The Characterization on Organic Sulfur Occurrence in Coking Coal and Mechanism of Microwave Actionon Thiophene
GE Tao,CAI Chuan-chuan,CHEN Ping,MIN Fan-fei,ZHANG Ming-xu
1. Institute of Material Science &Engineering, Anhui University of Science and Technology, Huainan 232001, China 2. Institute of Earth & Environment, Anhui University of Science and Technology, Huainan 232001, China 3. Department of Civil and Environmental Engineering, University of Houston, Houston Texas 77204, USA
Abstract To understand the endowment characteristics of the organic sulfur in coking coal and know about the chemical mechanism of microwave to organic sulfur in coal are of great importance to the enrichment of the coal desulfurization theory, optimization of the microwave desulfurization process of coal and development of new technology of fine coal processing. XPS and XANES were used to represent the main structure types of organic sulfur and the relative content of organic sulfur in coking coal. Based on the difference of coal density characteristics, we explored the types and content changes of organic sulfur. The benzothiophene and 3-thiophenecarboxylic acid were selected for microwave irradiation experiments at 915 and 2 450 MHz. The variation characteristics of sulfur structures in the model compounds were studied by Raman spectroscopy. The quantum chemistry simulation was performed using Materials Studio to calculate different directions. Materials Studio was used to simulate the configuration parameters of model compounds under energy fields in different directions. We compared the conformation changes of the model compounds after microwave radiating, and analyzed the response mechanism of the sulfur chemical bonds in the coal to the microwave. The XPS and XANES characterization results showed that sulfur in coking coal is dominated by organic sulfur, and thiophene is the most important form of organic sulfur. With the increase of the densities of coking, thiophenic content decreases relatively, a thiol (ether), and (sub) sulfone gradually increase, and the content of three types of organic sulfur tends to be average. After applying the applied energy fields in different directions, the chemical bonds length and bond level of sulfur in benzothiophene and 3-thiophenecarboxylic acid were not obvious, indicating that the microwave energy fields have limited stretching and torsion of chemical bonds. However, there had influence on the bonds angle and dipole moment in the molecular structures of the model compounds by different energy fields application directions. Raman spectrum showed that the vibration peaks of sulfur chemical bonds in the model compounds were red-shifted after microwave irradiation at 915 and 2 450 MHz. Therefore, microwave radiation affected the local structures of the model compounds microenvironment, and changed their molecular configuration and molecular polarity. At the same time, the vibration recovery force of the crystal lattice was reduced, the interaction force between atoms was weakened, and the fracture of sulfur chemical bonds and the dissociation of sulfur were promoted. It was found that the 915 MHz microwave radiation had more obvious effects than 2 450 MHz.
Keywords Coking Coal; Microwave; Thiophene; Mechanism of action
Introduction
Coking coal is an important raw material for the metallurgy, foundry and chemical industries. During the coking process, 60%~70% of the sulfur in coal is transferred to coke. They are results that brittleness of the pig iron and reduces the production capacity of the blast furnace. Therefore, the removal of sulfur in coking coal is an urgent problem to be solved.Organic sulphur is difficult to remove and it accounts for 30%~70% of the total sulphur in China high sulfur coal. Microwave has good application prospect among the primary coal desulfurization methods[1]. XPS has high ability to identify the chemical properties of the material surface, and it is an effective test method to analyze the characteristics of the main elements in addition to hydrogen of the coal surface structure[3-7]. The X-ray absorption near edge structure analysis (XANES) is very ingenious for chemical valence and spatial configuration of elements. It can be used as a fingerprint spectrum for compound recognition. In recent years, it has been gradually applied to the chemical forms of sulfur in mineral, petroleum, biological and heavy metal pollution analysis[8-10]. Raman spectroscopy has the advantages of no pollution, high sensitivity, rapid detection and low frequency messages[11]. It is widely used in minerals, food and medicine and composite materials structural information[12-17].
Coal density plays an important role in the process of coal processing and utilization. It is a macroscopic representation of the content of organic matter and minerals in coal. Base on coking coal with different densities to study the occurrence characteristics of organic sulfur in coal and explore the differences in its reaction characteristics. Analyze the chemical mechanism of microwave on the structure of main organic sulfur in coal. They are of great significance for saving coking coal resources to improve the desulfurization efficiency of coal by microwave and develop new technologies for fine coal processing.
1 Materials and experiment methods
1.1 Coal quality analysis
The coal sample was taken from Shanxi. The ultimate and sulfur composition analysis are shown in Table 1. Organic sulfur is the most important form of sulfur in coal,and it accounts 68.75% of the total sulfur. In order to reduce the interference of inorganic sulfur in spectroscopy characterization, coking coal was selected as the research object. The 6~13 mm particle size coals were subjected to floatation test and density classification.

Table 1 Ultimate, Sulfur composition of Shanxi coking coal
1.2 Experiment setup and test procedure
1.2.1 XPS,XANES and RAMAN analysis
To ensure homogeneity, the coal powder was ball milled before being pressed and sieved into particles with diameters between 75 and 100 μm.
Coal samples were subjected to XPS detection. The XPS experiments were carried out at room temperature in an ultra-high vacuum (UHV) system with the surface analysis system (EscaLab250Xi). The data processing was fittedwith XPS Peak fit v4.1 software.
The XANES of samples was performed for studying sulfur forms, at 4W1B endstation, Beijing synchrotron Irradiation Facility. The XANES data of the standard samples and model samples were processed by interpolating and normalizing, fitted by the Athena software.
This study was conducted using a LabRAM HR Evolution Raman spectrometer, equipped with 532 nm laser and 600 grooves·mm-1grating. The configuration provided a spectral resolution of 2.0 cm-1, repeat rate better than 0.2 cm-1.
1.2.2 Microwave irradiation experiment
The model compounds were subjected to microwave irradiation experiments at 2 450 MHz(MCR-3 Microwave generator)and 750~950 MHz. The microwave irradiation experimental platform of 750~950 MHz consists of vector network analyzer(Agilent E5071C), microwave reactor (BDS7595-200) and frequency-reconfigurable reaction chamber.
1.2.3 Density functional theory (DFT) calculation
The structural models of the thiophene sulfur model compounds were constructed and optimized by the Visualizer and Doml3 module respectively in the Materials Studio. The molecular structure and properties of the compounds were obtained by density functional quantum chemical calculation.
2 Results and discussion
2.1 XPS characterization of organic sulfur in coking coal of different density grades
Selected 162~167 eV in the XPS scan spectrums of the coal samples to fit the organic sulfur fitting. The results are shown in Fig.1.
In Fig.1, the coal samples with density of +1.8 cannot be baselined and have no fitted curve of organic sulfur. High mineral contentsin high density coals have large interference to the organic sulfur spectrum. It is the main reason for the spectral fitting failure of density +1.8. Pusz believes that pyrite is mainly distributed in high density coal[18]. According to this, the morphological sulfur analysis is carried out on coal samples with density of +1.8. The results are shown in Table 2. The content of iron and sulfur exceeds 50% and the inorganic sulfur contents account for 82.01% of total sulfur, which can explain the failure of coal sample organic sulfur XPS with density of +1.8 reasonably.

Fig.1 XPS fitting spectration of organic sulfur indifferent densities coking coals

Table 2 The speciation analysis data ofsulfur in density of +1.8
The forms of sulfur in other densities coal samples include thiol (ether), (sub) sulfone and thiophene. Among them, thiophene sulphur is the most important endowment form. The relative contents of three organic sulfur forms in high densities coals tend to be average. The quantitative analysis results are shown in Fig.2.
2.2 XANES Characterization of Sulfur Structurein Coking Coal
XANES tests were carried out with samples of 1.4~1.5, which are more samples in coking coal. Seventeen substances such as DMSO were selected as the sulfur standard to analyze the occurrence characteristics of sulfur in coking coal by XANES. The absorption edge position XANES spectrum of standard sulfur compounds are shown in Fig.3. The K-side XANES measured spectrum and fitting spectrum of sulfur in coal is shown in Fig.4.
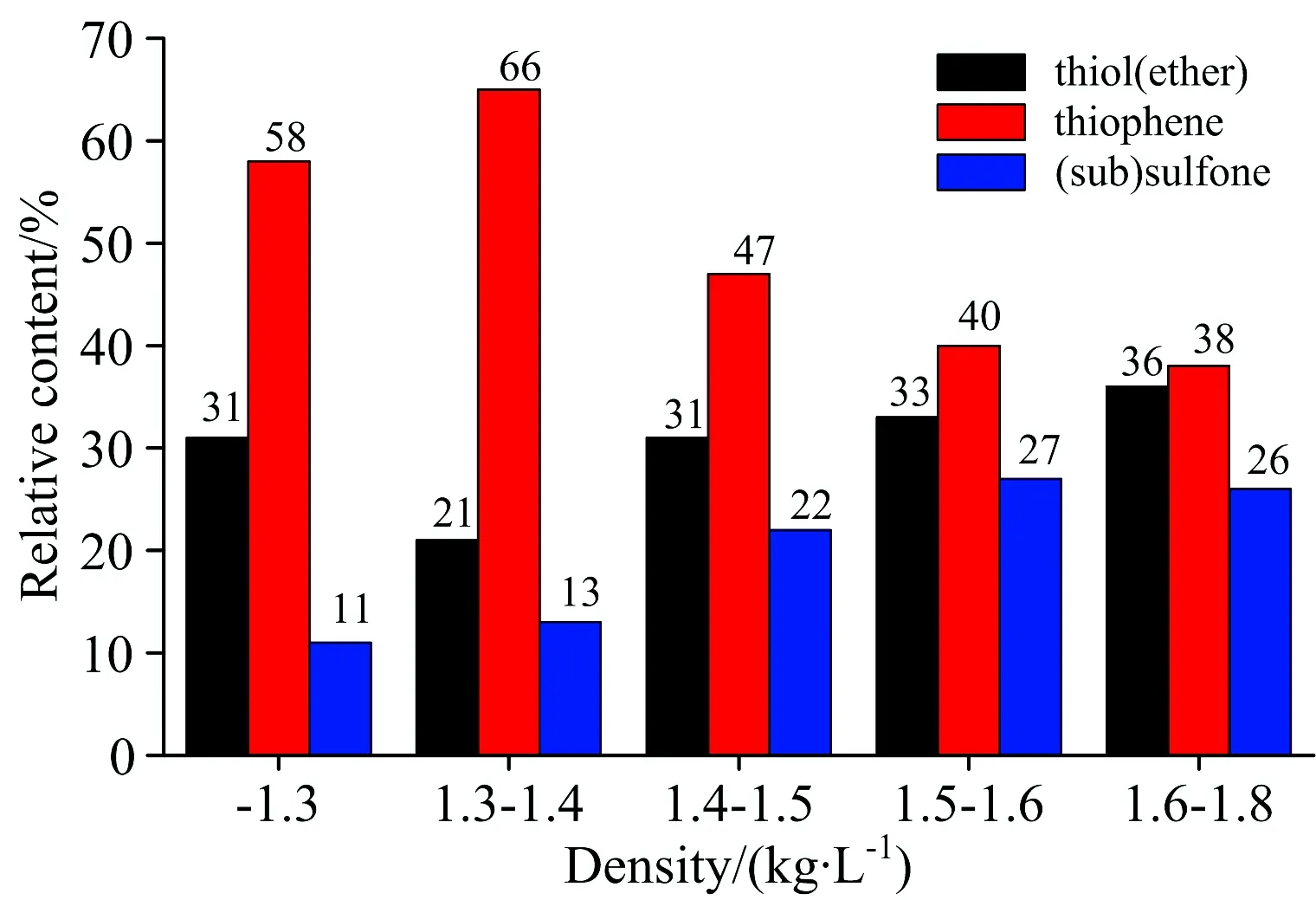
Fig.2 XPS analysis of sulfur occurrence ofdifferent densities coking coals

Fig.3 Standard sulfur compounds XANESspectral absorption edge dosition

Fig.4 The XANES spectrogram of K edge in the coking coal
The XANES measured spectrum has four absorption peaks with electron binding energy of 2.474, 2.476, 2.482 and 2.485 keV, respectively. Compared with the standard library, Na2S2O3, KFe3(SO4)2(OH)6, C12H8S and C3H7NO2S were selected for the sulfur K-side XANES spectrum fitting. The results are shown in Table 3. Thiophene is still the main form of organic sulfur in coal and accounts for more than 50%,followed by thiol (ether),again inorganic sulfur.

Table 3 The analysis of sulfur forms inthe the coking coal of XANES
2.3 XPS Study on the Effect of Microwave on Coking Coal
The 915 and 2 450 MHz are the most commonly used civilian microwave frequencies. The coal samples with density of 1.4~1.5 were also selected for radiating by microwave. The XPS analytical spectrum of the sample after irradiation are shown in Fig.5, and the types and contents of organic sulfur are shown in Fig.6. There is no change in the types of organic sulfur in coal after microwave irradiation. However, the content of thiophene sulphide and (sub) sulfone increases, and the thiol (ether) content decreases. After 915 and 2 450 MHz microwave radiation, the relative content of thiol (ether) in coal decreased from 31% to 15%, 22%. It can be seen that microwave radiation has an effect on the structure of organic sulfur in coal, which changes the relative content of organic sulfur components. Thiophene is the most stable structure of organic sulfur in coal. The Sulfur-containing bonds energy in thiol (ether) is lower than that of sulfone and thiophene. Therefore, microwave energy is relatively easy to change the chemical structure of thiol (ether).
2.4 Raman Study on the Effect of Microwave on Thiophene Model Compounds
According to the results of coal structure analysis, thio-phene is the most important organic sulfur component in raw coal and coal after microwave treatment. Benzothiophene and 3-thiophenecarboxylic acid were selected for microwave irradiation experiments at 750~950 and 2 450 MHz to study the mechanism of action of microwave on the structure of thiophene. The test conditions are shown in Table 4. Rapid heating and selective heating are special effects of microwave fields. Since the power and frequency of the two microwave reactors are different, the same temperature difference was chosen. The microwave irradiation time was controlledin order to avoid sample evaporation by excessive temperature.

Fig.5 XPS fitting spectration of organic sulfurin coal after microwave irradiation

Fig.6 The Change of organic sulfur componentsin coal by microwave irradiation

Table 4 The testconditions of model compounds in microwave irradiation
The Raman spectrum of the two model compounds before and after microwave irradiation are shown in Figures 7 and 8. Raman spectrum information is contained in the peaks and Raman absorption of common sulfur structure has three main regions. The wave numbers are 430~550, 630~780 and 1 050~1 100 cm-1. The functional groups are S—S, aliphatic carbon-sulfur bonds and aromatic carbon-sulfur bonds respectively[19].
The vibration peaks of sulfur-containing bonds of benzothiophene and 3-thiophenecarboxylic acid are red-shifted by microwave action. The vibrational peaks of carbon-sulfur bond in 3-thiophenecarboxylic acid and benzothiophene are red-shifted 26,20 cm-1by 853 MHz and 21, 26 cm-1by 2 150 MHz respectively. The movement of the Raman spectral vibration peaks indicate that the energy bands and structure have changed. In the thiophene model compounds, the sulfur-containing vibration peaks shift toward the long wavelength direction. It indicates that the wave number is reduced, the energy of the photons lower, the band gap is smaller and the groups are more unstable. Therefore, the energy of microwave changes the microscopic environmental structure of the model compounds molecules. As a result, the lattice vibration frequency of the model compound and the relative motion of the atom increases, and the vibration recovery force and the molecular stability decreases.

Fig.7 The Raman spectrum of Benzothiophenemicrowave activity

Fig.8 The Raman spectrum of beta-thiophenicacid microwave activity
2.5 Effect of simulated microwave energy field on structural properties of thiophene in coal
The essence of microwave action on the medium is that the medium responds to microwave energy and changes the chemical structure. The above experiments have shown that sulfurcontaining structure in coal and thiophene model compounds respond to microwaves. In order to study the influence of response on structural parameters, the simulated three-dimensional microwave energy field was added using the Materials Studio simulation platform[20]. The change in structural property parameters of benzothiophene and 3-thiophenecarboxylic acid was calculated.
According to the directivity of microwave transmission and the conversion relationship between microwave energy and electric field strength, setted electricfield 0.000 27,0.000 20,0.000 15 a.u. and electric field 0.000 20,0.000 15,0.000 27 a.u. as the energy and direction parameters of the two simulated energy fields. The configuration of the model compounds after the action of the simulated energy fields are shown in Fig.9, and the structural parameters are shown in Table 5.

Fig.9 The model compound configurationafter simulated energy fields

Table 5 Changes of structural parameters of model compounds after simulated energy fields
The bond length and bond level of sulfur containing chemical bonds in the benzothiophene and 3-thiophenecarboxylic acid are not change substantially after the action of the simulated energy field. Therefore, the energy field has a very limited effect on the stretching and torsion of chemical bonds. It does not have the ability to break the sulfur containing bonds in the thiophene structure. This is consistent with the stability of the thiophene. However, the bond angle and dipole moment in the molecular structureare changed in different directions energy field. The key angle always has a tendency to shift to the most stable form. The dipole moment is an important parameter for characterizing the spatial structure of a molecule and it is used to determine the polarity of a molecule. It can be seen that the simulated microwave energy field can change the molecular configuration and molecular polarity of the thiophene sulfur model compounds.
3 Conclusion
The content of organic sulfur accounts for 70% nearly of the total sulfur in coking coal. The forms of organic sulfur in coal include thiol (ether), (sub) sulfone and thiophene. Among them, thiophene is the most important organic sulfur structure in coal. With the increase of the densities, the contents of organic sulfur in three types tend to be average. The content of thiophene sulfur decreases and thiol (ether) , (sub)sulfone increase gradually in coal.
The organic sulfur in coal responds to microwave energy. After microwave action, there is no change of the organic sulfur types, but the relative content of organic sulfur has change in coal. The content of thiol sulfide with lower sulfur-bonding energy is decreased, and more stable (sub) sulfone and thiophene contents are increased. The microwave can change the local structure of the microenvironment of the model compounds molecules. The microwave field can change the local structure of the microenvironment of the model compound molecules. The microwave energy promotes the vibration recovery of the model compounds crystal lattice. At the same time,it exacerbates the relative motion between the atoms and enhances the vibration frequency. Therefore, the interaction force between atoms is weakened. It is possible to break the sulfurcontaining bonds in the thiophene sulfur structure and to dissociate sulfur.

