草地贪夜蛾对Bt玉米的抗性与治理对策思考
何康来 王振营

摘要 Bt玉米已在美洲廣泛种植20多年,成功控制了欧洲玉米螟Ostrinia nubilalis、草地贪夜蛾Spodoptera frugiperda等玉米重大害虫为害。然而,近年来相继报道在波多黎各、巴西、阿根廷因草地贪夜蛾产生抗性而导致一些Bt玉米抗虫性丧失。尤其是在热带和亚热带地区,多数Bt玉米品种商业化种植仅3年就丧失了对草地贪夜蛾的抗性。本文分析了草地贪夜蛾的生物学和生态学、对Bt杀虫蛋白抗性遗传特征和交互抗性特性、种群抗性基因频率等内因对抗性演化的影响,以及Bt玉米种植的生态环境、耕作栽培制度、Bt玉米种类、抗性治理策略实施情况等外部环境因素对抗性演化的影响。根据我国玉米种植的生态格局,提出了“整体布局,源头治理”的抗性治理对策。即在草地贪夜蛾周年繁殖区要谨慎种植Bt玉米,尤其是避免种植表达Cry1Ab杀虫蛋白的Bt玉米,以避免源头产生抗性而危及温带玉米主产区。遵循差异化(不同杀虫作用机理)选择Bt玉米品种原则,制定精准抗性监测计划,以高剂量庇护所为抗性治理基本策略,在Bt玉米资源有限的情况下,落实好庇护所尤为重要。
关键词 草地贪夜蛾; Bt玉米; 害虫抗性演化; 抗性治理; 对策
中图分类号: S 435.132
文献标识码: A
DOI: 10.16688/j.zwbh.2020224
Resistance evolution to Bt maize in the fall armyworm and
consideration on IRM strategy in China
HE Kanglai, WANG Zhenying
(State Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection,
Chinese Academy of Agricultural Sciences, Beijing 100193, China)
Abstract
Bt maize has been commercialized for over 20 years in the American countries, which was successfully used to control a number of economically important insect pests such as the European corn borer, Ostrinia nubilalis, fall armyworm (FAW), Spodoptera frugiperda, etc. However, a couple of resistance cases have threatened their efficacy and resulted in the failure of field control. Whats worse, most Bt maize events/stacks varieties lost their ability to control FAW in just 3 years after their release in the tropical and subtropical agricultural ecosystems. In this paper, we reviewed the internal and external factors which drove/facilitated the rapid evolution of FAW resistance, including the biology and ecology of FAW, its inheritance of resistance, cross resistance to different Bt proteins, initial allele frequency of resistance in the field populations, agroecosystem landscape of Bt maize planting, cropping systems, Bt maize events, adaptation of insect resistance management (IRM) practices, etc. Furthermore, we suggested a “Natal Source IRM” strategy based on the integral scenario of maize cropping landscape throughout the country, i.e., careful consideration should be taken in deploying Bt maize in the tropical and subtropical regions of China (winter maize region) where FAW reproduced year-round and is the source of adults to migrate to the Yangtze River Basin, Huanghuaihai summer maize region, even up to the northern spring maize region. If the resistance evolved in the Natal Source of FAW population, it will diminish the Bt maize efficacy in the rest regions of the country. Even more care should be taken in deploying Bt maize expressing Cry1Ab protein in the winter maize region. At least, different Bt maize varieties (without cross resistance) should be used between tropical and temperate regions. In addition, IRM plans should be designed to ensure a reliable resistance monitoring program, ensure refuge compliance to make high dose refuge as a corner stone tactics under limited Bt maize resources.
Key words
Spodoptera frugiperda; Bt maize; evolution of insect resistance; IRM; strategy
草地贪夜蛾Spodoptera frugiperda,又名秋黏虫(fall armyworm,FAW),2018年末入侵我國[1],2019年随着季节变暖及玉米等作物播种由热带亚热带向温带依次推进,寄主的可觅性由南向北也相应增加,草地贪夜蛾入侵逐渐向长江流域、黄淮海及西北地区蔓延,到10月份已迁移扩散至除黑、吉、辽、青、新外的26个省(市、区)1 518县(区、市),查见幼虫的省份22个,其中玉米草地贪夜蛾发生面积达到106.5 万hm2[2],对我国玉米生产构成严重威胁。与此同时,科学工作者积极应对,在入侵路径[3]、迁飞扩散轨迹[4]、化学防控[56]、生物防治[7]、玉米品种抗性利用等测报和防控技术方面开展了广泛的研究。及时提出了“分区治理、联防联控、综合治理”策略[8],有效地遏制了这一危险性害虫可能造成的重大灾害发生。
由于草地贪夜蛾繁殖潜力大[9],飞行与扩散能力极强[10],易引起异地突发和暴发。因此,化学防控因其速效、高效和易于实施是应急防控预案必不可少的手段。在其原发地美洲大陆草地贪夜蛾的防治已有悠久的历史。
对全球1910年-2019年文献大数据分析表明,美洲防控草地贪夜蛾以Bt玉米商业化应用为标志,可划分为化学防治和绿色综合防控两个时期。20世纪末之前是以化学杀虫剂为主的化学防治时期[11]。即使在其近年侵入的国家如南非、印度以及我国,亦多首选化学防治遏制其扩散为害。因而不可避免地会面临环境污染和抗药性发展等问题。李永平等分析总结了草地贪夜蛾抗药性的演化史,提出了化学防治策略[12]。
20世纪90年代后期,国外草地贪夜蛾防控进入了以Bt玉米为主,兼有天敌释放、生物农药使用等措施的绿色防控时期[11]。我国则经历了入侵风险分析预警,侵入后的监测、应急阻击防控,到目前以生态控制和农业防治为基础,生物防治和理化诱控为重点的持续治理探索。在天敌资源鉴定与应用[7,13],白僵菌等微生物农药应用,物理防控技术等方面取得进展,同时还明确了几个国产Bt玉米对草地贪夜蛾具有良好的杀虫效果[14]。2020年1月20日,随着北京大北农生物技术有限公司和瑞丰公司的两个国产转基因抗虫玉米获得生产应用安全证书,预示在我国玉米生产中应用这一现代生物技术产品防控草地贪夜蛾指日可待。然而值得注意的是,在美洲、南非等大面积商业化种植Bt玉米的国家,相继报道了草地贪夜蛾对不同类型的Bt玉米产生了抗性。本文就美洲Bt玉米的应用与草地贪夜蛾抗性演化、抗性产生的内外因进行了分析,结合草地贪夜蛾在我国的迁移扩散情况、玉米产区的生态环境、耕作栽培制度和种植的季节性规律、已获生产应用安全证书的国产Bt玉米资源等,针对未来生产中应用Bt玉米防治草地贪夜蛾,初步提出了抗性治理策略。
1 Bt玉米应用与草地贪夜蛾抗性的演化
20世纪90年代初,Koziel等[15]首次报道了表达Cry1Ab蛋白的Bt玉米田间高效杀虫效果。1996年,以欧洲玉米螟Ostrinia nubilalis和巨座玉米螟Diatraea grandiosella等为靶标害虫的Bt玉米在北美商业化种植,包括不同公司研发的‘176 ‘MON810和‘Bt11(表达Cry1Ab杀虫蛋白),‘DBT-418(表达Cry1Ac杀虫蛋白), ‘CBH-351(表达Cry9C杀虫蛋白)等产品,且‘MON810和‘Bt11持续种植或与后续新的转化事件聚合使用至今。这些Bt玉米对既定靶标害虫的防治效果可达99%以上[1621]。虽然草地贪夜蛾和玉米穗夜蛾(CEW),即美洲棉铃虫Helicoverpa zea等鳞翅目夜蛾科害虫没有被列为主要防治靶标对象,但在田间对这两种害虫亦有显著的防治效果,心叶期被害率降低90%以上,穗期被害率降低50%~80%[2227]。表达Cry1F杀虫蛋白的玉米‘TC1507于2001年在美国获得种植批准[28],2003年商业化种植,主要防治对象包括欧洲玉米螟[29],同时对晚播玉米上草地贪夜蛾有更好的防治效果[3032]。2013年,美国玉米带种植表达1种Bt杀虫蛋白的Bt玉米面积达到76%[33],美国玉米带的主要害虫欧洲玉米螟已得到有效控制[34],而草地贪夜蛾、美洲棉铃虫等害虫在美国南部各州偶发严重为害[24]。为了扩大杀虫谱以及实施多基因抗性治理策略,叠加多基因抗虫玉米‘MON89034(表达Cry2Aba+Cry1A.105,2009年)和聚合多基因抗虫玉米(如‘Bt11×MIR162,2009年;‘MON89034×TC1507‘MON89034×TC1507×MON88017×DAS-59122,2010年)在美国和加拿大种植,尤其是后者不仅能防治多种鳞翅目害虫,还能防治鞘翅目玉米切根叶甲Diabrotica[3536]。目前,全球商业化的Bt玉米多数都是叠加/聚合多个基因[37]。
波多黎各岛具有独特的热带农业生态系统,玉米周年连续种植,尤其是大面积错期月播青贮饲料玉米。草地贪夜蛾是当地最重要的害虫,周年发生且世代重叠,频繁造成严重为害损失。表达Cry1F杀虫蛋白的Bt玉米‘TC1507于2003年开始大面积商业化种植,2005年和2006年种植面积占整个玉米种植面积的80%。然而, 2006年田间出现‘TC1507玉米受草地贪夜蛾为害严重的情况,表明草地贪夜蛾已对‘TC1507玉米产生了田间抗性,抗性倍数超过1 000倍[3839]。抗性丧失的主要原因归结为岛屿热带农业生态环境、玉米周年持续播种、草地贪夜蛾周年繁殖和世代重叠,其次还与2006年草地贪夜蛾大暴发,以及当年天气干旱导致其他寄主植物源减少有关。靶标害虫田间产生抗性直接导致了‘TC1507玉米在当地退市停用[39]。随后数年持续监测数据表明,这一地区的草地贪夜蛾对‘TC1507持续呈高水平抗性,而同期监测美国南部各州草地贪夜蛾种群对‘Cry1F杀虫蛋白和‘TC1507玉米依然敏感[36,38,4041]。直到2013年,美国东南部的佛罗里达州和北卡罗来纳州田间草地贪夜蛾种群Cry1F抗性基因频率增高,田间出现非预期的存活幼虫[42],可能是由于波多黎各岛及巴西田间抗性草地贪夜蛾迁飞到美国东南部所致[4346]。
巴西玉米种植面积120万~150万hm2[4748],从南部的亚热带到中、北部的热带环境,年种植2~3季[4950],传统的第一季(夏)玉米面积占比由61.5%(2009年)下降到34.5%(2015年),而第二季(冬)玉米面积占比已由38.5%增加到65.5%[5152],且冬玉米播种时间由当年12月持续到翌年2月。草地贪夜蛾是当地最主要的害虫[53],发生高峰通常在夏玉米季节,减产可达17%~38%[54]。连续两季玉米,为草地贪夜蛾搭起了发生多代、世代重叠的寄主“绿色桥梁”[38, 5557],且其发生种群密度高[5859]。表达Cry1Ab(‘Bt11和‘MON810)、Cry1F(‘TC1507)、Cry2Aba+Cry1A.105(‘MON89034)杀虫蛋白Bt玉米先后于2008/2009、2009/2010、2010/2011生长季在巴西商业化种植[52, 6061],能高效控制草地贪夜蛾、美洲棉铃虫、小蔗螟Diatraea saccharalis等多种鳞翅目害虫的为害[48,6263]。然而同样由于热带、亚热带生态环境下连续种植多季玉米[49],害虫连续多世代重叠,且庇护所面积实施率低[50]等,仅在商业化种植1年后,发现田间草地贪夜蛾已对表达Cry1F的Bt玉米产生了抗性[50,6465],对表达Cry1Ab的Bt玉米的抗性也逐年上升[66]。草地贪夜蛾在Bt玉米和Bt棉花田转移为害[67],使选择压增加。
阿根廷玉米面积约300万hm2。先后商业化种植表达单基因cry1Ab(‘Bt11‘MON810和‘176,1998年), cry1F(‘TC1507,2005年),vip3Aa20(‘MIR162,2011年)玉米以及多基因‘MON89034(2010年),‘MON88017(‘TC1507×MON810, 2010年),‘MON89034×MON88017(2010年)玉米、‘Bt11×MIR162(2011年)[68],主要防治草地贪夜蛾、小蔗螟等鳞翅目害虫[6971]。由于在其北部大量种植晚季玉米,且庇护所有限,以及草地贪夜蛾发生多代且世代重叠等因素,增加了选择压和年选择世代数[7273]。2013年,在阿根廷多地田间发现草地贪夜蛾抗表达Cry1F的Bt玉米[7475],并对表达Cry1Ab的玉米有交互抗性[76]。
2 抗性產生的内因
2.1 草地贪夜蛾生物学、生态学及其对抗性演化的影响
害虫抗性演化与其生物学、生态学习性密切相关,如寄主范围、繁殖期和繁殖力、性比、迁移扩散行为等[77],尤其是年发生世代数[78]。草地贪夜蛾寄主植物广泛,多种主要农作物如玉米、棉花、大豆、高粱等都是其适宜寄主[56,67,79],草地贪夜蛾在这些作物上的生活史接近[80],在表达某一杀虫蛋白的一种作物(如Cry1F玉米)上产生的抗性个体,在表达相同蛋白的其他作物(如Cry1F棉花)上不会产生适合度劣势[81]。草地贪夜蛾在28℃时世代历期约30 d,在热带和亚热带地区可年发生10代[82]。美属波多黎各岛,地理环境相对隔离,属热带生态系统,草地贪夜蛾是当地玉米上的最主要害虫,适宜的其他寄主植物少。玉米周年种植,因而对草地贪夜蛾形成了多世代周年汰选。从商业化种植表达Cry1F的Bt玉米到监测出抗性产生的3~4年间,田间汰选已有30~40代[38]。中南美洲的巴西、阿根廷与波多黎各情况相似,草地贪夜蛾是当地最主要的玉米害虫,年平均发生8代以上[64,83]。加上草地贪夜蛾超强的繁殖力[9,82,84],极易形成高密度种群。如若出现对Bt玉米产生抗性的个体,就会在短时间产生大量的后代,且因其没有或很低的抗性适合度劣势[81,85],使群体的抗性基因频率大量繁衍扩充,促使抗性快速产生。
害虫的迁移能力直接影响其在不同生态系统种群间的基因交流,尤其是不同生产力管理水平下,如用药水平与绿色防控水平等,无疑会导致种群内的遗传结构如抗药性出现空间上的差异分化,如果害虫迁移能力弱,生境相对隔离,这种分化的长期存在就可能演化出特性差异显著的品系/生态型等。相反,如果害虫迁移能力强,个体在不同生境间迁移,使得不同管理水平生境下的抗性差异种群实现基因交流,其结果一方面迁移个体携带抗性基因在不同生境间得以传播扩散,另一方面,迁入的非抗性个体可以使迁入区域内的汰选富集基因得到稀释。可见,迁移能力直接影响害虫抗性的时空演化[77]。草地贪夜蛾成虫具有超强的迁移能力,在不同区域、洲际间入侵蔓延[8689],携带抗性基因传播扩散[42,44]。
在美国本土,草地贪夜蛾冬季一般仅能在佛罗里达和得克萨斯南端越冬,暖冬可在墨西哥湾沿岸地区过冬,夏季世代发育历期28~30 d,冬季可长达3个月[90]。4月-5月佛罗里达种群(FP)北迁到佛罗里达北部及佐治亚南部;得克萨斯种群(TP)向东北迁入路易斯安那、密西西比、亚拉巴马[4546,91];得克萨斯种群沿密西西比河流域北上到美国北部的宾夕法尼亚州等地,而佛罗里达种群则沿东海岸经南/北卡罗来纳、弗吉尼亚、宾夕法尼亚州,一般在夏末或秋初扩散至北部[92]。美国玉米主要分布在中北部玉米带,南部尤其是佛罗里达州玉米种植面积很小[91]。由于冬春季(1月-5月)在佛罗里达种植大面积(>12万hm2)的马铃薯Solanum tuberosum L.、甘蓝Brassica oleracea L.var.capitata等蔬菜,后续种植绿肥作物高粱苏丹草(高粱和苏丹草的杂交种)Sorghum bicolor(L.)[93],因此,在佛罗里达有大量的非玉米庇护所。南部极小面积玉米上越冬种群北迁到佛罗里达北部非玉米绿肥寄主时的死亡率高。由于草地贪夜蛾在美国温带玉米带不能越冬,而在美国本土,尤其是玉米带,玉米为单季种植,玉米生长期草地贪夜蛾仅能繁殖1~2代,仅在局地零星重发生,不会出现周年多世代频繁汰选。即使出现抗性个体,在冬季到来时不能完成下一世代,因而不能在种群中遗传。
值得注意的是,在巴西报道了草地贪夜蛾抗性问题后,很快在阿根廷、巴拉圭、乌拉圭、哥伦比亚等也发现田间Bt作物防治失效问题[53]。除当地热带环境、庇护所不足的高压频繁汰选外,外来携带抗性基因成虫迁入可能也是原因之一[94]。
害虫幼虫的转移扩散规律会显著影响其对Bt玉米抗性演化,尤其是在采用“种子混合”庇护所法的情景下。幼虫在Bt与非Bt植株间的转移为害将有利于其抗性产生[95]。一方面幼虫由非Bt玉米植株转移至Bt玉米植株,导致死亡率显著增加,从而减少了庇护所种群密度[9697];另一方面,抗性杂合个体或携带微小抗性基因的个体由Bt植株转移到非Bt植株,将导致其没有摄食足够的Bt蛋白,不能满足高剂量庇护所抗性治理策略的“高剂量”先决条件而存活[9899],从而富集种群中的抗性基因,加剧抗性演化[100]。此外,Bt蛋白在玉米组织的时空表达存在差异[101],幼虫在植株上的不同部位间的转移,尤其是对植株不同生长发育时期的器官组织有选择性时,亦会影响其存活率[102],以及在非Bt植株上的抗性杂合体(尤其是不完全隐性遗传)或携带微小抗性基因的大龄幼虫转移到Bt植株(即使对初孵幼虫是“高剂量”)也能存活,提高抗性基因频率[103]。由此可见,幼虫的迁移扩散行为将直接影响庇护所设置策略。草地贪夜蛾有显著的株间转移习性,但多数发生在同行(50%)及邻近(1.1 m)植株间(91.4%)[104]。
草地贪夜蛾对一些Bt蛋白的抗性间不存在交互抗性,如Cry1F、Cry1Ab与Vip3Aa20, Cry1F与Cry2Ab等,因此在多个不同Bt玉米同时商业化种植的景观生态下,其难以克服不同杀虫蛋白在时/空叠加(轮换)。这可能是美国玉米带转基因玉米防治草地贪夜蛾依然有效的原因之一[39,105106]。
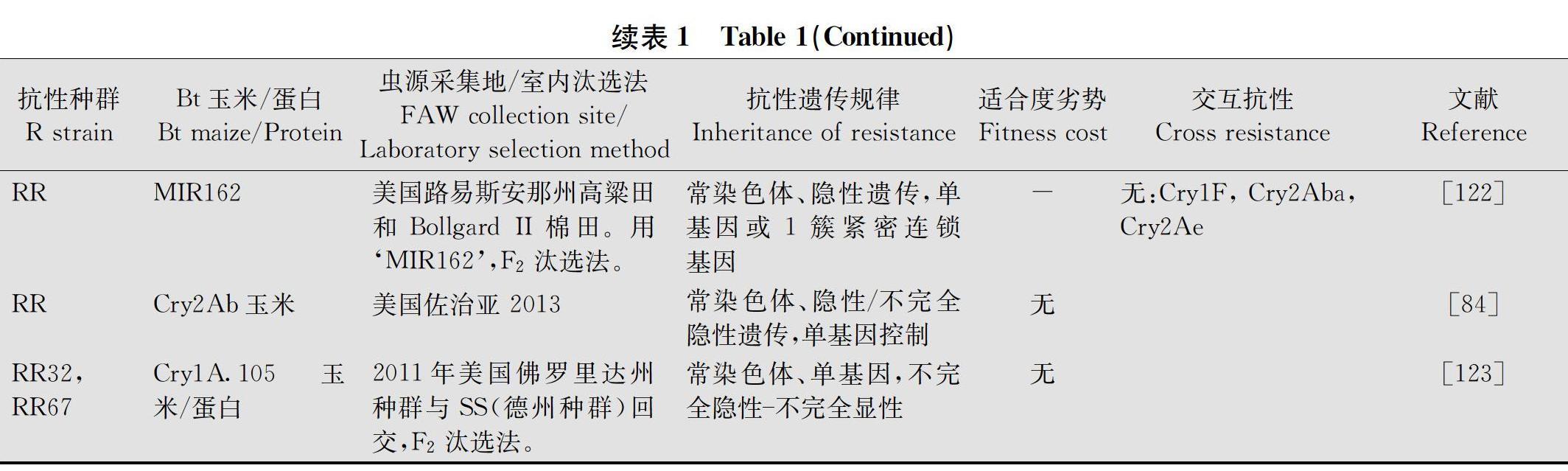
2.2 抗性遗传特征
抗性遗传学特征直接关系到抗性演化速率,是抗性治理的基础。在田间出现抗性之前,有关草地贪夜蛾对Bt玉米表达的主要杀虫蛋白Cry1Ab、Cry1Ac、 Cry1F、Vip3Aa、Cry2Ab等的抗性遗传特征几乎没有报道。自从Storer等[39]首次报道草地贪夜蛾在波多黎各田间对Cry1F玉米产生抗性以来,已有多个分离自巴西、阿根廷、美国本土东南部州及波多黎各岛等地的Cry1F抗性种群的遗传特征报道(表1)。总的来看,草地贪夜蛾对Cry1F玉米的抗性为常染色体、隐性或不完全隐性遗传,仅在美国北卡罗来纳州普利茅斯分离的种群为显性或不完全显性。无母系效应。抗性由单基因或1簇紧密连锁的基因控制。抗性产生较快,且田间抗性产生后,即使消除选择压,抗性依然能稳定遗传[39,81]。多地Cry1F抗性草地贪夜蛾种群没有抗性相关的适合度劣势。对Cry1Ab、Cry1Ac存在低水平交互抗性, 对Cry1Aa、Vip3Aa、Cry2Ab、Cry2Aa、Cry1Ba没有交互抗性[107]。相比之下,对表达Cry1Ab的Bt玉米的抗性报道较少,该抗性为常染色体1个以上基因控制的隐性遗传,有抗性适合度劣势。对‘MON89034玉米抗性为常染色体多基因控制的隐性遗传,存在抗性相关的适合度劣势。对表达Vip3Aa20玉米的抗性为常染色体单基因或1簇紧密连锁基因控制的隐性遗传,显著的抗性相关适合度劣势。
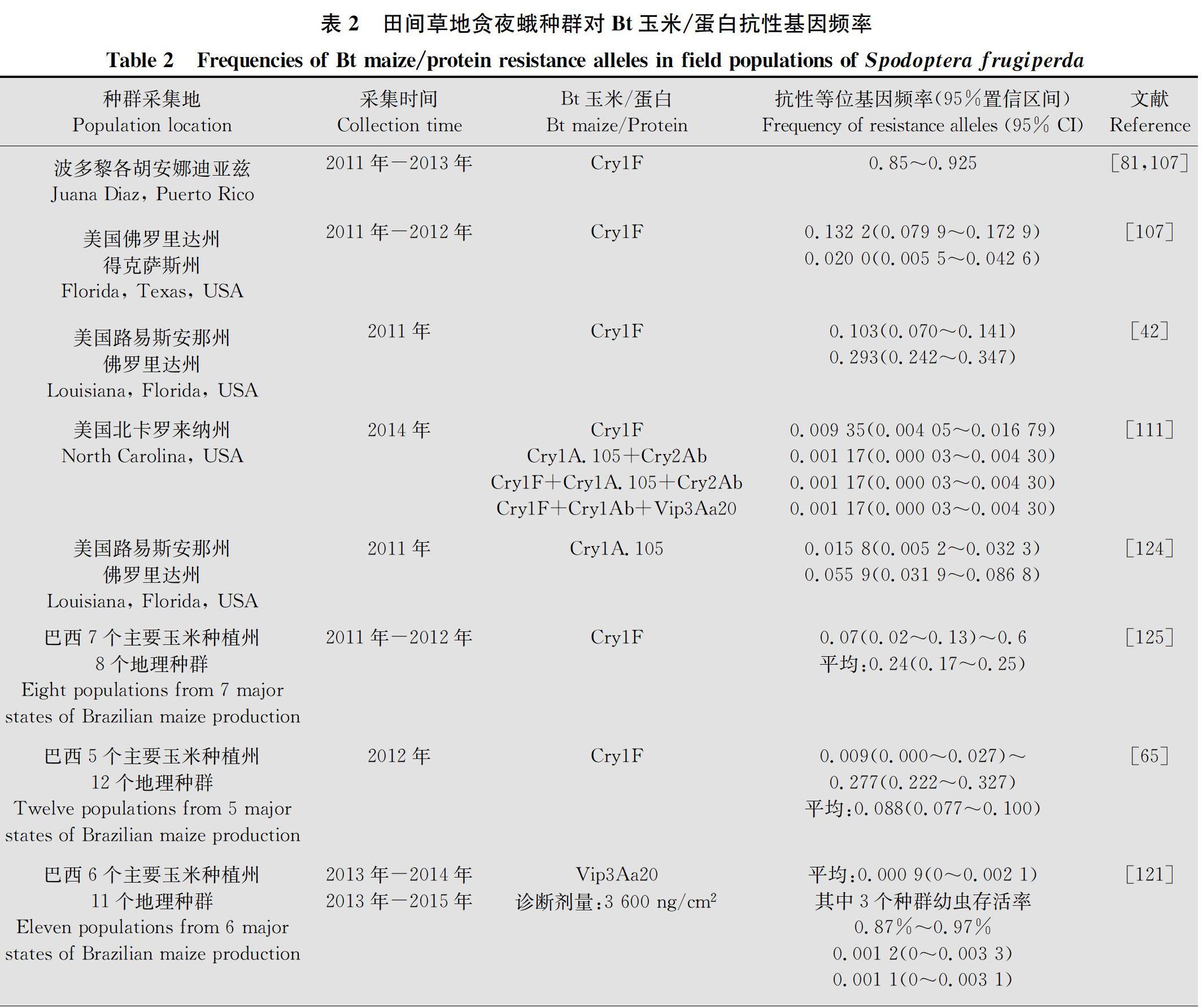
2.3 抗性基因频率
在草地贪夜蛾对‘TC1507玉米田间产生抗性被报道之前,自然种群的抗性基因频率并没有相关监测数据报道。自报道田间出现抗性之后,在巴西、美国多地对抗性基因频率进行了检测。虽然商业化种植前,草地贪夜蛾田间种群对Cry1F抗性的基因频率未知,但从后来各国多年监测数据可以看出,草地贪夜蛾对Cry1F抗性基因频率上升有明显的地理差异,在热带亚热带生态系统的波多黎各、巴西、阿根廷抗性基因频率上升非常快,其次是美国南部的佛罗里达等,而在美国北卡罗来纳则相对慢(表2)。这与年发生世代數相关。虽然草地贪夜蛾对Vip3Aa20的抗性基因频率监测数据较少,但可以看出其上升相对较慢。说明不同杀虫蛋白之间抗性基因频率有一定差异。
3 抗性产生的外因
3.1 热带亚热带农田生态系统有利于抗性产生
热带亚热带地区,一般降水丰富,温度适宜作物周年生长,复种指数高,常出现“反季”作物种植,如我国玉米等南繁育/制种、冬季种植鲜食甜糯玉米就是利用热带周年可种植的生态条件,在温带地区不能应季生产时的反季种植。由于制/育种、鲜食、饲用等不同目的需要,同一地区存在多个播期/生育期田块的景观格局,为草地贪夜蛾的周年连续繁殖提供了丰富的易觅寄主[126]。在巴西,不同作物组成嵌合周年生长农田景观,包括大豆、玉米、小麦、棉花等,其中玉米、棉花和大豆都是Bt作物[58]。尤其在同一地区不同播期连续种植同一转基因玉米或转相同或相似Bt基因作物,如表达Cry1Ab玉米、Cry1Ac棉花、Cry1Ac大豆、Cry1F玉米、Cry1Ac+Cry1F棉花、Vip3Aa20玉米以及Vip3Aa19棉花等[58,64,67,79],必然出现连续多代汰选,提高抗性演化风险。
3.2 表达蛋白种类及表达量
高剂量庇护所是目前应用最广、最有效的抗性治理策略。庇护所起作用的关键前提之一是产品表达的Bt蛋白达到“高剂量”,即表达的剂量能够杀死隐性敏感纯合(ss)个体和杂合(sr)个体,仅有抗性纯合个体可能存活[100]。这一措施有效延缓了多种害虫抗性产生,如烟芽夜蛾Heliothis virescens、棉红铃虫Pectinophora gossypiella[127128]、欧洲玉米螟等[34]。Cry1Ab蛋白对草地贪夜蛾等夜蛾科害虫毒力相对较低[129130],虽然表达Cry1Ab玉米(‘MON810和‘Bt11)能有效控制草地贪夜蛾的为害,抑制幼虫生长,尤其是在虫口密度高的情况下能显著减少产量损失,但最终幼虫死亡率与对照差异不显著[22,131133],即没有达到高剂量,甚或没有将草地贪夜蛾列为转cry1Ab基因Bt玉米的靶标害虫[134]。巴西2010年-2015年多地监测数据表明,尽管不同地区草地贪夜蛾种群对Cry1Ab的敏感性存在显著性差异,但平均敏感性逐年下降[66]。Cry1F蛋白对草地贪夜蛾有较高的毒力和更好的防治效果[3032],然而其在田间的表现也没有达到高剂量的要求[116]。聚合2个或多个具有不同杀虫谱且作用机理不同基因的Bt玉米,如‘MON89034‘TC1507×MON89034以及‘TC1507×MON810等被认为既能扩大杀虫谱,又能延缓抗性产生[35,135136],然而有研究结果表明单基因Cry1F和Cry1Ab玉米与聚合多基因玉米对草地贪夜蛾等的防效并没有显著差异[36,106]。值得注意的是,有研究报道Cry1F抗性草地贪夜蛾对‘MON89034产生了抗性[137],且Cry1F与Cry1A.105间存在交互抗性,将影响聚合多基因的抗性治理作用效果[118]。
‘MIR162玉米表达Vip3Aa20杀虫蛋白,其与Cry类蛋白作用机理不同[138],且对鳞翅目夜蛾科多种害虫如草地贪夜蛾、美洲棉铃虫有很高的毒力[139141],尤其是对Cry1F、Cry1Ab、Cry1Ac、Cry2Ab抗性草地贪夜蛾有很高的毒力,即没有交互抗性[142144]。值得关注的是,目前为止,表达单一Vip3Aa20或与Cry类聚合的玉米产品是唯一没有出现田间草地贪夜蛾产生抗性而防控失效的产品[58]。
3.3 有效的抗性治理措施缺失
自从McGaughey首次报道印度谷螟Plodia interpunctella(Hübner)对Bt制剂产生抗性[145],尤其是发现小菜蛾Plutella xylostella在田间对Bt制剂产生抗性后[145],害虫对Bt作物的抗性问题在商业化应用伊始就备受重视。有学者曾预测,如若缺乏相应的抗性治理对策,Bt作物种植2~4年靶标害虫将产生抗性[100,146]。因此,美国在转基因抗虫作物商业化种植的同时,提出实施“高剂量庇护所”的抗性治理策略[28,100]。转基因抗虫作物商业化种植初期,抗性治理策略的落实是自愿的,但很快成为美国环保署(USA-EPA)颁布的强制性要求,即在种植Bt作物的同时,要求种植5%~20%的非转基因作物作为庇护所。这一策略在北美的实施,有效地避免或延缓了靶标害虫产生抗性[147],被各国科学家普遍认同是转基因抗虫作物靶标害虫抗性治理的根本策略[148151]。
目前抗性治理策略主要包括高剂量庇护所策略、聚合多基因策略、IPM策略等。其中聚合多基因策略依赖于可用的Bt基因产品。而高剂量庇护所策略和IPM策略则需要种植者根据生产实际加以落实。
高剂量庇护所策略有效性的前提条件是种植必要面积的庇护所。
纵观转cry1F和cry1Ab玉米在波多黎各、巴西、阿根廷等中南美洲国家因草地贪夜蛾产生抗性而防治失效,其原因之一就是没有种植足够的庇护所,使得高剂量庇护所这一抗性治理基本策略得不到落实。虽然巴西生物技术行业协会制定并推广了科学的IRM策略[152],但庇护所在巴西种植业者中难以落实[58,64]。据巴西种业协会估计,庇护所落实率不到20%[58]。
4 我国Bt玉米利用与草地贪夜蛾抗性治理策略的思考
种植Bt玉米可为农民带来巨大的直接经济效益[34]。2020年初2个国产转基因抗虫玉米产品‘DBN9936(表达Cry1Ab杀虫蛋白)和‘瑞丰125(表达Cry1Ab/Cry2Aj杀虫蛋白)获得在北方春玉米区的生产应用安全证书[153],将我国转基因玉米的研发应用向前推进了一大步,为亚洲玉米螟Ostrinia furnacalis、黏虫Mythimna separata、草地贪夜蛾等我国玉米主要鳞翅目害虫提供了可供选择的防治新途径。基于全球种植Bt玉米的历史与害虫抗性演化与治理经验,尽早依据我国生产力水平、种植制度、农田景观生态类型以及虫害发生为害规律等,制定适宜的害虫抗性治理策略,是确保这一技术能长期持续有效应用的前提。笔者认为,应用Bt玉米防治草地贪夜蛾及其抗性治理策略应重点考虑以下几点。
4.1 整体布局,源头治理
我国玉米主产区分布北起东北(黑龙江)、穿越华北、南至西南(云贵高原南端)地带,由南到北面积递增,播期从春到冬,耕作栽培制度由多季到单季。由此构成了草地贪夜蛾以玉米为寄主的可觅性时空分布路線图。结合2019年我国草地贪夜蛾监测数据表明,其发生区可划分为周年繁殖区——北迁过渡区——重点防范区(黄淮海夏玉米区)——潜在为害区(北方玉米区)[2,4]。以发生时序划分为越冬区(南方冬玉米区)——春末迁入区(江淮)——夏季迁入区(黄淮海夏玉米区)——秋季迁入区(北方玉米区)。这与草地贪夜蛾在美国玉米带发生、迁移扩散为害规律相似[9192]。因此,应按照整体布局,源头治理的原则,做好周年繁殖虫源区抗性治理,以达到整体解决草地贪夜蛾对Bt玉米的抗性问题。
南方冬玉米面积约10万hm2 [2],主要种植鲜食玉米,是草地贪夜蛾的周年繁殖区,世代重叠,年发生6~8代。如果在此区域种植Bt玉米,草地贪夜蛾将会面临连续世代高频汰选抗性,导致抗性演化风险增加。加之境外虫源地越南和菲律宾是Bt玉米(‘Bt11‘MON810‘MON89034‘TC1507)种植国以及缅甸是Bt棉(表达Cry1Ac杀虫蛋白)种植国[2,3,37,51],草地贪夜蛾在这些国家可周年繁殖,多代的汰选压容易导致当地草地贪夜蛾对Bt玉米产生抗性,携带抗性基因的个体随季风迁入我国南方玉米区,使得春夏季迁移扩散虫源携带抗性基因北上,势必对其他玉米种植区尤其是夏玉米区Bt玉米的利用构成严重威胁。因此,在草地贪夜蛾周年繁殖区应谨慎种植Bt玉米(尤其是应避免种植表达Cry1Ab的Bt玉米),以避免源头产生抗性而危及黄淮海夏玉米区乃至北方春玉米主产区。在种植Bt玉米的情况下,尽管春、夏、秋迁入区草地贪夜蛾也会面临抗性汰选,但其后代不能越冬,因而抗性基因不能在种群中累积遗传,目前尚未见草地贪夜蛾在秋季回迁到周年繁殖区的文献报道。
如果要在南方冬玉米区应用Bt玉米防治草地贪夜蛾等害虫,在Bt玉米种类选择上应采取“差异化”原则,即选择与其他种植区没有交互抗性(杀虫作用机理不同)的Bt玉米品种(应避免选择表达Cry1Ab及其类似的Cry1类杀虫蛋白的Bt玉米),以使周年繁殖区北迁携带抗性基因的个体,在迁入区的不同Bt玉米上成为敏感个体。这与草地贪夜蛾抗药性治理要在不同省份尽量做到“药剂品种、施用时间和空间不同”[12]类似。
4.2 长期精准的抗性监测计划
技术上应针对生产上即将推广应用的Bt玉米种类,尽早摸清草地贪夜蛾自然种群的敏感基线、抗性基因频率(包括基因型检测[154156]和表型检测),明确其对不同Bt玉米产品表达Bt蛋白的抗性和交互抗性问题,建立抗性监测计划。同时应密切关注境外迁入虫源地Bt玉米种植情况,包括Bt玉米种类,种植制度,草地贪夜蛾的汰选史,当地监测抗性情况,外来虫源的敏感性测定等。精准的抗性监测将为抗性演化预测、抗性治理措施有效性评价提供及时可靠的科学依据。
4.3 切实落实好庇护所策略
美国20多年的成功实践经验证明,高剂量庇护所是靶标害虫抗性治理的根本性策略[147,157]。理论上高剂量是指Bt作物植株表达的Bt杀虫蛋白量能杀死靶标害虫种群中100%的ss个体和95%的sr个体[100]。在抗性产生前,这一量化指标实际是无法准确得到的。因此,提出以表达量≥25×LC99.9剂量作为可操作高剂量指标[148,158160]。评价一个产品是否高剂量,与评价方法有关。Burkness等报道了田间‘MIR162及‘MIR162×Bt11甜玉米上多年多点试验结果,草地贪夜蛾无存活幼虫,转基因玉米杀虫效果达到高剂量水平[141]。Niu等报道了2个草地贪夜蛾Cry1A.105抗性品系在Cry1A.105玉米离体叶片上表现为隐性或不完全隐性遗传,然而在整株玉米上表现为中等或不完全显性遗传[161],甚至在‘MON89034植株上也一样[162],说明了田间评价的重要性。目前,国产转基因玉米多以亚洲玉米螟、黏虫为靶标,而草地贪夜蛾作为新入侵害虫没有被列为靶标。张丹丹等报道了‘C0030.3.5(表达Cry1Ab蛋白)玉米离体叶片对草地贪夜蛾1龄幼虫的致死率<66%,‘DBN3601和‘DBN5608(表達Cry1Ab+Vip3Aa蛋白)对1~2龄幼虫的致死率达到100%,3龄幼虫的死亡率84%~95%[14]。是否达到高剂量,有待进一步田间试验评价。
由于有限的可利用Bt玉米资源,这一措施的实施重点在于庇护所的落实。我们知道,‘TC1507‘MON810‘Bt11等对于草地贪夜蛾都没有达到高剂量水平。但在美国本土应用已有20多年,现在依然有效,其中一个重要的原因是庇护所得到较好的落实[147]。
参考文献
[1] 郭井菲, 静大鹏, 太红坤, 等. 草地贪夜蛾形态特征及与3种玉米田为害特征和形态相近鳞翅目昆虫的比较[J]. 植物保护, 2019, 45(2): 712.
[2] 姜玉英, 刘 杰, 谢茂昌, 等. 2019年我国草地贪夜蛾扩散为害规律观测[J]. 植物保护, 2019, 45(6): 1019.
[3] 吴秋琳, 姜玉英, 吴孔明. 草地贪夜蛾缅甸虫源迁入中国的路径分析[J]. 植物保护, 2019, 45(2): 16.
[4] 吴秋琳, 姜玉英, 胡 高, 等. 中国热带和南亚热带地区草地贪夜蛾春夏两季迁飞轨迹的分析[J]. 植物保护, 2019, 45(3): 19.
[5] 赵胜园, 孙小旭, 张浩文, 等. 常用化学杀虫剂对草地贪夜蛾防效的室内测定[J]. 植物保护, 2019, 45(3): 1014.
[6] 赵胜园, 杨现明, 杨学礼, 等. 8种农药对草地贪夜蛾的田间防治效果[J]. 植物保护, 2019, 45(4): 7478.
[7] 赵旭, 朱凯辉, 张柱亭, 等. 夜蛾黑卵蜂对草地贪夜蛾田间防效的初步评价[J]. 植物保护, 2020, 46(1): 7477.
[8] 杨普云, 朱晓明, 郭井菲, 等. 我国草地贪夜蛾的防控对策与建议[J]. 植物保护, 2019, 45(4): 16.
[9] 赵胜园, 杨现明, 和伟, 等. 草地贪夜蛾卵巢发育分级与繁殖潜力预测方法 [J]. 植物保护, 2019, 45(6): 2834.
[10]葛世帅, 何莉梅, 和伟, 等. 草地贪夜蛾的飞行能力测定[J]. 植物保护, 2019, 45(4): 2833.
[11]李红梅, 万敏, 顾蕊, 等. 基于文献计量学的重大入侵害虫草地贪夜蛾的研究动态分析[J]. 植物保护, 2019, 45(4): 3442.
[12]李永平, 张帅, 王晓军, 等. 草地贪夜蛾抗药性现状及化学防治策略[J]. 植物保护, 2019, 45(4): 1419.
[13]宁素芳, 周金成, 张柱亭, 等. 贵州省黔东南地区发现草地贪夜蛾的5种寄生性天敌及其两种重寄生蜂[J]. 植物保护, 2019, 45(6): 3942.
[14]张丹丹, 吴孔明. 国产Bt-Cry1Ab和Bt-(Cry1Ab+Vip3Aa)玉米对草地贪夜蛾的抗性测定[J]. 植物保护, 2019, 45(4): 5460.
[15]KOZIEL M G, BELAND G L, BOWMAN C, et al. Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis [J]. Nature Biotechnology, 1993, 11(2): 194200.
[16]GRAEBER J V, NAFZIGER E D, MIES D W. Evaluation of transgenic, Bt-containing corn hybrids [J]. Journal of Production Agriculture, 1999, 12(4): 659663.
[17]LAUER J, WEDBERG J. Grain yield of initial Bt corn hybrid introductions to farmers in the northern corn belt [J]. Journal of Production Agriculture, 1999, 12(3): 373376.
[18]ARCHER T L, SCHUSTER G, PATRICK C, et al. Whorl and stalk damage by European and southwestern corn borers to four events of Bacillus thuringiensis transgenic maize [J]. Crop Protection, 2000, 19(3): 181190.
[19]ARCHER T L, PATRICK C, SCHUSTER G, et al. Ear and shank damage by corn borers and corn earworms to four events of Bacillus thuringiensis transgenic maize [J]. Crop Protection, 2001, 20(2): 139144.
[20]HE Kanglai, WANG Zhenying, ZHOU Darong, et al. Evaluation of transgenic Bt corn for resistance to the Asian corn borer (Lepidoptera: Pyralidae) [J]. Journal of Economic Entomology, 2003, 96(3): 935940.
[21]BURKNESS E C, HUTCHISON W D, BOLIN P C, et al. Field efficacy of sweet corn hybrids expressing a Bacillus thuringiensis toxin for management of Ostrinia nubilalis (Lepidoptera: Crambidae) and Helicoverpa zea (Lepidoptera: Noctuidae) [J]. Journal of Economic Entomology, 2001, 94(1): 197203.
[22]LYNCH R E, WISEMAN B R, PLAISTED D, et al. Evaluation of transgenic sweet corn hybrids expressing CryIA (b) toxin for resistance to corn earworm and fall armyworm (Lepidoptera: Noctuidae) [J]. Journal of Economic Entomology, 1999, 92(1): 246252.
[23]BUNTIN G D, ALL J N, LEE R D, et al. Plant-incorporated Bacillus thuringiensis resistance for control of fall armyworm and corn earworm (Lepidoptera: Noctuidae) in corn [J]. Journal of Economic Entomology, 2004, 97(5): 16031611.
[24]BUNTIN G D, FLANDERS K L, LYNCH R E. Assessment of experimental Bt events against fall armyworm and corn earworm in field corn [J]. Journal of Economic Entomology, 2004, 97(2): 259264.
[25]WILLIAMS W P, SAGERS J B, HANTEN J A, et al. Transgenic corn evaluated for resistance to fall armyworm and southwestern corn borer [J]. Crop Science, 1997, 37(3): 957962.
[26]WILLIAMS W P, BUCKLEY P M, SAGERS J B, et al. Evaluation of transgenic corn for resistance to corn earworm (Lepidoptera: Noctuidae), fall armyworm (Lepidoptera: Noctuidae), and southwestern corn borer (Lepidoptera: Crambidae) in a laboratory bioassay [J]. Journal of Agricultural Entomology, 1998, 15(2): 105112.
[27]STORER N P, VAN DUYN J W, KENNEDY G G. Life history traits of Helicoverpa zea (Lepidoptera: Noctuidae) on non-Bt and Bt transgenic corn hybrids in eastern North Carolina [J]. Journal of Economic Entomology, 2001, 94(5): 12681279.
[28]U S Environmental Protection Agency. Biopesticides registration action document for Bacillus thuringiensis plant-incorporated protectants (October 16, 2001) [EB/OL]. http:∥wwwepagov/pesticides/biopesticides/reds/brad_bt_ pip2htm, 2001.
[29]CHAMBERS J A, JELEN A, GILBERT M P, et al. Isolation and characterization of a novel insecticidal crystal protein gene from Bacillus thuringiensis subsp. aizawai [J]. Journal of Bacteriology, 1991, 173(13): 39663976.
[30]SIEBERT M W, BABOCK J M, NOLTING S, et al. Efficacy of Cry1F insecticidal protein in maize and cotton for control of fall armyworm (Lepidoptera: Noctuidae) [J]. Florida Entomologist, 2008, 91(4): 555565.
[31]SIEBERT M W, TINDAL K V, LEONARD B R, et al. Evaluation of corn hybrids expressing Cry1F (Herculex I insect protection) against fall armyworm (Lepidoptera: Noctuidae) in the southern United States [J]. Journal of Entomological Science, 2008, 43(1): 4151.
[32]BUNTIN G D. Corn expressing Cry1Ab or Cry1F endotoxin for fall armyworm and corn earworm (Lepidoptera: Noctuidae) management in field corn for grain production [J]. Florida Entomologist, 2008, 91(4): 523530.
[33]NAAS. National Agricultural Statistics Service. Acreage. ISSN: 19491522 [EB/OL]. United States Department of Agricultural, National Agricultural Statistics Service, Washington, DC, 2013. https:∥downloads.usda.library.cornell.edu/usdaesmis/files/jo98zb09z/3197xp47r/z603r0644/Acre-06-28-2013.txt.
[34]HUTCHISON W D, BURKNESS E C, MITCHELL P D, et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers [J]. Science, 2010, 330(6001): 222225.
[35]STORER N P, THOMPSON G D, HEAD G P. Application of pyramided traits against Lepidoptera in insect resistance management for Bt crops [J]. GM Crops Food, 2012, 3(3): 154162.
[36]SIEBERT M W, NOLTING S P, HENDRIX W, et al. Evaluation of corn hybrids expressing Cry1F, cry1A.105, Cry2Ab2, Cry34Ab1/Cry35Ab1, and Cry3Bb1 against southern United States insect pests [J]. Journal of Economic Entomology, 2012, 105(5): 18251834.
[37]ISAAA. Global status of commercialized biotech/GM crops: 2018 [J]. ISAAA Briefs, 2018, 54: iii+143.
[38]STORER N P, KUBISZAK M E, ED KING J, et al. Status of resistance to Bt maize in Spodoptera frugiperda: lessons from Puerto Rico [J]. Journal of Invertebrate Pathology, 2012, 110(3): 294300.
[39]STORER N P, BABCOCK J M, SCHLENZ M, et al. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico [J]. Journal of Economic Entomology, 2010, 103(4): 10311038.
[40]NIU Ying, MEAGHER R L, YANG Fei, et al. Susceptibility of field populations of the fall armyworm (Lepidoptera: Noctuidae) from Florida and Puerto Rico to purified Cry1F protein and corn leaf tissue containing single and pyramided Bt genes [J]. Florida Entomologist, 2013, 96(3): 701713.
[41]NIU Ying, YANG Fei, DANGAL V, et al. Larval survival and plant injury of Cry1 F-susceptible,-resistant, and-heterozygous fall armyworm (Lepidoptera: Noctuidae) on non-Bt and Bt corn containing single or pyramided genes [J]. Crop Protection, 2014, 59: 2228.
[42]HUANG Fangneng, QURESHI J A, MEAGHER R L, et al. Cry1F resistance in fall armyworm Spodoptera frugiperda: single gene versus pyramided Bt maize [J/OL]. PLoS ONE, 2014, 9(11): e112958.
[43]NAGOSHI R N, PIERRE S, MEAGHER R L, et al. Identification and comparison of fall armyworm (Lepidoptera: Noctuidae) host strains in Brazil, Texas, and Florida [J]. Annals of the Entomological Society of America, 2007, 100(3): 395402.
[44]NAGOSHI R N, MEAGHER R L, JENKINS D A. Puerto Rico fall armyworm has only limited interactions with those from Brazil or Texas but could have substantial exchanges with Florida populations [J]. Journal of Economic Entomology, 2010, 103(2): 360367.
[45]NAGOSHI R N, MEAGHER R L. Review of fall armyworm (Lepidoptera: Noctuidae) genetic complexity and migration [J]. Florida Entomologist, 2008, 91(4): 546554.
[46]NAGOSHI R N, MEAGHER R L, FLANDERS K, et al. Using haplotypes to monitor the migration of fall armyworm (Lepidopte-ra: Noctuidae) corn-strain populations from Texas and Florida [J]. Journal of Economic Entomology, 2008, 101(3): 742749.
[47]JAMES C. Global status of commercialized biotech/GM crops: 2011 [R]. ISAAA Briefs, 2011, 43: Viii+325.
[48]FARIAS J R, COSTA E C, GUEDES J V, et al. Managing the sugarcane borer, Diatraea saccharalis, and corn earworm, Helicoverpa zea, using Bt corn and insecticide treatments [J]. Journal of Insect Science, 2013, 13(109):110.
[49]MARTINELLI S, OMOTO C. Resistência de insetos a plantas geneticamente modificadas [J]. Biotecnol Ciênc Desenvolvimento, 2005, 34: 6777.
[50]FARIAS J R, HORIKOSHI R J, SANTOS A C, et al. Geographical and temporal variability in susceptibility to Cry1F toxin from Bacillus thuringiensis in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations in Brazil [J]. Journal of Economic Entomology, 2014, 107(6): 21822189.
[51]JAMES C. 20th anniversary (1996 to 2015) of the global commercialization of biotech crops and biotech crop highlights in 2015 [R]. ISAAA Briefs, 2015, 51: vi+272.
[52]JAMES C. Global status of commercialized biotech/GM crops: 2009 [R]. ISAAA Briefs, 2009, 41: x + 290.
[53]BLANCO C A, CHIARAVALLE W, DALLA-RIZZA M, et al. Current situation of pests targeted by Bt crops in Latin America [J]. Current Opinion in Insect Science, 2016, 15: 131138.
[54]FERNANDES O D, PARRA J R P, NETO A F, et al. Effect of the genetically modified corn MON810 on fall armyworm, Spodoptera frugiperda (J.E.Smith,1797) (Lepidoptera: Noctuidae) [J]. Revista Brasileira de Milho e Sorgo, 2003, 2: 2535.
[55]BUSATO O R, GRTZMACHER A D, GARCIA M S, et al. Compared biology of Spodoptera frugiperda (J.E.Smith) (Lepidoptera: Noctuidae) populations in corn and rice leaves [J]. Neotropical Entomology, 2005, 34: 743750.
[56]MONTEZANO D G, SPECHT A, SOSA-GMEZ D R, et al. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas [J]. African Entomology, 2018, 26(2): 286300.
[57]HORIKOSHI R J, BERNARDI D, BERNARDI O, et al. Effective dominance of resistance of Spodoptera frugiperda to Bt maize and cotton varieties: implications for resistance management [J/OL]. Scientific Reports, 2016, 6: 34864.
[58]FATORETTO J C, MICHEL A P, SILVA FILHO M C, et al. Adaptive potential of fall armyworm (Lepidoptera: Noctuidae) limits Bt trait durability in Brazil [J]. Journal of Integrated Pest Management, 2017, 8(1): 17; 110.
[59]PINHEIRO J C A, PDUA L E M, PORTELA G L F, et al. Biologia comparada de Spodoptera frugiperda (J.E. Smith, 1797) visando ao seu zoneamento ecológico no estado do Piauí [J]. Caatinga, 2008, 21: 197203.
[60]JAMES C. Global status of commercialized biotech/GM crops: 2008 [R]. ISAAA Briefs, 2008, 39: 243.
[61]CTNBIO. Liberacao comercial de milho geneticamente modificado resistente a insetos [J/OL]. Comissao Técnica Nacional de Biossegurana, 2011, http:∥www.ctnbio.gov.br/index.php/content/view/14784.html.
[62]OKUMURA R S, MARIANO D C, DALLACORT R, et al. Agronomic efficiency of Bacillus thuringiensis (Bt) maize hybrids in pests control on Lucas do Rio Verde city, State of Mato Grosso, Brazil [J]. African Journal of Agricultural Research, 2013, 8(19): 22322239.
[63]LOURENO A L F, FERNANDES M G. Evaluation of Cry1Ab and Cry1F Bt maize genotypes for the control of Spodoptera frugiperda (J.E. Smith. 1797) (Lepidoptera: Noctuidae) under field conditions [J]. Cient Jaboticabal, 2013, 41: 164188.
[64]FARIAS J R, ANDOW D A, HORIKOSHI R J, et al. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil [J]. Crop Protection, 2014, 64: 150158.
[65]FARIAS J R, ANDOW D A, HORIKOSHI R J, et al. Frequency of Cry1F resistance alleles in Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil [J]. Pest Management Science, 2016, 72(12): 22952302.
[66]OMOTO C, BERNARDI O, SALMERON E, et al. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil [J]. Pest Management Science, 2016, 72(9): 17271736.
[67]MARTINELLI S, BARATA R M, ZUCCHI M I, et al. Molecular variability of Spodoptera frugiperda (Lepidoptera: Noctuidae) populations associated to maize and cotton crops in Brazil [J]. Journal of Economic Entomology, 2006, 99(2): 519526.
[68]JAMES C. Global status of commercialized biotech/GM Crops: 2014 [R]. ISAAA Briefs, 2014(49): vi+259.
[69]TRIGO E J, CAP E J. Ten years of genetically modified crops in Argentine agriculture [R]. ArgenBio Report, 2006: 152.
[70]GRIMI D A, PARODY B, RAMOS M L, et al. Field-evolved resistance to Bt maize in sugarcane borer (Diatraea saccharalis) in Argentina [J]. Pest Management Science, 2018, 74(4): 905913.
[71]SIGNORINI A M, ABRATTI G, GRIMI D, et al. Management of field-evolved resistance to Bt maize in Argentina: a multi-institutional approach [J/OL]. Frontiers in Bioengineering and Biotechnology, 2018, 6.DOI:10.3389/fbioe.2018.00067.
[72]TRUMPER E V. Resistencia de insectos a cultivos transgénicos con propiedades insecticidas. Teoría, estado del arte y desafíos para la República Argentina [J]. Agriscientia, 2014, 31: 109126.
[73]ARGENBIO. Grfico de evolución de las superficies sembradas con OGM en la Argentina, enporcentajes [R/OL]. http:∥www.argenbio.org/adc/uploads/2017/Argentina_Evolucion_superficie_cultivos_GM_totalcada_cultivo.pdf.2019.
[74]BALBI E I, FLORES F. Evaluasssción del dao causado por el “Cogollero de maíz” (Spodoptera frugiperda) y presencia de la “Isoca de la espiga” (Helicoverpa zea) en diferentes híbridos de maíztransgénico [R/OL]. http:∥inta.gob.ar/documentos/evaluacion-del-danocausado-por-el-cogollero-de-maiz-spodoptera-frugiperda-y-presenciade-la-isoca-de-la-espiga-helicoverpa-zea-en-diferentes-hibridos-de-maiztransgenico.2015.
[75]CHANDRASENA D I, SIGNORINI A M, ABRATTI G, et al. Characterization of field-evolved resistance to Bacillus thuringiensis-derived Cry1F delta-endotoxin in Spodoptera frugiperda populations from Argentina [J]. Pest Management Science, 2017, 74(3): 746754.
[76]MURUA M G, VERA M A, MICHEL A, et al. Performance of field-collected Spodoptera frugiperda(Lepidoptera: Noctuidae) strains exposed to different transgenic and refuge maize hybrids in Argentina [J]. Journal of Insect Science, 2019, 19(6): 17.
[77]莫建初, 莊佩君, 唐振华. 迁移对害虫抗性演化的影响[J]. 昆虫学报, 2000, 43(2): 143151.
[78]TABASHNIK B E, CROFT B A. Managing pesticide resistance in crop-arthropod complexes: interactions between biological and operational factors [J]. Environmental Entomology, 1982, 11(6): 11371144.
[79]MARTINELLI S, CLARK P L, ZUCCHI M I, et al. Genetic structure and molecular variability of Spodoptera frugiperda (Lepidoptera: Noctuidae) collected in maize and cotton fields in Brazil [J]. Bulletin of Entomological Research, 2007, 97(3): 225231.
[80]BARROS E M, TORRES J B, BUENO A F. Oviposition, development, and reproduction of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) fed on different hosts of economic importance [J]. Neotropical Entomology, 2010, 39(6): 9961001.
[81]JAKKA S R, KNIGHT V R, JURAT-FUENTES J L. Fitness costs associated with field-evolved resistance to Bt maize in Spodoptera frugiperda (Lepidoptera: Noctuidae) [J]. Journal of Economic Entomology, 2014, 107(1): 342351.
[82]PRASANNA B M, HUESING J E, EDDY R, et al. Fall armyworm in Africa: a guide for integrated pest management. Manuals [M]. CABI, 2018.
[83]BUSATO G R, GRTZMACHER A D, GARCIA M S, et al. Biologia comparada de populaces de Spodoptera frugiperda (J.E.Smith) (Lepidoptera: Noctuidae) em folhas de milho e arroz [J]. Neotropical Entomology, 2005, 34: 743750.
[84]ACHARYA B, HEAD G P, PRICE P A, et al. Fitness costs and inheritance of Cry2Ab2 resistance in Spodoptera frugiperda (J.E.Smith) [J]. Journal of Invertebrate Pathology, 2017, 149: 814.
[85]SANTOS-AMAYA O F, TAVARES C S, RODRIGUES J V, et al. Fitness costs and stability of Cry1Fa resistance in Brazilian populations of Spodoptera frugiperda [J]. Pest Management Science, 2017, 73(1): 3543.
[86]PAIR S D, RAULSTON J R, SPARKS A N, et al. Fall armyworm distribution and population dynamics in the southeastern States [J]. Florida Entomologist, 1986, 69(3): 468487.
[87]GOERGEN G, KUMAR P L, SANKUNG S B, et al. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J.E.Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa [J/OL]. PLoS ONE, 2016, 11(10): e0165632.
[88]郭井非, 趙建周, 何康来, 等. 警惕危险性害虫草地贪夜蛾入侵中国[J]. 植物保护, 2018, 44(6): 110.
[89]NAGOSHI R N, ROSAS-GARCIA N M, MEAGHER R L, et al. Haplotype profile comparisons between Spodoptera frugiperda (Lepidoptera: Noctuidae) populations from Mexico with those from Puerto Rico, south America, and the United States and their implications to migratory behavior [J]. Journal of Economic Entomology, 2015, 108(1): 135144.
[90]SPARKS A N. A review of the biology of the fall armyworm [J]. Florida Entomologist, 1979, 62(2): 8287.
[91]WESTBROOK J K, NAGOSHI R N, MEAGHER R L, et al. Modeling seasonal migration of fall armyworm moths [J]. International Journal Biometeorology, 2016, 60(2): 255267.
[92]NAGOSHI R N, FLEISCHER S, MEAGHER R L. Texas is the overwintering source of fall armyworm in central Pennsylvania: implications for migration into the northeastern United States [J]. Environmental Entomology, 2009, 38(6): 15461554.
[93]MEAGHER R L, NAGOSHI R N, STUHL C, et al. Larval development of fall armyworm (Lepidoptera: Noctuidae) on different cover crop plants [J]. Florida Entomologist, 2004, 87(4): 454460.
[94]MONNERAT R, MARTINS E, QUEIROZ P, et al. Genetic variability of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) populations from Latin America is associated with variations in susceptibility to Bacillus thuringiensis cry toxins [J]. Applied and Environmental Microbiology, 2006, 72(11): 70297035.
[95]RAZZE J M, MASON C E, PIZZOLATO T D. Feeding behavior of neonate Ostrinia nubilalis (Lepidoptera: Crambidae) on Cry1Ab Bt corn: implications for resistance management [J]. Journal of Economic Entomology, 2011, 104(3): 806813.
[96]ONSTAD D W, GOULD F. Modeling the dynamics of adaptation to transgenic maize by European corn borer (Lepidoptera: Pyralidae) [J]. Journal of Economic Entomology, 1998, 91(3): 585593.
[97]DAVIS P M, ONSTAD D W. Seed mixtures as a resistance management strategy for European corn borers (Lepidoptera: Crambidae) infesting transgenic corn expressing Cry1Ab protein [J]. Journal of Economic Entomology, 2000, 93(3): 937948.
[98]CARROLL M W, HEAD G, CAPRIO M. When and where a seed mix refuge makes sense for managing insect resistance to Bt plants [J]. Crop Protection, 2012, 38: 7479.
[99]WANGILA D S, LEONARD B R, GHIMIRE M N, et al. Occurrence and larval movement of Diatraea saccharalis (Lepidoptera: Crambidae) in seed mixes of non-Bt and Bt pyramid corn [J]. Pest Management Science, 2013, 69(10): 11631172.
[100]GOULD F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology [J]. Annual Review of Entomology, 1998, 43: 701726.
[101]NGUYEN H T, JEHLE J A. Quantitative analysis of the seasonal and tissue-specific expression of Cry1Ab in transgenic maize Mon810 [J]. Journal of Plant Diseases and Protection, 2007, 114(2): 8287.
[102]PANNUTI L E R, BALDIN E L L, HUNT T E, et al. On-plant larval movement and feeding behavior of fall armyworm (Lepidoptera: Noctuidae) on reproductive corn stages [J]. Environmental Entomology, 2016, 45(1): 192200.
[103]MIRALDO L L, BERNARDI O, HORIKOSHI R J, et al. Functional dominance of different aged larvae of Bt-resistant Spodoptera frugiperda (Lepidoptera: Noctuidae) on transgenic maize expressing Vip3Aa20 protein [J]. Crop Protection, 2016, 88: 6571.
[104]PANNUTI L E R, PAULA-MORAES S V, HUNT T E, et al. Plant-to-plant movement of Striacosta albicosta (Lepidoptera: Noctuidae) and Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize (Zea mays) [J]. Journal of Economic Entomology, 2016, 109(3): 11251131.
[105]BERNARDI O, BERNARDI D, AMADO D, et al. Resistance risk assessment of Spodoptera frugiperda (Lepidoptera: Noctuidae) and Diatraea saccharalis (Lepidoptera: Crambidae) to Vip3Aa20 insecticidal protein expressed in corn [J]. Journal of Economic Entomology, 2015, 108(6): 27112719.
[106]REISIG D D, AKIN D S, ALL J N, et al. Lepidoptera (Crambidae, Noctuidae, and Pyralidae) injury to corn containing single and pyramided Bt traits, and blended or block refuge, in the southern United States [J]. Journal of Economic Entomology, 2015, 108(1): 157165.
[107]VELEZ A M, SPENCER T A, ALVES A P, et al. Inheritance of Cry1F resistance, cross-resistance and frequency of resistant alleles in Spodoptera frugiperda (Lepidoptera: Noctuidae) [J]. Bulletin of Entomological Research, 2013, 103(6): 700713.
[108]JAKKA S R, KNIGHT V R, JURAT-FUENTES J L. Spodoptera frugiperda (J.E. Smith) with field-evolved resistance to Bt maize are susceptible to Bt pesticides [J]. Journal of Invertebrate Pathology, 2014, 122: 5254.
[109]CAMARGO A M, CASTANERA P, FARINOS G P, et al. Comparative analysis of the genetic basis of Cry1F resistance in two strains of Spodoptera frugiperda originated from Puerto Rico and Florida [J]. Journal of Invertebrate Pathology, 2017, 146: 4752.
[110]DANGAL V, HUANG Fangneng. Fitness costs of Cry1F resistance in two populations of fall armyworm, Spodoptera frugiperda (J.E.Smith), collected from Puerto Rico and Florida [J]. Journal of Invertebrate Pathology, 2015, 127: 8186.
[111]LI Guoping, REISIG D, MIAO Jin, et al. Frequency of Cry1F non-recessive resistance alleles in North Carolina field populations of Spodoptera frugiperda (Lepidoptera: Noctuidae) [J/OL]. PLoS ONE, 2016, 11(4): e0154492.
[123]NIU Ying, HEAD G P, PRICE P A, et al. Inheritance and fitness costs of Cry1A.105 resistance in two strains of Spodoptera frugiperda (J.E. Smith) [J]. Crop Protection, 2018, 110: 229235.
[124]HUANG Fangneng, QURESHI J A, HEAD G P, et al. Frequency of Bacillus thuringiensis Cry1A.105 resistance alleles in field populations of the fall armyworm, Spodoptera frugiperda, in Louisiana and Florida [J]. Crop Protection, 2016, 83: 8389.
[125]SANTOS-AMAYA O F, TAVARES C S, RODRIGUES J V C, et al. Magnitude and allele frequency of Cry1F resistance in field populations of the fall armyworm (Lepidoptera: Noctuidae) in Brazil [J]. Journal of Economic Entomology, 2017, 110(4): 17701778.
[126]太紅坤, 郭井菲, 张峰, 等. 草地贪夜蛾在云南冬季甜玉米上的生物学习性及为害状观察[J]. 植物保护, 2019, 45(5): 9195.
[127]TABASHNIK B E, BREVAULT T, CARRIERE Y. Insect resistance to Bt crops: lessons from the first billion acres [J]. Nature Biotechnology, 2013, 31(6): 510521.
[128]WAN Peng, XU Dong, CONG Shengbo, et al. Hybridizing transgenic Bt cotton with non-Bt cotton counters resistance in pink bollworm [J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(21): 54135418.
[129]李国平, 姬婷婕, 孙小旭, 等. 入侵云南草地贪夜蛾种群对5种常用Bt蛋白的敏感性评价[J]. 植物保护, 2019, 45(3):1520.
[130]LUTTRELL R G, WAN L, KNIGHTEN K. Variation in susceptibility of Noctuid (Lepidoptera) larvae attacking cotton and soybean to purified endotoxin proteins and commercial formulations of Bacillus thuringiensis [J]. Journal of Economic Entomology, 1999, 92(1): 2132.
[131]CHILCUTT C F, ODVODY G N, CORREA J C, et al. Effects of Bacillus thuringiensis transgenic corn on corn earworm and fall armyworm (Lepidoptera: Noctuidae) densities [J]. Journal of Economic Entomology, 2007, 100(2): 327334.
[132]BUNTIN G D. Corn expressing Cry1Ab endotoxin for management of fall armyworm and corn earworm (Lepidoptera: Noctuidae) in silage production [J]. Journal of Entomological Science, 2010, 45(3): 283295.
[133]BUNTIN G D, LEE R D, WILLSON D M, et al. Evaluation of yieldgard transgenic resistance for control of fall armyworm and corn earworm (Lepidoptera: Noctuidae) on corn [J]. Florida Entomologist, 2001, 84(1): 3742.
[134]BOKONON-GANTA A, BERNAL J S, PIETRANTONIO P V, et al. Survivorship and development of fall armyworm, Spodoptera frugiperda (J.E.Smith) (Lepidoptera: Noctuidae), on conventional and transgenic maize cultivars expressing Bacillus thuringiensis Cry9C and Cry1A(b) endotoxins [J]. International Journal of Pest Management, 2003, 49(2): 169175.
[135]RULE D M, NOLTING S P, PRASIFKA P L, et al. Efficacy of pyramided Bt proteins Cry1F, Cry1A105, and Cry2Ab2 expressed in Smartstax corn hybrids against lepidopteran insect pests in the northern United States [J]. Journal of Economic Entomology, 2014, 107(1): 403409.
[136]WAQUIL J M, DOURADO P M, DE CARVALHO R A, et al. Management of lepidopteran pests in maize crop using the Bt pyramided event Cry1A.105 and Cry2Ab2 [J]. Pesquisa Agropecuaria Brasileira, 2013, 48(12): 15291537.
[137]SANTOS-AMAYA O F, RODRIGUES J V, SOUZA T C, et al. Resistance to dual-gene Bt maize in Spodoptera frugiperda: selection, inheritance, and cross-resistance to other transgenic events [J/OL]. Scientific Reports, 2015, 5: 18243.
[138]LEE M K, WALTERS F S, HART H, et al. Mode of action of the Bacillus thuringiensis vegetative insecticidal protein Vip3A differs from that of Cry1Ab delta-endotoxin [J]. Applied and Environmental Microbiology, 2003, 69(8): 46484657.
[139]HERNANDEZ-MARTINEZ P, HERNANDEZ-RODRIGUEZ C S, RIE J V, et al. Insecticidal activity of Vip3Aa, Vip3Ad, Vip3Ae, and Vip3Af from Bacillus thuringiensis against lepidopteran corn pests [J]. Journal of Invertebrate Pathology, 2013, 113(1): 7881.
[140]ESTRUCH J J, WARREN G W, MULLINS M A, et al. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects [J].Proceedings of the National Academy of Sciences of the United States of America, 1996, 93(11): 53895394.
[141]BURKNESS E C, DIVELY G, PATTON T, et al. Novel Vip3A Bacillus thuringiensis (Bt) maize approaches high-dose efficacy against Helicoverpa zea (Lepidoptera: Noctuidae) under field conditions: Implications for resistance management [J]. GM Crops, 2010, 1(5): 337343.
[142]YANG Fei, KERNS D L, HEAD G P, et al. Cross-resistance to purified Bt proteins, Bt corn and Bt cotton in a Cry2Ab2-corn resistant strain of Spodoptera frugiperda [J]. Pest Management Science, 2017, 73(12): 24952503.
[143]YANG Fei, HUANG Fangneng, QURESHI J A, et al. Susceptibility of Louisiana and Florida populations of Spodoptera frugiperda (Lepidoptera: Noctuidae) to transgenic Agrisure (R) Viptera (TM) 3111 corn [J]. Crop Protection, 2013, 50: 3739.
[144]ZHU Chengqi, NIU Ying, ZHOU Yiwan, et al. Survival and effective dominance level of a Cry1A.105/Cry2Ab2-dual gene resistant population of Spodoptera frugiperda (J.E.Smith) on common pyramided Bt corn traits [J]. Crop Protection, 2019, 115: 8491.
[145]MCGAUGHEY W H. Insect resistance to the biological insecticide Bacillus thuringiensis [J]. Science, 1985, 229(4709): 193195.
[146]TABASHNIK B E, CARRIERE Y, DENNEHY T J, et al. Insect resistance to transgenic Bt crops: Lessons from the laboratory and field [J]. Journal of Economic Entomology, 2003, 96(4): 10311038.
[147]HUANG Fangneng, ANDOW D A, BUSCHMAN L L. Success of the high-dose/refuge resistance management strategy after 15 years of Bt crop use in North America [J]. Entomologia Experimentalis et Applicata, 2011, 140(1): 116.
[148]BATES S L, ZHAO J Z, ROUSH R T, et al. Insect resistance management in GM crops: past, present and future [J]. Nature Biotechnology, 2005, 23(1): 5762.
[149]BAMBAWALE O M, TANWAR R K, SHARMA O P, et al. Impact of refugia and integrated pest management on the performance of transgenic (Bacillus thuringiensis) cotton (Gossypium hirsutum) [J]. Indian Journal of Agricultural Sciences, 2010, 80(8): 730736.
[150]TABASHNIK B E, GASSMANN A J, CROWDER D W, et al. Insect resistance to Bt crops: evidence versus theory [J]. Nature Biotechnology, 2008, 26(2): 199202.
[151]SANAHUJA G, BANAKAR R, TWYMAN R M, et al. Bacillus thuringiensis: a century of research, development and commercial applications [J]. Plant Biotechnology Journal, 2011, 9(3): 283300.
[152]AGROBIO. Brazilian Biotechnology Industry Association 2016. IRM advertising campaign [EB/OL].http:∥boaspraticasagronomicas.com.br/.2016.
[153]中華人民共和国农业农村部. 2019年农业转基因生物安全证书(生产应用)批准清单(一)[EB/OL].http:∥www.moa.gov.cn/ztzl/zjyqwgz/spxx/201912/P020200121588032501444.pdf.2020.
[154]ANDOW D A, ALSTAD D N, PANG Y H, et al. Using an F2 screen to search for resistance alleles to Bacillus thuringiensis toxin in European corn borer (Lepidoptera: Crambidae) [J]. Journal of Economic Entomology, 1998, 91(3): 579584.
[155]GOULD F, ANDERSON A, JONES A, et al. Initial frequency of alleles for resistance to Bacillus thuringiensis toxins in field populations of Heliothis virescens [J]. Proceedings of the National Academy of Sciences of the United States of America, 1997, 94(8): 35193523.
[156]MAHON R J, DOWNES S, JAMES W, et al. Why do F1 screens estimate higher frequencies of Cry2Ab resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) than do F2 screens? [J]. Journal of Economic Entomology, 2010, 103(2): 472481.
[157]REAY-JONES F P, BESSIN R T, BREWER M J, et al. Impact of Lepidoptera (Crambidae, Noctuidae, and Pyralidae) pests on corn containing pyramided Bt traits and a blended refuge in the southern United States [J]. Journal of Economic Entomology, 2016, 109(4): 18591871.
[158]GOULD F. Potential and problems with high-dose strategies for pesticidal engineered crops [J]. Biocontrol Science and Technology, 1994, 4(4): 451461.
[159]KURTZ R W, MCCAFFERY A, OREILLY D. Insect resistance management for Syngentas VipCot transgenic cotton [J]. Journal of Invertebrate Pathology, 2007, 95(3): 227230.
[160]CAPRIO M A, SUMERFORD D V, SIMS S R. Evaluating transgenic plants for suitability in pest and resistance management programs [M]∥LACEY L A, KAYA H K. Field manual of techniques in invertebrate pathology. Dordrecht, Netherlands; Kluwer Academic Press, 2000: 805828.
[161]NIU Ying, HEAD G P, PRICE P A, et al. Performance of Cry1A.105-selected fall armyworm (Lepidoptera: Noctuidae) on transgenic maize plants containing single or pyramided Bt genes [J]. Crop Protection, 2016, 88: 7987.
[162]NIU Ying, GUO Jianguo, HEAD G P, et al. Phenotypic performance of nine genotypes of Cry1A.105/Cry2Ab2 dual-gene resistant fall armyworm on non-Bt and MON 89034 maize [J]. Pest Management Science, 2019, 75(8): 21242132.
(責任编辑:张文蔚)
收稿日期: 20200429 修订日期: 20200502
基金项目:中国农业科学院重大科研任务(CAAS-ZDRW202007)
通信作者 E-mail:klhe@ippcaas.cn

