor in the Hypothalamic-pituitary-testicul
Zhiyu MA Ying ZHANG Junpei GUO Jinlong ZHANG

Abstract [Objectives] This study was conducted to investigate the effect of lipopolysaccharide (LPS) on the gene expression levels of neuromedin B (NMB) and its receptor (NMBR) in the hypothalamic-pituitary-testis (HPT) axis of male rabbits.
[Methods] Forty male New Zealand white rabbits were randomly divided into an experimental group injected with LPS and a control group. At 0, 1.5, 3, 6 and 12 h after LPS injection, four rabbits in each group were sacrificed, and the hypothalamus, pituitary and testicular tissues were collected. RT-PCR was carried out to detect the expression of rabbit NMB and NMBR mRNA in the HPT axis.
[Results] NMB and NMBR mRNA were expressed in the hypothalamus, pituitary gland and testis of rabbits, and there was a certain time effect, indicating that the NMB/NMBR system is involved in the activities of the hypothalamus-pituitary-testis (HPT) axis. At 1.5 h after LPS stimulation, the expression of NMB mRNA in the hypothalamus increased significantly, and showed an extremely significant difference from the control group (P<0.01), while the expression of NMBR mRNA in the hypothalamus increased, but was not significantly different from the control group (P>0.05). The expression levels of NMB and NMBR mRNA in the pituitary and testis tissues in the HPT axis at different time after the LPS treatment were not significantly different from those in the control group (P>0.05). Therefore, LPS stimulation still has a certain effect on the expression of NMB and its receptor mRNA in the HPT axis.
[Conclusions]This study is of great significance for the in-depth study on the mechanism of damage caused by immune stress to livestock and poultry reproduction.
Key words LPS; NMB; NMBR; Hypothalamic-pituitary-testicular axis
Received: July 27, 2020 Accepted: September 28, 2020
Supported by National Natural Science Foundation of China (31802149, 31372388); Natural Science Foundation of Jiangsu Province (BK20180919); Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Zhiyu MA (1988-), male, P. R. China, lecturer, PhD, devoted to research about neuropeptides and its receptors, neuroendocrine and reproduction.
*Corresponding author. E-mail: zjl@yzu.edu.cn.
Neuromedin B (NMB) is a neuropeptide isolated and purified from porcine spinal cord in 1983[1]. It belongs to the bombesin family and plays a variety of important roles in animals through its receptor NMBR. NMB has an important physiological role in regulating food intake, stress, endocrine and reproduction[2-3]. In 2015, Guo et al.[4] successfully cloned rabbit NMB and NMBR genes, and found that NMB and its receptor are expressed in the hypothalamic-pituitary-testicular axis. In addition, studies have found that NMB can promote the secretion of a variety of hormones in the HPT axis of male rats[2]. It has also been found that NMB and NMBR are highly expressed in the reproductive axis of pigs and can affect the secretion of testosterone[5]. All these indicate that NMB has a regulatory effect on reproduction. Lipopolysaccharide (LPS) is a characteristic component of the cell wall of gram-negative bacteria. Studies have found that the immune stress induced by LPS can disrupt the reproductive function of animals, and affect normal sexual behavior by destroying the neuroendocrine function of animals[6]. The hypothalamic-pituitary-adrenal (HPA) axis is an important neuroendocrine immune system of the body, which plays an important role in the immune stress regulatory network[7]. However, there are few reports about the effect of immune stress on the expression of NMB and NMBR mRNA in the hypothalamic-pituitary-gonad axis. Therefore, studying the relationship between the neuropeptide NMB/NMBR system and immune stress is of great significance for in-depth study of the mechanism of damage caused by immune stress to livestock and poultry reproduction.
Materials and Methods
Experimental animals
Forty healthy male New Zealand white rabbits of 3 months old, weighing about 2 kg, were purchased from Nanjing Shengmin Scientific Research Animal Farm. They were raised in the animal breeding room of Yangzhou University, and the adaptation period was 5 d.
Main instruments and reagents
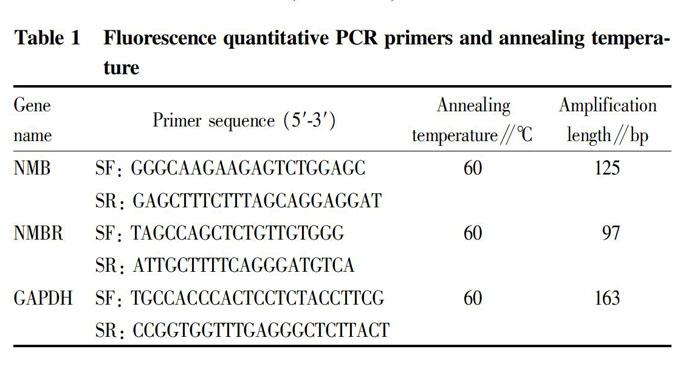
The instruments used included 4 ℃ desk type high-speed freezing centrifuge (Thermo, USA), ABI PRISM 7300 fluorescent PCR machine (Applied Biosystems, USA) and electrophoresis gel imaging system GIS 2500 (Tanon). LPS was purchased from Sigma-Aldrich; RNA isolater, 2 × Taq Plus Master Mix, Hiscript Q RT SuperMix for qPCR(+gDNA wiper)and SYBR Green Master Mix Kit were purchased from Vazyme Biotech Co., Ltd.; rabbit NMB, NMBR and GAPDH primers were synthesized by Shanghai Invitrogen Company; and other reagents were all domestically pure.
Experimental design and sample collection
The experiment was carried out at room temperature (24 ℃). Forty male New Zealand white rabbits were randomly divided into an experimental group (20) injected with LPS and a control group (20) injected with saline. The experimental group was injected with LPS diluted with normal saline at a dose of 0.5 mg LPS/kg into the ear vein, and the control group was injected with the same amount of normal saline through the ear vein. At 0, 1.5, 3, 6 and 12 h after injection, four rabbits were taken from each group, anesthetized with ether, and sacrificed. The hypothalamus, pituitary and testicular tissues were quickly collected and put into liquid nitrogen for quick freezing, and stored in a refrigerator at -80 ℃ for later use.
Total RNA extraction and cDNA preparation
The Trizol method (according to the RNA isolater instruction) was used to extract total RNA from the hypothalamus, pituitary gland and testis. The concentration and purity of the total RNA were determined by UV spectrophotometer, and the integrity of RNA was tested by gel electrophoresis. According to the measured RNA concentration, the extracted RNA was diluted to 1 μg/μl, and stored at -80 ℃ for later use.
Fluorescence quantitative PCR (RT-PCR)
Fluorescence quantitative PCR was carried out by the RT-PCR method[3] with GAPDH as the endogenous reference to detect the expression levels of NMB and NMBR mRNA in the rabbit hypothalamus, pituitary gland and testis. According to the SYBR Green Master Mix Kit instruction, the reaction conditions were as follows: 95 ℃ for 5 min, and 40 cycles of 95 ℃ for 5 s, 60 ℃ for 30 min and 72 ℃ for 15 s. The primers for the fluorescence quantitative PCR reaction are shown in Table 1. After the PCR reaction, the melting curve analysis and gel electrophoresis detection were conducted to determine the specificity of the amplified product. The 2-ΔΔCt method was used to calculate the relative expression of target mRNA at different time.
Statistical analysis
All data was expressed as mean±standard error (mean±SEM), and the SPSS Statistics 17.0 statistical software was used for statistical analysis and processing. The significance of the difference was detected by one-way analysis of variance (One-Way ANOVA, LSD), in which P<0.05 indicates a significant difference, and P<0.01 indicates an extremely significant difference.
Results and Analysis
Expression of rabbit NMB and its receptor on the hypothalamic-pituitary-testis (HPT) axis
General PCR was performed with rabbit GAPDH, NMB and NMBR specific primers. The amplified products were detected by agarose gel electrophoresis, obtaining specific fragments, respectively. It showed that GAPDH, NMB and NMBR gene amplification obtained specific products in the hypothalamus, pituitary gland and testis, proving that NMB and NMBR mRNA were expressed in the HPT axis of rabbits.
Effect of LPS on the expression of NMB and NMBR mRNA in the hypothalamus of rabbit HPT axis
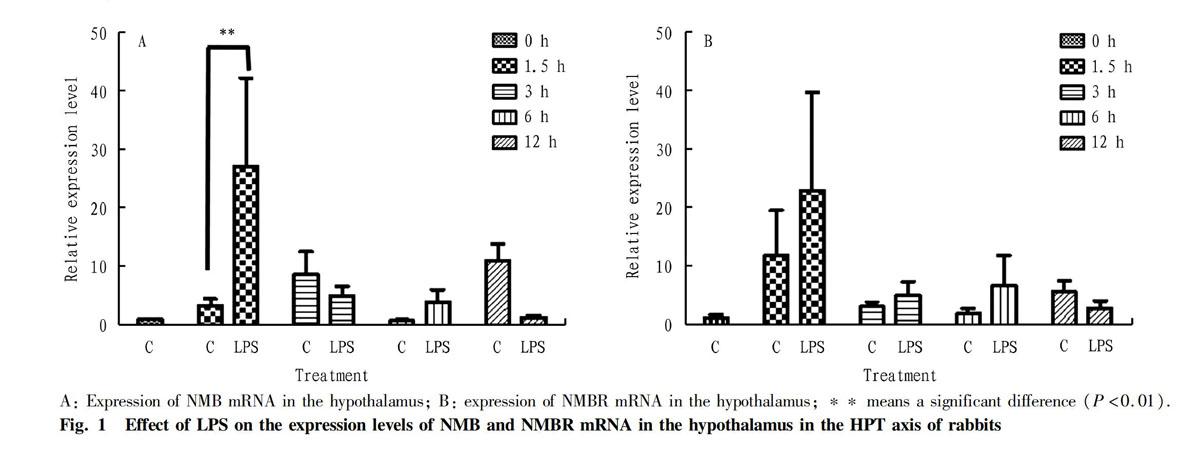
The expression of NMB and NMBR mRNA in the hypothalamic tissue in the HPT axis of rabbits at different times after LPS treatment is shown in Fig. 1. In the hypothalamic tissue, the expression of NMB (Fig. 1-A) and NMBR (Fig. 1-B) mRNA after LPS stimulation was different from the corresponding normal saline control group. Especially, when the rabbits were stimulated with LPS for 1.5 h, the expression levels of NMB and NMBR mRNA were the highest, and the expression of NMB gene increased significantly (P<0.01), which had an extremely significant difference from the control group, but was not significantly different from that at other time (P>0.05).
Effect of LPS on the expression of NMB and NMBR mRNA in the pituitary gland of rabbit HPT axis
The expression of NMB and NMBR mRNA in the pituitary tissue in the HPT axis at different time after the LPS treatment is shown in Fig. 2. In the pituitary tissue, after LPS stimulation, NMB (Fig. 2-A) and NMBR (Fig. 2-B) mRNA increased at 1.5 h, decreased at 3 h, then increased at 6 h and then decreased at 12 h. The expression levels of NMB and NMBR mRNA were the highest when the rabbits were stimulated with LPS for 6 h, but they were not significantly different from the control group (P>0.05).
Zhiyu MA et al. Effects of LPS on the Gene Expression of NMB and Its Receptor in the Hypothalamic-pituitary-testicular Axis of Rabbits
Effect of LPS on the expression of NMB and NMBR mRNA in the testis of rabbit HPT axis
The expression of NMB and NMBR mRNA in the testis tissue in the HPT axis at different time after the LPS treatment is shown in Fig. 3. In the testis, the expression of NMB mRNA (Fig. 3-A) was the highest at 1.5 h after the LPS stimulation, while the expression of NMBR mRNA (Fig. 3-B) was the highest at 3 h. It was slightly different from the expression of NMB and NMBR mRNA in the hypothalamus and pituitary, but the differences from the control group were not significant (P>0.05).
Conclusions and Discussion
As a neurotransmitter and/or neuromediator, NMB plays an important role in regulating the physiological functions of the body. NMB and its receptor are widely expressed in the hypothalamus, pituitary gland and testis of rats and pigs[2], our research also found that NMB and NMBR were expressed in the hypothalamus, pituitary gland and testis of rabbits, and there was a certain time effect, indicating that the NMB/NMBR system is involved in the activities of the hypothalamus-pituitary-testis (HPT) axis.
The results of this study showed that the hypothalamus was the first to respond after LPS stimulation, and the expression of NMB mRNA in the hypothalamus increased significantly at 1.5 h after LPS stimulation and had an extremely significant difference from the control group (P<0.01), while its physiological significance needs to be further studied. Although the expression of NMBR mRNA in the hypothalamus increased, the difference from the control group was not significant (P>0.05). The expression levels of NMB and NMBR mRNA in the pituitary and testis tissues in the HPT axis at different time after LPS treatment were not significantly different from those in the control group (P>0.05). Therefore, LPS stimulation still has a certain effect on the expression of NMB and its receptor mRNA in the HPT axis.
References
[1] NAOTO MINAMINO, KENJI KANGAWA, HISAYUKI MATSUO. Neuromedin B: A novel bombesin-like peptide identified in porcine spinal cord[J]. Biochemical and Biophysical Research Communications, 1983, 114(2): 541-548.
[2] BOUGHTON CK, PATEL SA, THOMPSON EL, et al. Neuromedin B stimulates the hypothalamic-pituitary-gonadal axis in male rats[J]. Regulatory Peptides, 2013: 6-11.
[3] MA ZY, ZHANG Y, CHEN FL, et al. Effects of heat stress on the expression of NMB and its receptor genes and testosterone secretion in rabbit hypothalamic-pituitary-testicular axis[J]. Hubei Agricultural Sciences, 2019, 58(12): 141-146. (in Chinese)
[4] GUO TT, SU J, MA ZY, et al. Cloning of neuromedin B and its receptor in the rabbit and generating a polyclonal antibody to the neuromedin B protein[J]. Gene, 2015, 564(1): 21-28.
[5] MA ZY, ZHANG Y, SU J, et al. Effects of neuromedin B on steroidogenesis, cell proliferation and apoptosis in porcine Leydig cells[J]. Journal of Molecular Endocrinology, 2018, 61(1): 13-23.
[6] BATTAGLIA DF, BOWEN JM, KRASA HB, et al. Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: a simultaneous view from hypophyseal portal and peripheral blood[J]. Endocrinology, 1997, 138(10): 4273-4281.
[7] HADDAD, JOHN1. Oxygen-sensitive pro-inflammatory cytokines, apoptosis signaling and redox-responsive transcription factors in development and pathophysiology[J]. Cytokines, Cellular and Molecular Therapy, 2002, 7(1): 1-14.
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Comparative Nutritional Analysis on Fish Meal and Meat and Bone Meal of Harmless Treatment of Dead Pig Carcass
- A Monophyletic Status of Axis Genus in Subfamily Cervinae Supported by the Complete Mitochondrial Genome of Chinese Hog Deer (Axis porcinus)
- Prevention and Control Measures of the Occurrence of Ceracris kiangsu Tsai in Sugarcane Areas of Yunnan Province
- Study on the Accuracy of Different CASA Systems in the Quality Detection of Fresh Boar Semen
- Evaluation and Selection of Appropriate Tobacco Varieties for Badong Hubei Province
- Damage and Control of Blueberry Canker

