Tb(Ⅲ)-Based Metal-Organic Framework for Simultaneously Luminescent Detection of Cu2+and Fe3+Ions
WU Qi-QiWEN Yi-Hang*,,2
(1Key Laboratory of the Ministry of Education for Advanced Catalysis Materials,Institute of Physical Chemistry,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
(2State Key Laboratory of Structural Chemistry,Fujian Institute of Research on the Structure of Matter,Chinese Academy of Sciences,Fuzhou 350002,China)
Abstract:A novel metal-organic framework(MOF),namely[TbL(H2O)2]n(1)(H3L=4,4′,4″-((1,3,5-triazine-2,4,6-triyl)tris(sulfanediyl))tribenzoic acid),has been successfully synthesized under solvothermal conditions.All L3-ligands bridge metal ions to form a 3D framework with 1D open channels.In addition,luminescent studies revealed that complex 1 exhibited a unique turn-off luminescent response to Cu2+and Fe3+in CH3CN solution.CCDC:1936537.
Keywords:metal-organic frameworks;4,4′,4″-((1,3,5-triazine-2,4,6-triyl)tris(sulfanediyl))tribenzoic acid;crystal structure;luminescence property
Recently,environmental pollution problem has drawn more and more attentions.Metal ions,particularly copper and iron ions,are common pollutants.Although copper and iron perform outstanding functions in many industrial applications[1-4],it is easily to cause massive copper and iron ions entering human body through foods and drinking water,if industrial waste is not discharged as required.An adequate concentration of Cu2+ions is needed in bone development,cellular metabolism,and plays an important role in significant catalytic cofactor for iron[5-6].Much like copper,iron plays a critical role in human metabolism.Most importantly,iron has significant function in oxygen transport and metabolism of mitochondrial[7].However,overloading of copper and iron ions can cause a series of diseases,such as anemia,braindysfunction,liver cirrhosis,renal insufficiency hepatic cirrhosis,anemia,hereditary hemochromatosis and even death[8-13].Thus,it is extremely urgent to develop an efficient method to detect Cu2+and Fe3+ions in the areas of environmental and medical sciences and the nuclear industry.
Luminescence sensing has been demonstrated to be an effective approach to detect Cu2+and Fe3+ions for their obvious advantages,including high sensitivity and selectivity,low cost,rapid response time,simple manipulation,as well as the possibility to be applied to either solid phase or solution[14-16].Metal-organic frameworks(MOFs),as an outstanding class of microporous and mesoporous materials,have superior functional properties and applications in the fields of gas storage and separation[17-19],heterogeneous catalysis[20-22],photoluminescence[23-24],magnetic materials[25-26],electrocatalysis[27-28],molecular recognition[29]and drug delivery[30].Ln MOFs-based luminescence sensors have successfully attracted enormous attention,due to their unique luminescent properties of visible pure colors,large Stokes′shift values and relatively long luminescence lifetimes,which attributed to f-f transitions via an “antenna effect”[31-35].However,rare Ln MOFs could simultaneously probe Cu2+and Fe3+ions.
In this work,a new Ln-MOFs [Tb(L)(H2O)2]n(1)(H3L=4,4′,4″-((1,3,5-triazine-2,4,6-triyl)tris(sulfanediyl))tribenzoic acid)had been obtained.All L3-ligands bridge metal ions to form a novel 3D framework with 1D open channels(1.550 73(6)nm×0.926 97(4)nm).In addition,luminescent studies revealed that complex 1 exhibited a unique turn-off luminescent response to Cu2+and Fe3+in CH3CN solution.
1 Experimental
1.1 Materials and measurements
All other commercially available chemical reagents and solvents were of reagent grade and used without further purification.Powder X-ray diffraction(PXRD)was studied on a Rigaku UltimaⅣdiffractometer(Cu Kα radiation,λ=0.154 06 nm,40 kV,40 mA,2θ=5°~45°)at room temperature.The IR spectra were recorded on a FTIR-8700 spectrometer using KBr pellets in a range of 4 000~400 cm-1.Elemental analysis(EA)was carried out on a Perkin-Elmer 2400(Ⅱ) element analysis instrument.Thermogravimetric analyses(TGA)were taken on a Mettler-Toledo TGA/SDTA 851e in a range of 25~700 ℃ (the heating rate was 10℃·min-1)under air atmosphere.The fluorescent spectra were performed on a HITACHI F-7000 fluorescence spectrometer at room temperature.The UV-Vis spectra were recorded by using a UV-visible spectrophotometer(Evolution 500LC).
1.2 Preparation of complex 1
A mixture of Tb(NO3)3·2H2O(0.190 5 g,0.5 mmol)and H3L(0.267 3 g,0.5 mmol)was dissolved in 2 mL deionized water and 2 mL CH3CN.And the whole solution was transferred in a Teflon-lined stainless steel container and heated to 100℃for 3 days,and then it was slowly cooled to room temperature over 3 days.After filtered and washed with CH3CN,colorless and block single crystals were obtained in 45%yield based on H3L.Elemental Anal.Calcd.for complex 1(%):C,39.48;H,2.19;N,5.76.Found(%):C,39.62;H,2.28;N,5.25.IR(KBr,cm-1):3 190(m,br),1 687(s),1 631(m),1 594(m),1 557(s),1 478(vs),1 424(s),1 397(s),1 254(vs),1 172(w),1 075(w),1 000(w),906(w),844(s),761(s),678(m),662(w),575(m).
1.3 X-ray crystallography
The single-crystal X-ray diffraction data collection was performed on a Bruker SMART APEX-ⅡCCD detector diffractometer equipped with Mo Kα radiation(λ=0.071 073 nm)at room temperature.The absorption correction was performed by using the SADABS program[36].Data intensity was corrected by Lorentz-polarization factors and empirical absorption.The structure of the complex was measured by direct methods and expanded with Fourier techniques.Anisotropic displacement parameters based on F2were applied to all non-hydrogen atoms in full-matrix leastsquares refinements[37].The hydrogen atoms were assigned with isotropic displacement factors and included in the final refinement cycles by the use of geometrical restrains.All calculations were performed with SHELX-2014 package[38].The details of all pertinent crystallographic data and structure refinement for complex 1 are summarized in Table 1.The selected bond lengths and angles are listed in Table 2.
CCDC:1936537.

Table 1 Crystal data and structure refinement for complex 1

Table 2 Selected bond lengths(nm)and angles(°)for complex 1
2 Results and discussion
2.1 Crystal structure of the complex
Single crystal X-ray diffraction result of complex 1 reveals that it crystallizes in the monoclinic system with C2/c space group.The coordination environment of the metal center is shown in Fig.1a.The asymmetric unit is composed of one metal center,one fully deprotonated L3-anion,and two H2O molecules.Each Tb3+center is eight-coordinated by six O atoms from six different L3-ligands(Tb-O 0.226 4(5)~0.259 3(5)nm)and two O atoms from two H2O molecules(Tb-O 0.241 7(5)~0.244 5(5)nm),resulting in a dodecahedron geometry.The bond angles around Tb(Ⅲ)in complex 1 are from 51.60(15)°to 153.95(18)°(Table 2).
As to L3-ligand,each ligand coordinates to four Tb(Ⅲ)ions by two different carboxylate groups adopting μ1-η1∶η1and μ2-η1∶η1coordination modes (Fig.2).Carboxylate groups from different L3-ligands can be viewed as μ2-bridging ligands,which bridge contiguously two metal atoms to form an infinite 1D chains(Fig.1b).Furthermore,L3-ligands link two infinite 1D chains to make up a 2D layer parallel to ac plane(Fig.1c).In the complex,the layers are further extended by the L3-ligands,resulting in an open 3D network structure with 1D channels of 1.550 73(6)nm×0.926 97(4)nm along the c-axis(Fig.1d)(the size is calculated by the distance between the pair of the symmetric Tb1 atoms and C19 atoms in the pore).Note that,PLATON analysis reveals a moderate void of 1.337 5 nm3,which retains 22.1%of the volume of unit cell(6.049 5 nm3).
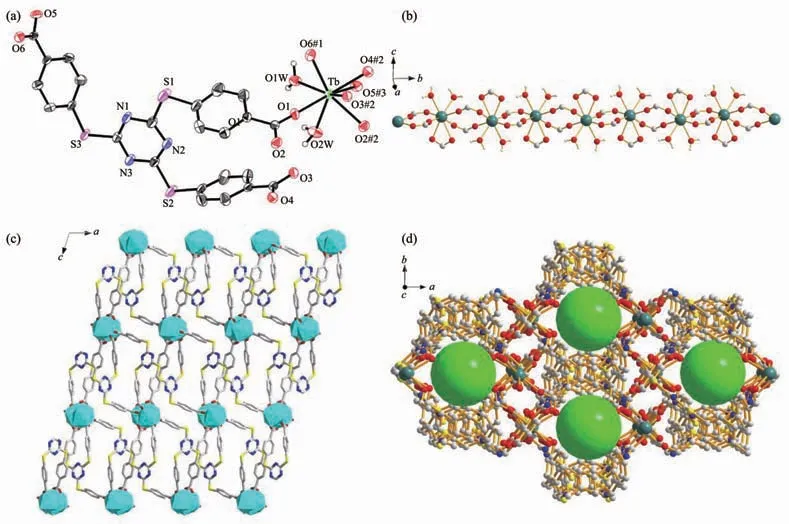
Fig.1 (a)Coordination environments of Tb(Ⅲ)ions in complex 1 showing 50%probability displacement ellipsoids;(b)1D helical chains in complex 1;(c)2D layer of complex 1 viewed along b axis;(d)3D network in complex 1 with green spheres representing pores
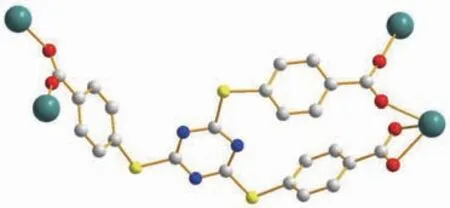
Fig.2 Binding mode of the linker of complex 1
2.2 Powder X-ray diffraction(PXRD)and thermogravimetric analysis
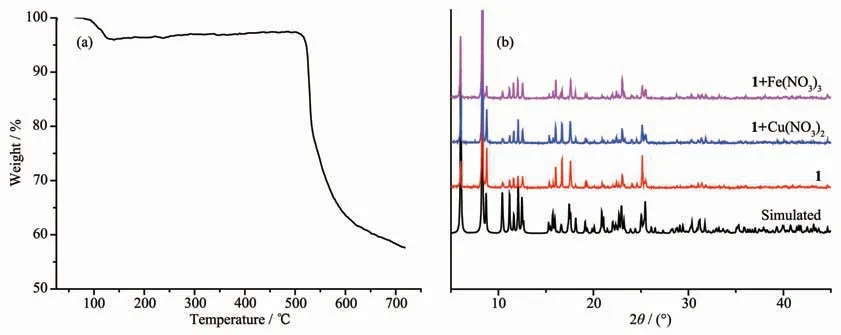
Fig.3 (a)TGA curve of complex 1;(b)PXRD patterns of complex 1 and complex 1 after immersing in Cu(NO3)2or Fe(NO3)3
To check the framework stability of complex 1,PXRD determination and thermogravimetric analysis(TGA)of complex 1 were performed (Fig.3).The PXRD pattern of 1 shows that complex 1 is almost similar to the simulated one.The result confirms the phase purity and good stability of complex 1.In TGA,complex 1 was heated to 700℃ in a rate of 10℃·min-1under air atmosphere.The TGA curve displayed the first weight loss of 4.17%before 125℃,which may correspond to the loss of H2O molecules(Calcd.4.93%).Subsequently,the ligand began to decompose and the framework of complex 1 was destroyed at 525℃.
2.3 Luminescence property
The solid-state fluorescent spectra of the free ligand (H3L)and complex 1 were measured at room temperature,respectively(Fig.4).Complex 1 exhibited four characteristic luminescent emission peaks at 490,545,585 and 622 nm,upon excitation at 330 nm,which are contributed to the5D4→7F6,5D4→7F5,5D4→7F4,and5D4→7F3f-f transitions,respectively[39].
To evaluate the influence of different kinds of metal cations on the luminescence sensing ability of complex 1,the powder of complex 1 (5.00 mg)was dispersed and immersed in different CH3CN solution(5 mL)containing 10 mmol·L-1of M(NO3)x(M=Na+,K+,Mg2+,Ca2+,Sr2+,Ba2+,Pb2+,Zn2+,Mn2+,Cd2+,Ni2+,Co2+,Fe3+and Cu2+)to form stable suspension by ultrasound.The corresponding luminescence curves still showed four characteristic emission peaks of Tb3+ions,and only the strongest emission bands at 545 nm were monitored under the adding of various cations(Fig.5).The results of fluorescent sensing measurements show that Zn2+and Pb2+could improve the emission intensity of complex 1 with small enhancement effects.As for Mn2+,Sr2+,K+,Mg2+,Ca2+,Ni2+,Cd2+,Ba2+,Na+and Co2+,the emission intensities exhibited no differences from each other.However,Cu2+and Fe3+exhibited a relatively high quenching effect on the luminescence of complex 1,which indicates that complex 1 can be considered as a promising fluorescent probe for Cu2+or Fe3+.

Fig.4 (a)Excitation(black line)and emission(red line)spectra of complex 1;(b)Emission spectrum of free ligand H3L(λex=320 nm)

Fig.5 (a)Luminescence intensities of complex 1 in the presence of different metal ions in CH3CN solution when excited at 330 nm;(b)Bar diagram of luminescence intensities at 545 nm of complex 1 in the presence of different metal ions in CH3CN solution
Moreover,to further explore the relationship between Cu2+concentration and quenching effect,a series of suspensions of Cu2+-1 in CH3CN solutions with different concentrations of Cu2+was made.A plot of the luminescence intensities of Cu2+-1 clearly shows the decrease of the luminescence intensity with increasing concentration of Cu2+(Fig.6a).The quenching effect was calculated by the Stern-Volmer equation:I0/I=1+KsvcM,where I0and I are the luminescence intensity of 1 in the absence and presence of metal ion,respectively;cMis the concentration of metal ion(0~1 mmol·L-1);Ksvis the quenching effect coefficient of metal ion (Fig.7a).Same as Cu2+,the corresponding luminescence intensity with different concentrations of Fe(NO3)3and Stern-Volmer plots for Fe3+are shown in Fig.6b and Fig.7b,respectively.They cannot be well fitted by the Stern-Volmer equation,indicating that the dynamic and static quenching processes coexist[40].
Interfering experiments of mixed other metal cations(10 mmol·L-1)with Cu2+or Fe3+(10 mmol·L-1)on the emission of complex 1 were studied.Significant quenching is also seen in Fig.8.The results show that the quenching effect of Cu2+or Fe3+on 1 is not influenced by other metal cations,suggesting that complex 1 exhibits high selectivity for Cu2+and Fe3+sensing.
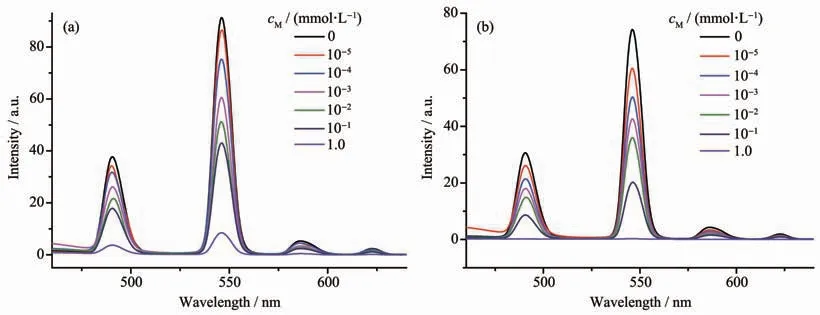
Fig.6 Variation of luminescence intensity of complex 1 suspension with different concentrations of Cu(NO3)2(a)and Fe(NO3)3(b)
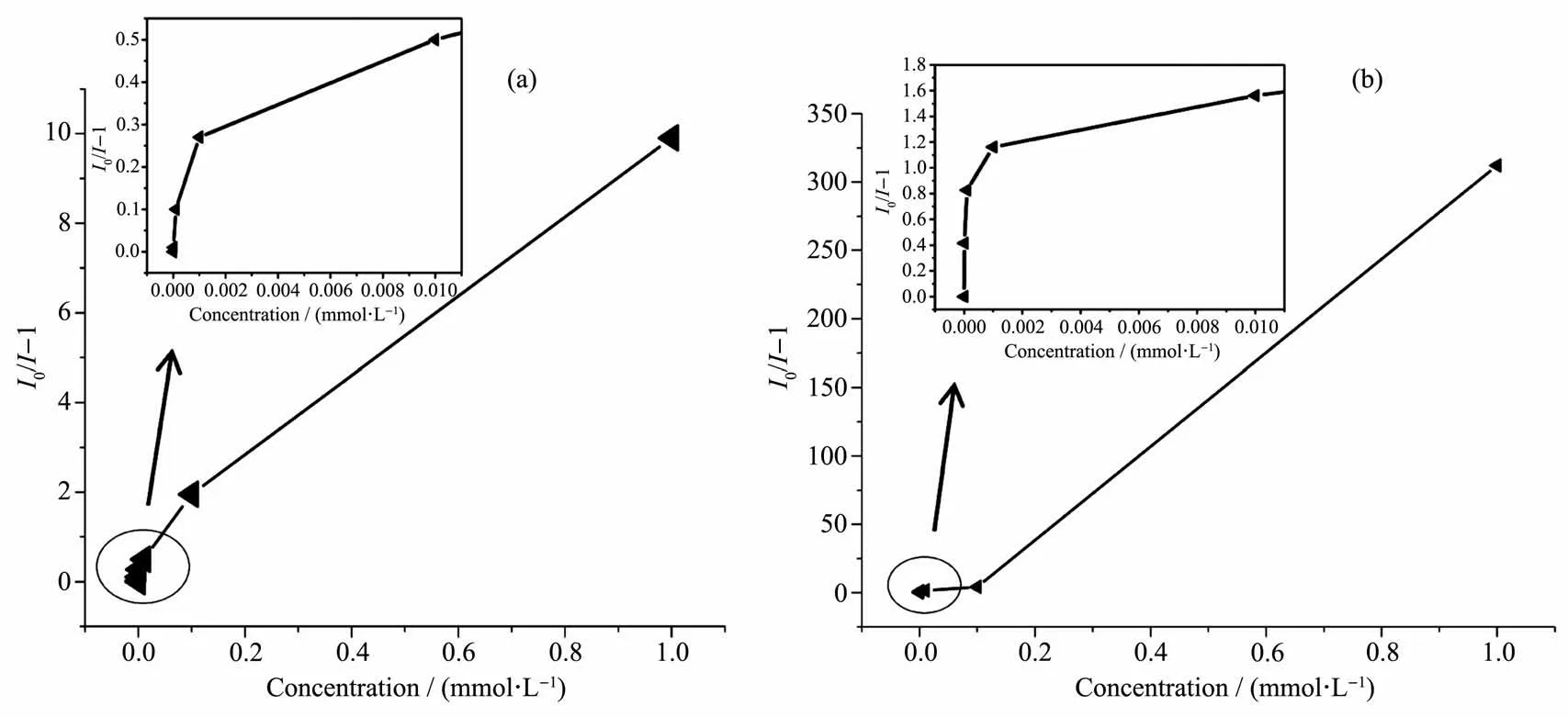
Fig.7 Corresponding Stern-Volmer plots for complex 1 suspension with different concentrations of Cu(NO3)2(a)and Fe(NO3)3(b)

Fig.8 Bar diagram of relative luminescence intensity of complex 1 with mixed metal ions
To explain the probing mechanism of complex 1 toward Cu2+and Fe3+,the PXRD of Cu2+-1 and Fe3+-1 and UV-Vis spectra of complex 1,Fe(NO3)3and Cu(NO3)2were studied.The discussions about probable mechanism are given as follows: (1)the result of PXRD shows good agreement and proves that the basic 3D framework of complex 1 still remains(Fig.3b);(2)it can be seen that the UV-Vis absorption band of a CH3CN solution of Cu2+(260~390 nm)or Fe3+(240~440 nm)covers the excitation spectrum of complex 1(330 nm)(Fig.9),which decreases the essential energy requirement for the “antenna effect”.Therefore,the existence of competitive absorption between metal ions(Cu2+and Fe3+)and complex 1 might be responsible for the luminescence quenching behavior[41-43].
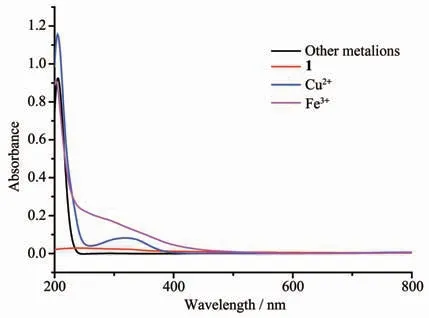
Fig.9 UV-Vis spectra of complex 1,Fe(NO3)3and Cu(NO3)2
3 Conclusions
In summary,we demonstrates an unusual metalorganic framework[Tb(L)(H2O)2]nupon solvothermal conditions.Each L3-ligand bridge metal atoms to form a 3D framework with 1D open channels(1.550 73(6)nm×0.926 97(4)nm).The emission spectra of Cu2+-1 and Fe3+-1 show almost complete fluorescence quenching.Clearly,complex 1 can be considered as a promising fluorescent probe for Cu2+and Fe3+.
- 无机化学学报的其它文章
- Effect of Promoter on the Ni-Al Alloy&the Corresponding Raney-Ni Catalyst and Hydrogenation Performance of 1,4-Butylenediol
- Hierarchically Porous Nanosized Red Phosphorus with Enhanced Photo-Oxidation and Photo-Reduction Activities
- Stretch-Induced Luminescent Changes of a Self-Healing Elastic Polymer Functionalized with Platinum Complex
- Solvent-Induced Syntheses,Crystal Structures and Magnetic Properties of Two Mononuclear Er(Ⅲ)Complexes
- Synthesis,Crystal Structures of Two Lanthanides Complexes with 2,6-Difluorobenzoic Acid
- Hydration Mechanism and Stability Regulation of Hemihydrate Calcium Sulfate Whiskers

