Hollow fiber-based liquid phase microextraction followed by analytical instrumental techniques for quantitative analysis of heavy metal ions and pharmaceuticals
Wjid Ali Khn , Muhmmd Bll Arin ,b,**, Ydollh Ymini , Nsrullh Shh ,
Tasneem Gul Kazi d, Stig Pedersen-Bjergaard e,*, Mohammad Tajik c
a Department of Chemistry, Abdul Wali Khan University Mardan, 23200, KPK, Pakistan
b Department of Chemistry, University of Karachi, 75270, Karachi, Pakistan
c Department of Chemistry, Tarbiat Modares University, P. O. Box 14115-175, Tehran, Iran
d National Center of Excellence in Analytical Chemistry, University of Sindh, Jamshoro, 76080, Sindh, Pakistan
e School of Pharmacy, University of Oslo, Oslo, Norway
Keywords:
ABSTRACTHollow-fiber liquid-phase microextraction (HF-LPME) and electromembrane extraction (EME) are miniaturized extraction techniques,and have been coupled with various analytical instruments for trace analysis of heavy metals,drugs and other organic compounds,in recent years.HF-LPME and EME provide high selectivity, efficient sample cleanup and enrichment, and reduce the consumption of organic solvents to a few micro-liters per sample. HF-LPME and EME are compatible with different analytical instruments for chromatography, electrophoresis, atomic spectroscopy, mass spectrometry, and electrochemical detection.HF-LPME and EME have gained significant popularity during the recent years.This review focuses on hollow fiber based techniques(especially HF-LPME and EME)of heavy metals and pharmaceuticals (published 2017 to May 2019), and their combinations with atomic spectroscopy, UVVIS spectrophotometry, high performance liquid chromatography, gas chromatography, capillary electrophoresis, and voltammetry.
1. Introduction
Most analytical methods include sample preparation prior to the instrumental analysis.The purpose of the sample preparation is to make the sample compatible with the analytical instrument, to remove interfering matrix components and to enrich the target analytes to a detectable level [1]. Different sample preparation techniques are available, but liquid-liquid extraction (LLE) and solid-phase extraction (SPE) have been the most popular ones [2].For several decades, different microextraction variants of LLE and SPE have been developed. This was originally motivated by reduction or elimination of organic solvents[3]and was initiated by the invention of solid-phase microextraction (SPME) by Pawliszyn et al. [4] and liquid-phase microextraction (LPME) by Jeannot and Cantwell [5]. Since the introduction of these innovative sample preparation methods, a variety of SPME and LPME methods have been reported in the literature. Head-space solid phase microextraction (HS-SPME), in-tube solid phase microextraction (ITSPME), thin-film solid phase microextraction (TF-SPME), headspace liquid phase microextraction (HS-LPME), liquid-liquid microextraction based on solidification of floating organic droplet(LLME-SFO), and dispersive liquid-liquid microextraction (DLLME)are examples in this direction [6-11]. While SPME has become commercially available, LPME is still performed with laboratory built devices. In spite of this, LPME is still a very active area of research for reasons including 1) simplicity, 2) costs, 3) green chemistry,4)selectivity,and 5)method sensitivity.Fig.1 illustrates different configurations and approaches to LPME. LPME was originally performed as single drop microextraction(SDME)[12].SDME is still under development,but in this approach the stability of the droplet of organic solvent may be an issue.

Fig.1. Classification of liquid phase microextraction techniques.
Considering these limitations, hollow fiber protected liquid phase microextraction (HF-LPME) was introduced in 1999 [13]. In the first paper, HF-LPME was utilized for preconcentration and extraction of pharmaceuticals prior to electrophoretic identification and determination.An aqueous extraction solvent was injected into the lumen of a porous polypropylene hollow fiber membrane,while the porous wall was impregnated by an organic supported liquid membrane(SLM).As the extraction solvent(acceptor phase)was not in direct contact with the sample solution, the extraction system was stable, and highly efficient sample cleanup was achieved with this innovative strategy. HF-LPME can be utilized for quantitative analysis from very complex environmental and biological samples [14].
A typical HF-LPME setup consists of a porous hollow fiber membrane made of polypropylene, polytetrafluoroethylene, and polyvinylidene fluoride, which is impregnated with an organic solvent. The solvent is immobilized in the pores in the wall of the hollow fiber membrane and serves as supported liquid membrane(SLM)[15].The acceptor phase can be either an aqueous solution or an organic solvent. Due to this flexibility, HF-LPME is compatible with most analytical instruments for chromatography, electrophoresis, molecular and atomic spectrometry, and electrochemistry.The current review discusses recent applications of HF-based microextraction for pharmaceutical and heavy metal applications,and on their compatibility with different instrumental methods.
2. Principles and possible modes of the HF-LPME
In HF-based LPME methods,the extraction phase is placed in the lumen of a porous hollow fiber.In such a way,the acceptor phase is protected by the SLM. To perform HF-LPME, a polypropylene hollow fiber membrane may first be sonicated in acetone to remove polymer impurities[16].Then the hollow fiber membrane is soaked in an impregnating solvent to fill the pores of the HF membrane by capillary forces, and excess solvent may be removed by washing with distilled water [17]. After this step, the lumen of the hollow fiber is filled with the acceptor phase. As is thoroughly discussed below, the acceptor phase can be the same organic solvent as the SLM (the two-phase mode) or can be an aqueous solution (the three-phase mode). Finally, the hollow fiber is immersed in the sample solution and the analytes are extracted from the sample,through the SLM and into the acceptor phase [18]. Four different arrangements for HF-LPME have been reported, i.e., rod like, ushaped, hollow-fiber solvent bar, and knotted hollow-fiber.Considering the physicochemical characteristics of the desired analytes and levels of complexity of the sample, HF-LPME can be performed in a two- or three-phase mode.
2.1. Two-phase HF-LPME
In two-phase HF-LPME,the pores in the wall of the hollow fiber and the lumen are filled with an organic solvent, which is immiscible with the aqueous sample solution.Solvents like 1-octanol and dihexyl ether are commonly used [19]. Two-phase HF-LPME is applied for extraction and preconcentration of analytes with low polarity such as polycyclic aromatic hydrocarbons(PAHs).Since the analytes are extracted into an organic solvent, two-phase LPME is compatible with gas chromatography.Fig.2 illustrates the principle of two-phase HF-LPME.According to this the SLM and the organic acceptor phase are the same in two-phase HF-LPME.
2.2. Three-phase HF-LPME
In three-phase LPME, the pores in the wall of the hollow fiber are filled with an organic solvent immiscible with water,while the lumen is filled with aqueous solution(acceptor solution).1-Octanol and Dihexylether are common solvents [20]. The mechanism of extraction is based on pH adjustment of the sample solution and the aqueous acceptor phase. For instance, extraction of acidic compounds is achieved by acidification of the sample to suppress ionization of the target analytes. This results in successful transfer of analytes towards the acceptor phase through the organic SLM.pH of the aqueous acceptor solution should be adjusted to a pH value 2-3 units above pKaof the analyte.
Carrier mediated three-phase HF-LPME was introduced where a hydrophobic carrier was dissolved in the organic SLM before the impregnation of the HF pores[21].The applied carrier is an organic compound capable of ion-pairing with analytes of interest.Hence,at the contact region between the sample solution and the SLM,the desired ion-pair complexes are formed, leading to successful extraction of target analytes. At the contact region between the SLM and the aqueous acceptor phase,the analyte is exchanged with a suitable inorganic counter ion dissolved in the acceptor solution,and is released into the acceptor solution.
As mentioned, three-phase HF-LPME can be applied for ionizable compounds(acids and bases).Two-phase HF-LPME offers high PFs and extraction efficiencies for non-polar analytes. However,because the SLM and the acceptor phase are the same organic solvent, there is no phase boundary. Therefore, cleanup is limited.Interestingly, Ghambarian et al. introduced a variant where ndodecane was used as the organic SLM,while organic solvents like methanol,ethanol,or acetonitrile,which are all immiscible with ndodecane,were used as acceptor phase[22].This strategy provided improved sample clean-up, while extraction efficiency was not sacrificed. Fig.3 illustrates the principles of three-phase HF-LPME.
HF-LPME can be carried out through static, dynamic, and even fully automated strategies.One of the most interesting approaches for dynamic HF-LPME was introduced by Esrafili et al. called TTextraction [23]. Between two T connectors, a stainless-steel tube was mounted, housing the hollow fiber membrane. The sample solution was pumped into the TT-extractor through the two T connectors, while the acceptor phase was injected into the hollow fiber membraneviaa syringe pump.This configuration was capable of carrying out two-phase HF-LPME. Fig. 4 illustrates this innovative approach with all the details.In another strategy offered by the same research group, a fully automated HF-LPME with capability for two- and three-phase HF-LPME procedures was developed. In this setup, a syringe pump was used for washing, filling, and ejection of different solvents. Four containers were used for washing solvent, SLM, extraction solvent, and elution solvent. The extraction system was successfully connected with liquid chromatography. Fig. 5 shows the graphical design of this HF-LPME instrument [24].

Fig. 2. A schematic mechanism of the two-phase HF-LPME.

Fig. 3. Schematic mechanisms of two possible modes of three-phase HF-LPME.
2.3. Electromembrane extraction
In HF-LPME,mass transfer is by passive diffusion and this results in relatively slow extraction kinetics. Alternatively, ionizable compounds can be extracted efficiently and with outstanding sample cleanup by electromembrane extraction (EME) [25]. The principle of EME is similar to that of HF-LPME.However,in EME the analytes are extracted selectively in their charged form under the influence of an electrical field. Thus mass transfer is by electrokinetic migration across the SLM, and 2-nitrophenyl octyl ether (NPOE) is often used as SLM.The setup for EME is very similar to three-phase HF-LPME, but in EME two electrodes (usually platinum wires) are utilized. One electrode is placed in the acceptor phase into the hollow fiber lumen,while the second electrode is inserted into the sample solution. Fig. 6 shows the principle of EME.
For extraction of basic analytes,the cathode (-) is placed inside the acceptor phase, while for acidic analytes the direction of the electrical field is reversed[18].After extraction,the acceptor phase is collected with a micro-syringe and injected into an analytical instrument for quantitative analysis. Due to the electrical field across the SLM, bubble formation, Joule heating, and even punctuation of SLM may occur in EME systems operated under nonoptimal conditions. These phenomena occur in longer extraction times as a result of excessive migration of ions across SLM. Also,when the electrical field is applied, electrical double layers and local pH gradients are formed in the interfaces between the SLM and the aqueous solutions on both side.These may impact the mass transfer. To reduce the impact of these phenomena, application of constant direct electrical current and application of pulsed voltage have been reported[26].Although the utilization of a stabilized and constant direct current improved the efficiency and stability of EME,this approach is still not common in EME publications.Pulsed electromembrane extraction (PEME) was introduced as a strategy to enhance the efficiency of EME using a simple and inexpensive extraction setup [27]. Pulsed voltage was found to improve extraction efficiency and system stability.

Fig. 4. Schematic configuration of the dynamic HF-LPME. Reprinted with permission from Ref. [23].
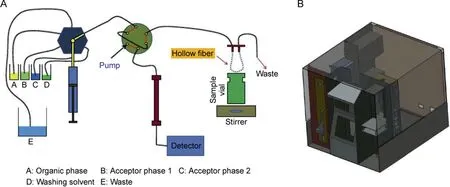
Fig. 5. (A) Illustrative configuration and (B) schematic structure of the automated HF-LPME offered by Esrafili et al. Reprinted with permissions from Ref. [24].
3. Operational parameters of HF-LPME and EME
The type of hollow-fiber membrane, type of extraction solvent,pH of donor and acceptor phases,extraction time,stirring rate,salt addition, and temperature are important parameters in HF-LPME and EME. Applied voltage and electrode material, thickness, and distance are additional parameters affecting EME performance.

Fig. 6. A schematic mechanism for electromembrane extraction.
3.1. Hollow-fiber membrane
The porous wall of the hollow-fiber serves as support for the SLM. The SLM solvent should be of low polarity and immiscible with water. For immobilization of such low-polarity solvents, the hollow-fiber should be of hydrophobic material[28].In most cases,polypropylene hollow-fiber membranes have been used. Such hollow-fibers are commercially available at a low price. Therefore,each hollow-fiber membrane is used only for a single extraction and is then discarded. Alternatively, polyvinylidene difluoride hollow-fiber membranes can be used [14].
3.2. Supported liquid membrane (SLM)
The solvent selected for the SLM plays a major role for the stability and the efficiency of the extraction system both in HF-LPME and EME. First, the solvent should be non-volatile in order to avoid evaporation, and solvents with boiling point exceeding 200°C are recommended.Second,the solvent should be immiscible with water in order to avoid leakage to the sample.Usually solvents with water solubility less than 0.5 mg/mL are recommended.Third,the solvent should facilitate efficient mass transfer of target analyte[14]. In HF-LPME, high efficiency can often be obtained with a variety of organic solvents. Common organic solvents for HF-LPME include n-dihexyl ether, 1-octanol, dodecyl acetate, and toluene.Ionic liquids have also been used as SLM solvent in HF-LPME[28].In EME, the type of solvent used for the SLM is much more critical[29].This is due to the fact that analyte molecules are charged when entering the organic SLM, and partition is voltage dependent.Common organic solvents used in EME are 2-nitrophenyloctyl ether (NPOE) and 1-octanol.
3.3. Sample and acceptor pH
Sample and acceptor pH plays a major role both in HF-LPME and EME.In HF-LPME,the analyte is extracted in neutral form into the SLM,while it is ionized in contact with the acceptor.Therefore,for extraction of basic analytes, the sample should be alkaline, while the acceptor should be acidic or neutral(depending on analyte pKavalue).For HF-LPME of acidic analytes,the pH gradient is reversed using acidic conditions in the sample and alkaline or neutral conditions in the acceptor [30,31]. In EME, the analytes are ionized in the entire extraction system. Thus, for basic analytes, both the sample and the acceptor are neutral or acidic. For acidic analytes,EME is conducted with neutral or alkaline conditions in sample and acceptor [32]. Due to local pH effects [33] at the SLM boundary layers, pH in the acceptor solution is critical in EME, and for basic analytes pH should be no less than three units above pKa. In addition, pH may change in the sample and acceptor during EME due to electrolysis[34],and therefore buffers are recommended in both sample and acceptor.
3.4. Extraction time
Both HF-LPME and EME are equilibrium techniques.This implies that extraction recovery increases rapidly versus time during initial extraction, and after a certain period of time, the systems enter equilibrium. Thus, from a theoretical point of view, recovery is constant from this point forward and extraction is terminated.Equilibrium time is typically in the range 30-60 min for HF-LPME depending on the chemical properties of the analytes and the geometry of the system[35].For EME,equilibrium time is shorter,due to the electro-kinetic mass transfer, and ranges typically from 5 to 15 min. In EME, recoveries may drop during prolonged extraction due to pH changes in sample and acceptor,and due to instability of the SLM [36].
3.5. Stirring/agitation
HF-LPME and EME are performed under stirring or agitation conditions. By such, convection is induced in the sample and analyte mass transfer to the SLM is facilitated. In addition, stirring or agitation reduces the boundary layer thickness at the sample/SLM interface,and this is very important for efficient mass transfer[31].The optimal stirring or agitation rate is geometry dependent,and is normally optimized during method development. Typically, stirring or agitation in the range 500-1000 rpm is used. At higher rates, air bubbles are often formed, and this can reduce extraction performance [37].
3.6. Salt addition
Addition of salt to the sample affects the efficiency of HF-LPME and EME. In HF-LPME, the addition of salt to the sample solution often increases the extraction efficiency [38,39]. The increase in extraction efficiency is due to increased ionic strength in the sample, which increases analyte partition into the organic SLM.However, negative effects of salt in the samples are also reported[40,41]. This may be due to changes in the Nernst diffusion film controlling the diffusion rate of analytes into the acceptor phase[42,43]. Typical salt additions are 5%-30% (w/v) [44]. In EME,addition of salt to the sample is normally not performed. For efficient EME, a low ion balance may be favorable under certain conditions[45-47].The ion balance is defined as the ratio between the total amount of ions in the sample and the total amount of ions in the acceptor phase.
3.7. Temperature
HF-LPME and EME are normally performed at room temperature. Both extraction modes are affected by temperature. Partition coefficients decrease with the increase in temperature, while diffusion across the SLM increases. Temperatures up to 40 C have been used, but above this temperature SLMs tend to be less stable[37,48].
3.8. Electrodes (EME)
Platinum is normally used as inert electrode material in EME.The electrode thickness plays a key role in EME. The internal diameter of the electrodes should be substantially less than the inner diameter of the hollow fiber in order not to displace acceptor phase. The distance between the electrodes is another important parameter[49].
3.9. Applied voltage (EME)
Generally, the extraction efficiency in EME increases with the increasing voltage up to a certain level. Above this, there is no further gain in extraction efficiency,and mass transfer is no longer limited by the voltage. Voltages applied in EME normally range from 5 to 300 V. Voltages exceeding 300 V are not recommended,due to instability of the extraction system and generation of gas bubbles (electrolysis) [37].
4. Instrumental techniques coupled with HF-LPME and EME
4.1. Heavy metals determination
4.1.1. Atomic spectrometry
Combinations of hollow fiber based techniques and atomic spectroscopic techniques have mainly been used for detection of heavy metals in environmental, clinical, and biological samples,petroleum products,pharmaceuticals,and in food[50].The hollow fiber based techniques coupled with atomic spectrometric techniques for preconcentration and determination of different heavy metals are summarized in Table 1 [51-60]. HF-LPME has been coupled to flame atomic absorption spectroscopy (FAAS) and graphite furnace atomic absorption spectrometry (GFAAS) for metal analysis [51-55]. In one example, Tahmasebi et al. introduced a new approach to the extraction of Cr (VI) based on EME coupled with electrothermal atomic absorption spectrometry(ETAAS). Before using the hollow fiber membrane as SLM, polyaniline nanoparticles were coated on the surface to increase the selectivity for the extraction of Cr(VI). The polyaniline reinforced hollow fiber selectively extracted Cr (VI) from real samples via anion exchange. The surface area of the nanostructure coated polyaniline increased the preconcentration of the target analyte [56].
In another example Alahmad et al. introduced an HF-LPME microfluidic paper-based analytical device (μPAD) for extraction of Cr(VI).The membrane was impregnated in a mixture of Aliquat 336 and 1-octanol for 1 min. The acceptor phase was collected for colorimetric analysis after EME, and DPC reagent was added. For quantitative analysis a scanner was used and colored images were analyzed with RGB. The results were compared with ICP-AES and found in good agreement [57].
4.1.2. UV-visible spectrophotometry
UV-visible spectrophotometry has been commonly selected as instrumental technique for quantitative analysis of heavy metals,highly conjugated organic compounds, and biological macromolecules due to easy availability,simplicity,versatility,speed,accuracy,precision,and cost-effectiveness.However,in complex samples the analytes cannot be measured directly with a UV-visible spectrophotometer due to low concentration. Therefore a sample preparation step is necessary to improve selectivity and sensitivity.Atikarnsakul et al. presented an EME procedure for preconcentration and extraction of Cr(VI).Polypropylene hollow fiber membrane was impregnated with NPOE and used for EME. For quantitative analysis, a specific volume of acceptor phase was mixed with a specific concentration of DPC and acidified with dilute H2SO4. The resultant red-violet solution was analyzed with a fiber optic ultraviolet-visible spectrophotometer with a Z-flow cell at 544 nm[61].
Onac et al.introduced a new approach to preconcentration and extraction of Cr(VI). A polymer inclusion membrane was synthesized and used as SLM for EME. The polymer inclusion membrane impregnated with NPOE contained calix[4]arene.The EME process was performed under constant current instead of constant voltage to control the extraction of charged species. The use of constant current in EME provided high reproducibility and mechanical stability. The formation of metal-calix [4]arene ion pair at the membrane-aqueous phase interface enhanced the transport of metal-complex ion through the membrane. Bath-water of chrome plating industry was used as real sample for analysis to check the selectivity of calix[4]arene towards Cr(VI)[62].Table 1 summarizes examples on research combining hollow fiber based extraction techniques with UV-Vis spectrophotometry [61-65].
4.2. Pharmaceuticals
4.2.1. High performance liquid chromatography (HPLC)
Three-phase HF-LPME and EME are ideal for acidic and basic drugs. In three-phase HF-LPME, the acceptor phase is an aqueous solution, and this can be injected directly into HPLC systems without previous evaporation and reconstitution [30]. For extraction of basic analytes in HF-LPME,pH of donor phase must be kept alkaline to suppress the ionization and solubility of the analyte,whereas pH for acceptor phase should be acidic. In case of acidic analytes, the pH gradient across the SLM is reversed [30,31]. For extraction of basic analytes in EME, pH of donor and acceptor phases are kept acidic to ionize the analytes.The anode is placed in the donor phase,while the cathode is placed in the acceptor phase to facilitate the migration of analyte.In case of acidic analytes,the donor and acceptor phases are made alkaline and the direction of the electrical field is reversed[30,32].Three-phase systems provide better degree of clean-up than two phase systems due to the fact that most water soluble components do not pass through the SLM.
Khan et al. presented a new strategy for selective and simultaneous extraction of three drugs with different hydrophobicity property. The EME process was carried out in a microfluidic chip device. The microfluidic device consisted of three PMMA plates having M shaped microchannels.The middle PMMA plate provided a flow path for sample solution and was in contact with two different SLMs and acceptor phases.A stainless steel electrode was embedded in the middle plate, dedicated for the flow of sample solution.Stainless steel electrodes were also embedded in the two PMMA plates dedicated for the two acceptor phases. Based on different SLMs, drugs of different hydrophobicity were extracted into two acceptor phases and analyzed with HPLC-UV [66].

Ref.[51][52][53][54][55][56][57][58][59][60][61][62][63][64][65]technique mode Instrumental FAAS FAAS FAAS GFAAS GFAAS ETAAS RGB/ ICP-AES ETAAS ETAAS ETAAS UV-vis spectrometry UV-vis spectrometry UV-vis spectrometry UV-vis spectrometry UV-vis spectrometry LODaEF/PFb LRc 0.4-12 ng/mL 0.6-3000 μg/mL 10-600 ng/mL 1-50 ng/mL 0.5-10 μg/L 0.02-2.0 ng/mL 10-90 μg/L 1-200 ng/mL 0.5-14 ng/mL 0.5-7.5 10-80 μg/L-2.3-950 μg/L for water and 40-9500 μg/kg for fish sample 20-2000 μg/L 4.9-800 μg/L 18.7 23 790 280-21.3 102-108 106-33 9.1 103 80--176 200 151 Atomic spectrophotometric and UV-Vis spectrophotometric techniques coupled with hollow fiber based extraction techniques for heavy metals determination.Blood, urine samples 0.001 0.5 μg/L 0.08 ng/mL 3 ng/mL mL -0.002 ng/0.2 μg/L 0.02 ng/mL 3 μg/L 0.1 ng/mL 0.143 2.3-7 μg/L 0.7-12 μg/kg 130 4.5 μg/L 1.47 μg/L Real applications Rice, milk, water Environmental water samples Environmental water samples Tap water Spring, sea and distilled water Drinking, mineral and tap water Blood samples Wastewater samples 0.06 ng/mL Water and freshwater fish Environmental water samples Industrial water-River, tap water and fish sample Tap, river and ground water Plasma, water Complexing agent/Carrier SLM composition TiO2 + Caprylic acid Triton X-100 1-octanol Kronenether + oleic acid DEHP + 1-octanol PANI + 1-octanol Methyltrialkylammonium chloride + 1-octanol CTAB + 1-octanol Tetra alkyl ammonium chloride+ 1-octanol DDTC + toluene NPOE NPOE + calix[4]arene 1-octanol 1-octanol DEHP + 1-octanol Methyltrialkylammonium TiO2 chloride DEHP PANI CTAB Kronenether Tetra alkyl ammonium chloride DDTC 1,5-diphenylcarbazide Calix[4]arene DEHP PAN DEHP Preconcentratio/EME Separation method EME HFSLPME APDC HFLPME IEMEHFLPME HFLPME CM-HFLPME μEME HFLPME EME EME EME EME EME Metal/Analyte As Cr (VI)Pb Pb Hg Cr(VI)Cr(VI)Pb Cr (VI)Hg Cr (VI)Cr (VI)Hg Au Bi +3 Table 1 Instrumental techniques Atomic spectroscopic techniques UV-visible spectrometry EME: Electro-membrane extraction, HFLPME: Hollow fiber liquid phase microextraction, IEME: In-tube electro-membrane extraction, CM-HFLPME: carrier-mediated hollow fiber liquid phase microextraction, DEHP: bis(2-ethylhexyl) phosphate, CTAB: N,N,N-cetyltrimethyl ammonium bromide, APDC: Ammonium pyrroldinedithiocarbamate, PANI: Polyanniline, DDTC: Diethyldithiocarbamate, PAN: 1-(2-pyridylazo)-2-naphthol), NPOE: 2-nitrophenyl octyl ether, FAAS: Flame atomic absorption spectroscopy, GFAAS: Graphite furnace atomic absorption spectrometry, ETV-ICP-OES: Electrothermal vaporization inductive couple plasma optical emission spectroscopy, RGB/ ICP-AES: Red Green Blue Analysis/ inductive couple plasma atomic emission spectroscopy.a Limit of detection.b Enrichment factor/Pre-concentration factor.c Linear range.

Ref.[66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91]HPLC-UV-MS Instrumental tec.HPLC-UV HPLC-DAD HPLC-UV HPLC-UV HPLC-UV HPLC-MS HPLC-UV HPLC-UV HPLC-UV HPLC-UV HPLC-DAD HPLC-UV HPLC-UV HPLC-UV HPLC-UV HPLC-UV UPLC-MS HPLC-DAD HPLC-UV HPLC-DAD HPLC-UV HPLC-UV HPLC-UV HPLC-UV HPLC-UV LODaEF/PFbLRc -10-850 μg/L--10-50,000 μg/L 0.02-20 mg/L 0.13-1000 ng/mL-10.-500 μg/L 5-1000 ng/mL 173.3-4000 μg/L 10-500 μg/L 1.0-1000 μg/L 15-500 ng/mL 0.5-500 8.0-500 ng/mL 5-500 μg/L 0.8-500 ng/mL 61-500 μg/L 1-200 ng/mL 10-1000 ng/mL 0.68-8.4 μg/mL 0.5-10 μg/mL 10-2000 μg/L 2.2-6--172-195 0.11-12.83-301 and 265 210-312----12-19 110-150 166-188 62-86 6-4177-43.2-96.8 110-135-23.1-29.5 75>125 98.89-130.3 1.8-5 μg/L High performance liquid chromatography (HPLC) technique coupled with hollow fiber based extraction techniques for pharmaceutical drugs.4-10 μg/L-0.31-1.61 μg/kg 0.95-3.6 μg/L 0.04 and 0.14 ng/mL-2-10 μg/L 1.5-1.8 ng/mL-112-402.3 μg/L 0.007-0.009 μg/mL<11 μg/L 0.1-0.4 μg/L 9-10 ng/mL 0.14-0.42 2.4 ng/mL>44 1.09-98.15 ng/L 1.6-4.3 μg/L 0.3-0.8 ng/mL 18-100 μg/L 0.3-6.0 ng/mL 3 ng/mL 0.20-0.27 μg/mL-0.5 μg/mL 3.3-5 μg/L Real 2-3 μg/L applications Urine, plasma Urine Urine Urine Fish, water Milk Urine Water and urine samples Plasma, urine Wastewater Urine samples Urine, plasma River and tap water Urine River and tap water Wastewater and urine samples Water samples Urine Urine, plasma, pharmaceutical wastewater Urine samples Breast milk, wastewater Plasma, urine Urine Plasma Wastewater, plasma, urine Membrane /SLM composition Chitosan membrane = 60%TFPBA + DEHPi NPOE + DEHP + TEHP Aliquat®336 / 1-octanol Aliquat-336 + 1-octanol 1-octanol 1-octanol + TBP RGO + NPOE DEHP + NPOE 1-octanol + toluene Acrylic nanofibers Agarose gel as SLM NPOE, TBPmembrane/Aliquat®336 + 1-octanol DEHP + NPOE 1-hexyl-3-methylimidazolium hexafluorophosphate C60 fullerene + 1-octanol Agarose film as membrane/Agarose film / 1-octanol 2-ethylhexanol 1-octanol DHE Choline chloride + 1-phenylethanol PIM = 29% CTA + 71%Polyacrylamide gel as membrane chitosan + 40%Aliquat®336 / 1-octanol 2-ethylhexanol 1-octanol 1-octanol 2-ethylhexanol Preconcentration/Separation method Microfluidic EME EME Complexation mediate-EME, EME HF-DLLME HFLPME HF-LPME HFLSPME EME EME PEME EME EME On chip-EME μEME EME EME CF-EME HFLPME HFLPME HFLPME EME EME EME EME EME EME Table 2 Analyte Atenolol,betaxolol,propranolol Amoxicillin, nicotinic acid,hippuric acid, salicylic acid,anthranilic acid, ketoprofen,naproxen, ibuprofen Epinephrine, norepinephrine dopamine Tetracycline, chlortetracycline,doxycycline, oxytetracycline 2-methyl hippuric acid, 3-methyl hippuric acid, 4-methyl hippuric acid Hippuric acid, mandelic acid 54 care products + pharmaceutical drugs Pramipexole Haloperidol, loperamide,methadone, nortriptyline,pethidine Codeine, naloxone, naltrexone Rivastigmine, verapamil,amlodipine, morphine Nicotinic acid, amoxicillin,hippuric acid, salicylic acid Ephedrine, clonidine Imipramine, amitriptyline,chlorpromazine Ibuprofen, sodium diclofenac Ketoprofen, diclofenac,ibuprofen, mefenamic acid Verapamil, Haloperidol,rivastigmine, clomipramine Various drugs and other contaminants Ketoprofen, ibuprofen,naproxen, diclofenac Propranolol, carvedilol,verapamil, amlodipine Salicylic acid, ketoprofen,naproxen, ibuprofen,anthranilic acid, nicotinic acid, amoxicillin, hippuric acid Pseudoephedrine, lidocaine,propranolol Zolpidem Indoprofen, ketoprofen,naproxen, ibuprofen Valproic acid
[92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109]HPLC-UV HPLC-UV HPLC-UV HPLC-UV HPLC-DAD HPLC-DAD UHPLC-FLD HPLC-DAD HPLC-PAD HPLC-DAD/FLD HPLC-UV HPLC-UV HPLC-MS HPLC-UV HPLC-UV HPLC-DAD HPLC-MS HPLC-UV-0.25-10 and 0.06-5 μg/mL respectively 50-2000 ng/mL 0.9-200 μg/L 0.2-2.0 μg/mL 0.01-10 μg/L 0.05-5 μg/L 0.02-2 mg/L 0.5-1000 ng/mL 0.5-750 ng/mL-1.5-500 ng/mL 5-2500 ng/g 2-500 μg/L 9.0-500 μg/L 0.3363-25 μg/L-20-5000 μg/L--170 152-411 1.5-13-14-60 85-133-123.6-146.6-88 17-19--53-86 Plasma-16-75 μg/L 2.8 ng/mL 0.3-0.6 μg/L 0.2 μg/mL 0.02 μg/L 3.1-11.2 ng/L 0.004 mg/kg-0.15 ng/mL-0.1-1.5 ng/mL-1.5-2.5 ng/mL 0.6-10 ng/g 1 μg/L 3-4 μg/L 0.363-0.49 μg/L 0.1-10 μg/L 5-10 μg/L Urine Urine and plasma Urine Plasma Urine Environmental water Orange or lemon peel samples Rabbit plasma Urine Lemon juice, soft drinks Urine, wastewater Blood, urine, liver Blood Urine Plasma, urine Environmental water, urine Pharmaceutical waste water Phthalate- and nitrilebased organic solvents 1-octanol 1-octanol n-dodecane + TOPO 1-octanol 1-octanol 1-octanol-1-octanol + 2-ethyl hexanol + DEHP 1-octanol 1-octanol, 2-ethylhexanol Mixture of 2-ethyl hexanol and 1-otanol 2-nonanone 1-octanol NPOE + DEHP and 1-octanol Cetyl alcohol + 1-octanol Toluene 1-octanol + CTAB EME μEME / LPME HFLPME HFLPME HFLPME HFSLPME HFPLME HFM-MI-MSPE EME EME EME EME FMLPME On chip PEME On chip-EME CA-HF-SLPME MIP-HFME HFLPME Amlodipine, verapamil,clomipramine Metaraminol, benzamidine,sotalol, ephedrine,trimethoprim, pethidine hydrochloride, quetiapine,haloperidol, nortriptyline hydrochloride, methadone hydrochloride, loperamide hydrochloride Five fluoroquinolones and four parabens Diclofenac Exemestane, letrozole,paclitaxel Omeprazole, pantoprazole,lansoprazole Phenazopyridine Different sulfonamides Thiabendazole Leuprolide, triptorelin Salicylic acid, naproxen,ketoprofen, diclofenac,ibuprofen Naproxen, verapamil Verapamil, riluzole Barbital, phenobarbital,pentobarbital Ciprofloxacin Nalmefene, diclofenac Ezetimibe, simvastatin Fosdanofloxacin, norfloxacin,enrofloxacin, ciprofloxacin Raloxifene, ethinylestradiol HFDLLME:Hollow fiber dispersive liquid-liquid microextraction,HFLSPME:Hollow fiber liquid solid phase microextraction,PEME:Pulsed electromembrane extraction,CF-EME:Continuous-flow electromembrane extraction,LPME:Liquid phase microextraction,HFM-MI-MSPE:Hollow fiber membrane-molecular imprinted-micro solid phase microextraction,FMLPE:Flat membrane based liquid phase microextraction,CA-HF-SLPME:Cetyl-alcoholreinforced hollow fiber solid/ liquid phase microextraction, MIP-HFME: Molecular imprinted polymer hollow fiber microextraction, DEHPi: Bis(2-ethylhexyl) phosphite, TFPBA: 4-(trifluoromethyl)phenylboronic acid, RGO:Reduced graphene oxide,TBP:Tributyl phosphate,DHE:Dihexyl ether,PIM:Polymer inclusion membrane,TOPO:Trioctylphosphine oxide HPLC-UV:High performance liquid chromatography with UV detector,HPLC-MS:High performance liquid chromatography with mass spectrometry, HPLC-DAD-FLD: High performance liquid chromatography with diode array detector and fluorescence detector, HPLC-UV-MS: High performance liquid chromatography with UV detector and mass spectrometry, UHPLC-MS: Ultra high performance liquid chromatography with mass spectrometry, HPLC-DAD: High performance liquid chromatography with diode array detector,Fluorometer/RGB/ HPLC-UV: Fluorometer/ Red Green Blue analysis/ High performance liquid chromatography with UV detector.a Limit of detection.b Enrichment factor/Pre-concentration factor.c Linear range.

Ref.[110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130][131]CE-UV Instrumental technique mode V:Fast Fourier transform GC-FID CE-DAD GC-FID CE-UV CE-UV GC-FID CE-UV CE-UV CE-UV CE-UV CE-UV CE-UV CE-UV CE/HPLC-DA FFSSWV SFFTCCV DPV FFTSWV DPV FFTSWV DPV Gas chromatography, Capillary electrophoresis and Voltammetric techniques coupled with Hollow fiber based extraction techniques for determination of pharmaceutical drugs.LODaEF/PF/EFb LRc 0.3-200 and 0.5-750 ng/mL 0.5-750 ng/mL 260-370 3.5-1000 ng/mL 1-20 mg/L 1-40 μg/mL 25-1500 ng/mL 0.5-10 μg/mL-25-1000 ng/mL 50-2500 ng/mL 132-190 1-1000 μg/L 0.5-30 μg/mL-0.1-10 and 10-1000 ng/mL 5-1000 ng/mL 0.010-200 μmol/L 0.1-1300 and 160-188 5-2000 μg/L 1300-10,000 ng/mL 0.5-5 μg/mL 0.02-1000 and 0.2-1000 ng/mL 0.5-100 μmol/L 166-203 10-1000 ng/mL-770.3 0.075-1.5 μg/mL-18-29.1 78 61≤0.15 μg/mL--200-≤0.15 mg/L 0.15 ng/mL 1.0-3.0 ng/L 6.24 ng/mL 0.30-0.91 μg/L 2.10-4.50 μg/L 6.4-9.3 ng/mL-3.03-6.06 ng/mL 4-20 ng/mL 0.02-0.15 μg/mL-0.07-5 μg/mL-0.05 ng/mL 1 ng/mL-7.39× 10-3 μmol/L -2 0.01 ng/mL 0.02 μg/mL 0.001-0.01 ng/mL 35-54 0.5 μmol/L Real applications Urine Urine, plasma Urine, wastewater Urine Plasma Urine Plasma-Water, urine, plasma 01-1.1 ng/mLUrine, plasma Urine Plasma Urine, water Urine, serum,wastewater Urine, plasma Urine Whole blood Whole blood Serum, urine Whole blood Urine Urine, whole blood Urine SLM +/Carrier/FLM/Membrane ENB NPOE Agarose gel ENB 2-ethylhexanol NPOE + MWCNT/ZnO coated by 1-octyl-3-methylimidazolium bromide ionic liquid 4-nitrocumene NPOE NPOE + MIL-110(Cr)NPOE + span 80 1-octanol NPOE 1-octanol ENB Undecanol + NPOE NPOE 1-octanol 1-octanol Nitrobenzene + CuNPs NPOE Butyl benzoate Preconcentration/Separation method EM-SPME G-EME/DLLME EME-EALLME μEME EME EME μEME Pa-EME EME EME HF-SLPME S/EME μEME SLM extraction On chip-EME EME EME HFSPMEEME EME EME HFLPME Instrumental technique Drug Cyproheptadine, ketotifen Trimipramine, clomipramine Clomipramine, imipramine Nort [112]riptyline, papaverine,haloperidol Nortriptyline, haloperidol,loperamide, ketoprofen,naproxen Imatinibmesylate Nortriptyline, papaverine Amitriptyline, haloperidol,bupivacaine, propranolol Methadone, methamphetamine,tramadol Tramadol, pseudoephedrine Capecitabine, 5-Fluorouracil Tranylcypromine Ibuprofen, naproxen, ketoprofen,diclofenac Nortriptyline, papaverine,haloperidol and loperamide Ibuprofen, diclofenac, naproxen,ketoprofen, salicylic acid Amlodipine Diclofenec Imatinib Estradiol valerate Propylthiouracil Imipramine Vanillylmandelic acid V:Fast Fourier transform stripping square wave voltammetry, SFFTCCV:Stripping fast Fourier transform continuous cyclic voltammetry,DPV: Differential pulse voltammetry,FFTSW Table 3 Gaschromatography Capillary electrophoresis Voltammetry EME-EALLME:Electromembrane extraction and electro-assisted liquid-liquid microextraction,EM-SPME:Electromembrane surrounded solid phase microextraction,G-EME/DLLME:Gel-electromembrane exraction/dispersive liquid-liquid microextraction,Pa-EME:Parallel electromembrane extraction,ENB:1-ethyl-2-nitrobenzene,MWCNTs:Multi-walled carbon nanotubes,MIL-110(Cr):Chromium terephthalate metal-organic framework,Span-80:Mono-(9Z)-9-octadecenoate,CuNPs:Copper nanoparticles,GC-FID:Gas chromatography with flame ionization detector,CE-UV:Capillary electrophoresis with UV detector,CE-DAD:Capillary electrophoresis with diode array detector,FFSSW square wave voltammetry.a Limit of detection.b Enrichment factor.c Linear range.
In another example, Romˊan-Hidalgo et al. introduced a new biopolymeric membrane for EME of NSAIDs and acidic compounds.The chitosan biopolymeric membrane was synthesized by mixing chitosan 60% (w/w) and Aliquat®336 40% (w/w). The synthesized membrane was characterized with scanning electron microscopy(SEM). The prepared membrane was impregnated with a suitable organic solvent and used as SLM in a home-made device.After EME,the acceptor phase was analyzed with HPLC-DAD for quantitative analysis[67].Table 2 summarizes examples on research combining hollow fiber based extraction techniques with HPLC [66-109].
4.2.2. Gas chromatography (GC)
GC has been coupled with two-phase HF-LPME and EME for analysis of neutral, low and medium polar analytes. In two phase systems the acceptor solution is the same organic solvent as used for impregnation of membrane. Due to the fact that the acceptor phase is organic, it can be injected directly in GC [109]. An EMESPME technique were introduced by Shamsayei et al. [110] for the extraction and determination of cyproheptadine and ketotifen. In this work,the surface of a stainless steel wire was electrochemically deposited with polypyrrole and manganese dioxide using cyclic voltammetry. A segment of polypropylene membrane was impregnated with a suitable organic solvent and filled with water as an acceptor phase. The prepared SPME fiber (electrochemically prepared stainless steel wire) was introduced into the membrane and used as a cathode. The cathode comprising the SLM and the acceptor solution was directed into donor solution. A platinum anode was directed into the sample solution.After EME,the SPME fiber was analyzed with GC [110].
Similarly Tabani et al. presented a new approach to preconcentration and determination of basic drugs. An agarose gel EME method was combined with dispersive liquid-liquid microextraction(DLLME)technique.For extraction of target analytes,an agarose-based gel membrane was synthesized and used for EME.After EME, acceptor phase was collected and mixed with 1 mM NaOH solution. DLLME was carried out from this solution and the final organic extract was analyzed with GC [111]. Table 3 summarizes information on research combining HF-LPME and EME with GC [110-131].
4.2.3. Capillary electrophoresis (CE)
CE is another analytical instrument which has frequently been coupled with three-phase HF-LPME and EME [30]. To avoid band broadening (de-stacking), the ionic strength of the acceptor phase should not exceed the ionic strength of the CE separation buffer[132]. Dvoˇrˊak et al. presented a new semi-automated-μEME technique for extraction of drugs coupled with CE. A three phase disposable microextraction unit was constructed, in which a free liquid membrane was sandwiched between acceptor and donor phases. All liquid samples (acceptor phase, FLM, and donor phase)were handled with a programmable syringe pump. After EME, the acceptor phase was collected by switching the syringe pump to infusion mode. The collected acceptor phase was quantitatively analyzed with CE [113].
Ryˇsavˊa et al. investigated the effect of membrane thickness on the extraction efficiency in EME [123]. Three polypropylene membranes with different thickness were used as SLMs for EME preconcentration of basic drugs. In this experiment, it was concluded that the transfer of analytes across the membrane is inversely proportional to the membrane thickness. Recent studies of EME and HF-LPME coupled with CE are mentioned in Table 3[113-124].
4.2.4. Voltammetry
One of the most important and widely used electroanalytical techniques is voltammetry [133], and for quantification of heavy metals anodic stripping voltammetry (ASV) has been combined with HF-LPME. The major advantage of ASV over other voltammetric techniques is the step of pre-concentration onto the electrode surface [134]. Anodic stripping voltammetry has low detection limits, high sensitivity and selectivity as compared to other electrochemical techniques. Detection limits with anodic stripping voltammetry of heavy metals are in the ppm range[135].To the best of our knowledge(2017 to May-2019),there is only one research article on voltammetric instrumental technique for quantification of heavy metals using HF-LPME or EME preconcentration. For pharmaceuticals, several new approaches based on hollow fiber based techniques have been reported.In one example,Hrdliˇcka et al. presented an HF-LPME method for extraction and determination of vanillylmandelic acid combined with DPV.In this study,a 20 mm polypropylene hollow fiber membrane was soaked in an organic solvent to impregnate the pores. The excess organic solvent was removed by air with a syringe. The lumen of the fiber was filled with acceptor phase and sealed in both ends. The prepared membrane was fully immersed in sample solution and stirred with a magnetic bar. After EME, the acceptor phase was collected and transferred to the surface of working electrode for DPV analysis[131].
Tahmasebi et al. reported a novel approach in EME for preconcentration and determination of propylthiouracil coupled with DPV. CuNPs were synthesized for selective extraction of propylthiouracil, and coated on hollow fiber membrane. The coated CuNPs increased mass transfer and selectivity of propylthiouracil due to the high selectivity of CuNPs toward thiol group/sulfhydryl compounds. The high affinity/selectivity of propylthiouracil towards CuNPs was explained by the hard and soft acid-base theory.It was concluded that nitrobenzene was the best choice for propylthiouracil extraction because alcoholic solvents such as 1-octanol and 2-ethylhexanol dissolved CuNPs. It was pointed out that this approach was not applicable for acidic compounds[129].Research combining HF-LPME and EME with voltammetry is summarized in Table 3 [125-131].
5. Conclusions and looking into the future
Since 1999, a larger number of reports have been published on the development of HF-LPME. These efforts have documented HFLPME as a green sample preparation technique requiring only a few microliters of organic solvent per sample. HF-LPME enables high enrichment and excellent sample clean-up from biological and environmental samples. In the three-phase mode, HF-LPME provides aqueous extracts which are directly injectable in HPLC.Due to the protection of the acceptor phase by the SLM, HF-LPME is amenable to highly complex samples such as plasma,whole blood,urine, saliva, breast milk, tap water, surface water, pond water,seawater, and soil slurries. HF-LPME shows great potential for routine applications, but to enter this area commercial products and automation are required. Also, generic methods have to be developed to simplify method development.
EME has been developed in parallel to HF-LPME,but EME is less mature.Although,EME provides the same advantages as HF-LPME,the electrical field open for additional perspectives. Thus, the extraction is controlled by an external electrical field, and selectivity may be tuned by the direction and magnitude of the electrical field,by the chemical composition of the SLM,and by pH in sample and acceptor phase.
Both HF-LPME and EME are highly efficient for extraction of heavy metals and pharmaceuticals.The techniques are compatible with a broad spectrum of chromatographic, spectroscopic, and electroanalytical techniques. Future work should focus on automation and commercialization. In addition, the future may see research on the use of green solvents, and SLMs modified with metal nanoparticles (MNPs) and carbon nanotubes (CNTs), which may increase the extraction efficiency of targeted analytes.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the Higher education commission of Pakistan (NRPU No.20-3925/R&D/NRPU/HEC/2014), PAK-US science and technology cooperation (Pak-US No6-4/PAK-US/HEC/2015/04) and Pakistan science foundation joint research projects with MSRT, Iran(No. PSF-MSRT/Env/KP-AWKUM).
 Journal of Pharmaceutical Analysis2020年2期
Journal of Pharmaceutical Analysis2020年2期
- Journal of Pharmaceutical Analysis的其它文章
- Recent advances and perspectives of nucleic acid detection for coronavirus
- Molecular immune pathogenesis and diagnosis of COVID-19
- Quantitative computed tomography analysis for stratifying the severity of Coronavirus Disease 2019
- Erucic acid from Isatis indigotica Fort. suppresses influenza A virus replication and inflammation in vitro and in vivo through modulation of NF-κB and p38 MAPK pathway
- Metabolic profiling of four synthetic stimulants, including the novel indanyl-cathinone 5-PPDi, after human hepatocyte incubation
- Analysis of TRPA1 antagonist, A-967079, in plasma using highperformance liquid chromatography tandem mass-spectrometry
