Study on Virus-free and Rapid Propagation Technology of Artemisia selengensis sp. in Yangxin County
Shunwen XU Yihe LI Anhuai MING Rongxing YAN Xianai GAO Jin ZHENG Wei KANG

Abstract [Objectives]This study was conducted to culture virus-free tissue culture plantlets of mid-maturing green-stalk Artemisia selengensis sp. varieties in Yangxin County.
[Methods] With bud tips of A. selengensis in Yangxin as explants and MS as the basal medium, screening and proportioning of plant growth regulators were performed to establish a virus-free and rapid propagation system for mid-maturing green-stalk varieties of A. selengensis in Yangxin.
[Results] The optimal callus induction medium, adventitious shoot differentiation medium, adventitious shoot elongation medium and rooting medium for explants were MS+6-BA 1.0 mg/L+NAA 0.5 mg/L+sucrose 25 g/L+agar 7 g/L, MS+6-BA 0.1 mg/L+NAA 0.1 mg/L+sucrose 25 g/L+agar 7 g/L, MS+6-BA 0.02 mg/L+NAA 0.02 mg/L+sucrose 25 g/L+agar 7 g/L and 1/2 MS+NAA 0.05 mg/L+sucrose 25 g/L+agar 7 g/L, respectively, and the seedling rate reached more than 95%. The culture conditions were as follows: temperature (25±2) ℃, relative humidity 85%, illumination intensity 3 000 lx, and illumination time 14 h/d.
[Conclusions] This study has important practical significance for the purification and rejuvenation and large-scale industrial breeding of Yangxin A. selengensis seedlings.
Key words Artemisia selengensis sp.; Culture in vitro; Virus free; Rapid propagation
Received: August 18, 2020 Accepted: October 27, 2020
Supported by Hubei Polytechnic University and Yangxin Vegetable Office School-Enterprise Technology Cooperation (KY2018-049).
Shunwen XU (1966-), male, P. R. China, agronomist, devoted to research about vegetable planting and promotion.
*Corresponding author. E-mail: kangwei@hbpu.edu.cn.
Artemisia selengensis sp. is a perennial herbaceous plant belonging to Artemisia of Compositae. It is widely favored by consumers because of its rich nutrition, crisp taste, and medicinal value. Yangxin County, Hubei Province is the main producing area of A. selengensis, with a long planting history. In 2018, A. selengensis was listed as a National Geographical Indication Product by the Ministry of Agriculture and Rural Affairs. It is an important pillar industry that promotes local farmers income and wealth. At present, the planting area of Baota Village, Xingguo Town, Yangxin County alone has reached 800 hm2, making it the first village for A. selengensis production in Hubei[1].
Rhizome cutting propagation is the traditional planting method of A. selengensis in Yangxin. Long-term continuous cutting propagation has caused problems in Yangxin A. selengensis such as yield decline, deterioration of quality, and uneven quality[1-2]. Therefore, it is urgent to purify and rejuvenate seedlings, so as to ensure the inherent flavor restoration, quality improvement, yield increase and income increase of A. selengensis in Yangxin and guarantee the needs of large-scale planting. At present, there have been some reports on the in vitro culture of Artemisia plants, such as Artemisia carvifolia, Artemisia annua, A. selengensis, Artemisia dracunculus L., Artemisia argyi and so on. The use of in-vitro plant culture technology to cultivate virus-free seedlings has important practical significance for achieving the purification and rejuvenation of Yangxin A. selengensis in and strengthening the protection of Yangxin A. selengensis germplasm resources[3]. So far, there is no report on the rapid propagation of A. selengensis in Yangxin County.
Materials and Methods
Plant material selection and explant preparation
The excellent mid-mature green-stem individuals of Yangxin A. selengensis used in this experiment were provided by the Vegetable Office of Yangxin County, and were collected from the A. selengensis plantation base in Baota Village, Xingguo Town, Yangxin County in March 2018.
The excellent plants of Yangxin A. selengensis were planted in flowerpots. After the plants survived and grew out new shoots, and the tips of the shoots were as explants, which were rinsed in sterile water for 3-5 times, treated with 0.1% mercury for 1-2 min, and then rinsed with sterile water for 3-5 times. The treated explants were put on sterile filter paper to absorb water.
Initiation of explant culture and callus induction
With MS+sucrose 25 g/L+agar 7 g/L (pH 6.0) as the basic medium, different concentrations of 6-BA (0.5, 1.0, 2.0 mg/L) and NAA (0.1, 0.5, 1.0 mg/L) were added to carry out a proportioning experiment. The treated sterile explants were put into the culture media for initiation culture, and the callus induction rate was calculated after 20 d to screen the most suitable medium for the callus induction of the explants: Callus induction rate = (Number of explants producing calli/Total number of inoculated explants)×100%. Each treatment was inoculated with 25 explants, repeated 3 times, and the culture conditions were as follows: temperature (25±2) ℃, relative humidity 85%, light intensity 3 000 lx, and illumination time 12 h/d (the same below).
Adventitious buds differentiated from calli
With MS+sucrose 25 g/L+agar 7 g/L (pH 6.0) as the basic medium, different concentrations of 6-BA (0.1, 0.3, 0.6 mg/L) and NAA (0.05, 0.1, 0.2 mg/L) were added to carry out a proportioning experiment. The explants were cultured and observed for 30 d, and the differentiation rates of explants and adventitious buds were calculated to screen the most suitable medium for the differentiation of adventitious buds of explants: Explant differentiation rate (%)=(Number of differentiated explants/Total number of inoculated explants)×100%, Adventitious bud differentiation rate=Total number of differentiated adventitious buds/Total number of inoculated explants.
Adventitious bud elongation culture
With MS+sucrose 25 g/L+agar 7 g/L (pH 6.0) as the basic medium, different concentrations of 6-BA (0.02, 0.04, 0.08 mg/L) and NAA (0.01, 0.02, 0.03 mg/L) were added to carry out a proportioning experiment. The elongation of the adventitious buds was measured after 20 d of culture to select the most suitable medium for adventitious bud growth.
Rooting culture
With 1/2 MS +sucrose 25 g/L+agar 7 g/L (pH 6.0) as the basic medium, different concentrations of NAA (0.01, 0.05, 0.1, 0.2, 0.4 mg/L) were added for the rooting test, and the rooting situation was observed 15 d after culture to screen the most suitable rooting medium for adventitious buds.
Seedling training and transplanting
The seedlings were trained in a well-ventilated room with no direct light, and then transplanted into a sterile substrate. Before transplanting, the medium on the roots of the tissue cultured plantlets was rinsed off. The transplanting substrate should be prepared according to the vegetable garden soil: vermiculite in a volume ratio of 3∶1. The survival rate was calculated 7 d after transplanting.
Results and Analysis
Effects of 6-BA and NAA at different concentrations on initiation of explants and callus induction
With the sprouts of Yangxin A. selengensis as explants, after starting the culture, it was found that the induction of explants differed significantly with different plant hormone concentrations and ratios. It can be seen from Table 1 that the concentration of 6-BA had a significant effect on the initiation culture of axillary buds. When the concentration of 6-BA was 1.0 mg/L, the number of days for the initiation culture was significantly less than other concentrations, only 6-9 d (treatments IV, V, IV), and the calli were yellow-green in color, softer in texture, compact in structure, and faster in growth. The overall induction rate was as high as 95%. The concentration of 6-BA at 0.5 mg/L took the second place. When the concentration of 6-BA was 2.0 mg/L, it took 12-15 d to initiate the culture (treatment VII, VIII, IX), and the calli were yellowish brown, harder in texture, loose in structure, had a faster growth rate in the early stage, and browned rapidly in the late stage, showing a low induction rate of only about 50%. Generally speaking, if calli are soft in texture and compact in structure, they will differentiate into adventitious buds easier in the later stage[4]. Different 6-BA and NAA ratios also had a certain effect on the callus induction of explants. At the 6-BA concentration of 1.0 mg/L, when the concentration of NAA was 0.5 mg/L, the callus induction rate was the highest, reaching 100%, which was slightly higher than those obtained at other two concentrations. Based on the above results, with the shoots of Yangxin A. selengensis as explants, the appropriate plant hormone ratio for culture initiation and callus induction was 6-BA 1.0 mg/L+NAA 0.5 mg/L.
Effects of different ratios of 6-BA to NAA on adventitious bud differentiation of calli
The calli were inoculated on the adventitious bud differentiation media of different treatments. After 30 d, the statistical results showed that each treatment had adventitious bud differentiation, but the degree of differentiation showed great differences (Table 2). In general, with the increase of 6-BA concentration, the differentiation rate and differentiation coefficient of adventitious buds decreased significantly, and the ratio of plant hormones of different concentrations also affected the differentiation of adventitious buds. When the concentrations of 6-BA and NAA were both 0.1 mg/L (treatment ②), a large number of adventitious buds began to appear after 14 d of culture, and the adventitious bud differentiation rate and differentiation coefficient were as high as 70.6% and 7.42, respectively. Treatment ① in which the concentrations of 6-BA and NAA were, respectively, 0.1 and 0.05 mg/L, took the second place, and its adventitious bud differentiation rate and differentiation coefficient were 57.3% and 4.87, respectively. When the concentrations of 6-BA and NAA were 0.6 and 0.05 mg/L, respectively, sporadic adventitious buds did not appear until about 26 d after culture, and the adventitious bud differentiation rate and differentiation coefficient were the lowest, only 2.7% and 0.2 (treatment ⑦), respectively. Considering comprehensively, adding 6-BA 0.1 mg/L and NAA 0.1 mg/L was the appropriate hormone ratio for the calli to differentiate adventitious buds, and the adventitious bud differentiation was optimal at this concentration ratio.
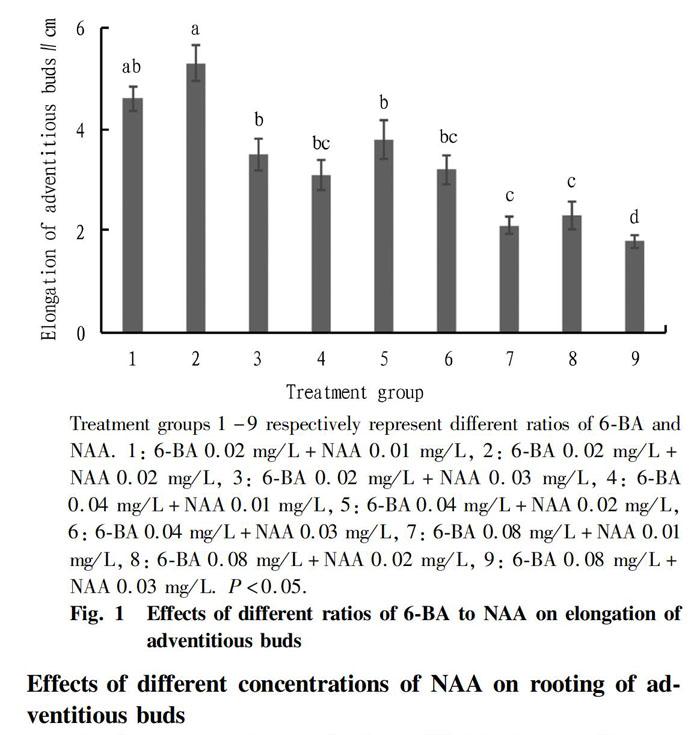
Effects of different ratios of 6-BA to NAA on elongation of adventitious buds
After calli have differentiated into adventitious bud clusters, since the concentration of plant hormones in the medium is an appropriate ratio for adventitious bud differentiation, the hormone ratio for adventitious bud elongation is also required to be optimal. It was observed that the concentrations of 6-BA and NAA had a significant effect on the elongation of buds (Fig. 1). Low concentrations of 6-BA and NAA were beneficial to the elongation of adventitious buds. On the contrary, high concentrations would inhibit the growth of buds. When the concentrations of 6-BA and NAA were both 0.02 mg/L (treatment 2), after 20 d of culture, the adventitious buds grew fastest, reaching 5.3 cm, and the adventitious buds had strong stems, stretched leaves and were better at this concentration ratio. When the concentrations of 6-BA and NAA were 0.02 and 0.01 mg/L, respectively (treatment 1), the adventitious buds grew by 4.6 cm, ranking second. With further increase of the two concentrations, the growth rate of adventitious buds decreased significantly. The 6-BA and NAA concentrations were both the highest in treatment group 9 at 0.08 and 0.03 mg/L, respectively, and the adventitious buds only elongated 1.8 cm, with thin stems, curled leaves and poor growth potential. The above results indicated that the appropriate hormone concentrations for adventitious bud growth were 6-BA 0.02 mg/L and NAA 0.02 mg/L.
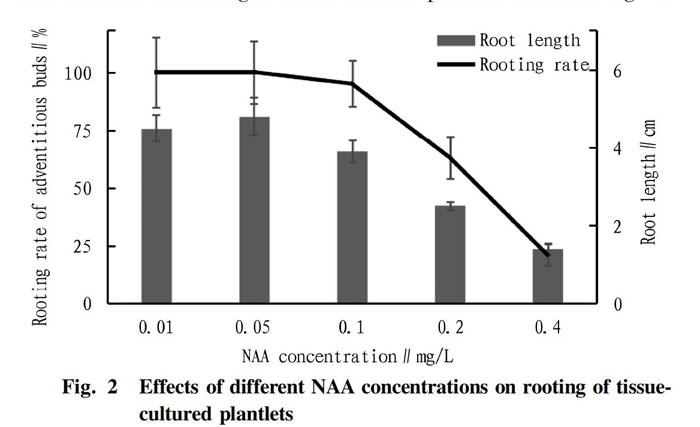
Effects of different concentrations of NAA on rooting of adventitious buds
In plant tissue culture technology, NAA is often used for root differentiation. Rootless tissue culture plantlets with a stem length of 4-5 cm and strong growth were selected and inoculated on the rooting medium. After 15 d, the statistical analysis showed that the low concentration of NAA promoted the rooting of tissue culture plantlets significantly (Fig. 2). When the NAA concentration was less than 0.1 mg/L, the rooting rate of tissue culture plantlets was above 95%. When the NAA concentration was in the range of 0.01-0.05 mg/L, the rooting rate was as high as 100%. As the concentration further increased, the rooting rate dropped sharply. When the NAA concentration was 0.4 mg/L, the rooting rate was only 21%. Similarly, when the NAA concentration was 0.05 mg/L, the root length of the tissue cultured shoots was the longest, with an average of 4.8 cm; when the NAA concentration was 0.05 mg/L, the root length was 4.5 cm, ranking second; and when the NAA concentration was 0.4 mg/L, the average root length was the shortest, only 1.4 cm. The results showed that the appropriate NAA concentration for rooting of tissue cultured plantlets was 0.05 mg/L.
Transplanting of rooted plantlets
After the rooted seedlings grew to 5-8 cm, the tissue culture plantlets with stretched leaves and good growth were moved to the artificial climate box for plantlet training together with the tissue culture bottles. After 7-10 d of training, they were transplanted to the seedbed. One week later, the survival rate of the plantlets reached more than 95%.
Conclusions and Discussion
The shoot tips of the mid-maturing green-stalk varieties of Yangxin A. selengensis were used as explants, which were subjected to sterilization treatment and cultured to successfully screen suitable media for explant initiation, callus induction, adventitious bud differentiation, adventitious bud elongation and rooting through proportioning of plant hormones. The results showed that the suitable medium for callus induction was MS+6-BA 1.0 mg/L+NAA 0.5 mg/L+sucrose 25 g/L+agar 7 g/L; the suitable medium for adventitious bud differentiation was MS+6-BA 0.1 mg/L+NAA 0.1 mg/L+sucrose 25 g/L+agar 7 g/L; the appropriate medium for adventitious shoot elongation was MS+6-BA 0.02 mg/L+NAA 0.02 mg/L+sucrose 25 g/L+agar 7 g/L; and rooting medium was 1/2 MS+NAA 0.05 mg/L+sucrose 25 g/L+agar 7 g/L. The establishment of the virus-free and rapid propagation system for mid-maturing green-stalk varieties of Yangxin A. selengensis has laid a foundation for the purification and rejuvenation and large-scale industrial seedling breeding of Yangxin A. selengensis seedlings.
References
[1] MING AH, LI YH, ZHOU XY, et al. Overview of Artemisia selengensis sp. industry development[J]. Rural Economy and Science-Technology, 2019, 19(30): 173-175. (in Chinese)
[2] FANG DF, XU YH, FANG XL, et al. Prominent problems and countermeasures faced by Longhao Base in Baota Village, Yangxin County[J]. Rural Economy and Science-Technology, 2011, 11(22): 108-109, 118. (in Chinese)
[3] JIANG XH, FU M, SHE CW, et al. Induction of multiple buds in Artemisia selengensis Turcz.[J]. Journal of Changjiang Vegetables, 2008(9b): 65-67. (in Chinese)
[4] ZHANG J, JIANG XW. Research on rapid propagation for three cultivars of Artemisia selengensis[J]. Journal of Changjiang Vegetables, 2014(24): 17-20. (in Chinese)
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Effects of LPS on the Gene Expression of NMB and Its Receptor in the Hypothalamic-pituitary-testicul
- Comparative Nutritional Analysis on Fish Meal and Meat and Bone Meal of Harmless Treatment of Dead Pig Carcass
- A Monophyletic Status of Axis Genus in Subfamily Cervinae Supported by the Complete Mitochondrial Genome of Chinese Hog Deer (Axis porcinus)
- Prevention and Control Measures of the Occurrence of Ceracris kiangsu Tsai in Sugarcane Areas of Yunnan Province
- Study on the Accuracy of Different CASA Systems in the Quality Detection of Fresh Boar Semen
- Evaluation and Selection of Appropriate Tobacco Varieties for Badong Hubei Province

