Validation of the six-and-twelve criteria among patients with hepatocellular carcinoma and performance score 1 receiving transarterial chemoembolization
Zhe-Xuan Wang, Jing Li, En-Xin Wang, Dong-Dong Xia, Wei Bai, Qiu-He Wang, Jie Yuan, Xiao-Mei Li,Jing Niu, Zhan-Xin Yin, Jie-Lai Xia, Dai-Ming Fan, Guo-Hong Han
Abstract BACKGROUND Transarterial chemoembolization (TACE) is recommended for patients with intermediate hepatocellular carcinoma (HCC) according to treatment guidelines.However, a large number of patients with advanced HCC also receive TACE in clinical practice, especially for those with liver-confined HCC and Eastern Cooperative Oncology Group score (ECOG) 1. In view of previous studies, such patients have different prognoses from advanced HCC patients with macrovascular invasion or extrahepatic spread; therefore, patients with ECOG 1 alone might be classified into the intermediate stage and benefit from TACE treatment, but a study particularly focusing on such patients and exploring the effectiveness of TACE therapy is lacking.AIM To investigate treatment outcomes of TACE in HCC patients with ECOG 1 alone and propose a specific prognostic model.METHODS Patients from 24 Chinese tertiary hospitals were selected in this nationwide multicenter observational study from January 2010 to May 2016. Overall survival(OS) was estimated using Kaplan–Meier curves and compared by the log-rank test. Multivariate Cox regression was used to develop the potential prognostic models. The discriminatory ability of the models was compared and validated in various patient subgroups. The individual survival prediction for six-and-twelve(6&12) criteria, defined as the algebraic sum of tumor size (cm) and tumor number, was illustrated by contour plot of 3-year survival probability and nomogram.RESULTS A total of 792 eligible patients were included. During follow-up, median OS reached 18.9 mo [95% confidence interval (CI): 16.9-21.0]. Three independent multivariate analyses demonstrated that tumor size, tumor number, α-fetoprotein level, albumin–bilirubin grade and total bilirubin were prognostic factors of OS(P < 0.05). The previously proposed 6&12 criteria was comparable or even better than currently proposed with the highest predictive ability. In addition, the 6&12 criteria was correlated with OS in various subgroups of patients. The patients were stratified into three strata with score ≤ 6, > 6 but ≤ 12, and > 12 with different median OS of 39.8 mo (95%CI: 23.9-55.7), 21.1 mo (95%CI: 18.4-23.8) and 9.8 mo (95%CI: 8.3-11.3), respectively (P < 0.001).CONCLUSION TACE is effective for advanced HCC patients with ECOG 1 alone, and the 6&12 criteria may help with clinical decision-making.
Key words: Transarterial chemoembolization; Hepatocellular carcinoma; Overall survival;Predictive factors; Prognostic model; Risk stratification
INTRODUCTION
According to the Barcelona Clinic Liver Cancer (BCLC) staging system and treatment guidelines, transarterial chemoembolization (TACE) is currently the only recommended therapy for patients with hepatocellular carcinoma (HCC) of intermediate stage[1-3]. However, the application of TACE is beyond such recommendations in clinical practice especially for advanced diseases[4-6]. In the BCLC system,advanced HCC is characteristic of macrovascular invasion (MVI), extrahepatic spread(EHS) and tumor-related symptoms based on Eastern Cooperative Oncology Group(ECOG) scoring. With at least one of these features, except preserved liver function,the patients should be stratified into advanced stage[1,7]. However, the population is of high heterogeneity because of such definitions.
Previously, it has been demonstrated that advanced HCC patients with ECOG 1 alone are different from those with MVI and/or EHS[8]. The presence of mild tumorrelated symptoms (ECOG 1) should not be considered as an independent feature of advanced HCC[9]. Therefore, it is unclear whether patients with liver-confined HCC and mild symptoms ought to be included in intermediate or advanced stage. This group of patients is considered to be substage B4 according to the substratification of BCLC, which is different from its original definitions[10]. The Hong Kong Liver Cancer system regards patients with asymptomatic and mild symptomatic HCC to be the same and recommends that patients with ECOG 1 receive TACE[11]. Similarly,advanced HCC patients with ECOG 1 have been recruited for evaluation of TACE in several observational studies and randomized controlled trials[12-15].
Consequently, whether advanced HCC patients with ECOG 1 alone should be classified into the intermediate stage and recommended for TACE remains inconclusive. This study focused on such patients and investigated the treatment outcomes of TACE and the independent predictive factors of survival and proposed a special prognostic score for patient stratification and individual prediction.
MATERIALS AND METHODS
Study population
A total of 3819 consecutive patients from 24 tertiary Chinese centers treated with TACE between January 2010 and May 2016 were retrospectively selected. HCC was diagnosed by histological or imaging evaluation according to the American Association for the Study of Liver Diseases/European Association for the Study of the Liver guidelines and was initially treated with TACE without any prior management[2,3]. Patients meeting one of the following criteria were excluded: (1)Presence of MVI and/or EHS; (2) Child–Pugh score > 7 or decompensation; (3) ECOG performance status score 0; (4) Tumor rupture; (5) Additional systemic treatment; (6)Other malignancies; and (7) Absence of image information. Finally, 792 advanced HCC patients with exclusive ECOG 1 were included (Figure 1). Written informed consent was obtained from all patients before treatment initiation. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Boards of participating centers.
TACE treatment and follow-up
All participating centers had specific expertise in the management of HCC and the practice of TACE. Treatment decisions were made at the discretion of the multidisciplinary liver tumor boards in each institution. Before TACE, digital subtraction angiography of the hepatic artery was performed to assess the vascular anatomy and tumor vascularity. During TACE, a vascular catheter was inserted selectively or super-selectively into the tumor-feeding artery followed by injection containing a mixture of doxorubicin (10-50 mg), cisplatin (10-110 mg), epirubicin (10-50 mg) or oxaliplatin (100-200 mg), which was selected according to the practice of each center and then embolization using gelatin sponge or polyvinyl alcohol foam particles. “On-demand” TACE procedures, based on laboratory assessment and radiological evaluation performed by contrast-enhanced computed tomography or magnetic resonance imaging at an interval of 6-12 wk, were scheduled after the procedure. However, in clinical practice, the intensity of follow-up depended on individuals’ baseline characteristics and responses to the last treatment,i.e. on demand. Thus, not all patients strictly stuck to this imaging follow-up schedule.
Statistical analysis
Categorical variables were described as frequencies and percentages; continuous data were shown as mean values with standard deviation or median with interquartile range (IQR). Overall survival (OS) was defined as the time interval between initial TACE and all-cause death or the last clinical follow-up and was estimated using Kaplan–Meier curves and compared by the log-rank test. Patients who survived at last follow-up date (December 15, 2017) or who were lost to follow-up were censored.To disclose the prognostic factors, univariate analyses for OS were applied to the cohort, then significant variables (P< 0.05) were entered into three Cox multivariate regression analysis models. Variables related to liver function [Child–Pugh class,albumin–bilirubin (ALBI) grade and total bilirubin (TBIL)] were separately included in the multivariate model 1, model 2 and model 3 with stepwise manners for analyses.According to the different accompanying hazard ratio (HR) estimated for each model,a linear predictor was calculated by adding each independent prognostic factor assigned its own weight. Comparison of the performance and discriminating abilities of the proposed models were measured by C index (measure of goodness of fit for binary outcomes in a logistic regression model), likelihood ratioχ2, area under timedependent receiving operator characteristic curve, and R2. Statistical analysis was conducted using SPSS version 17.0 (SPSS Inc., Chicago, IL, United States) and R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
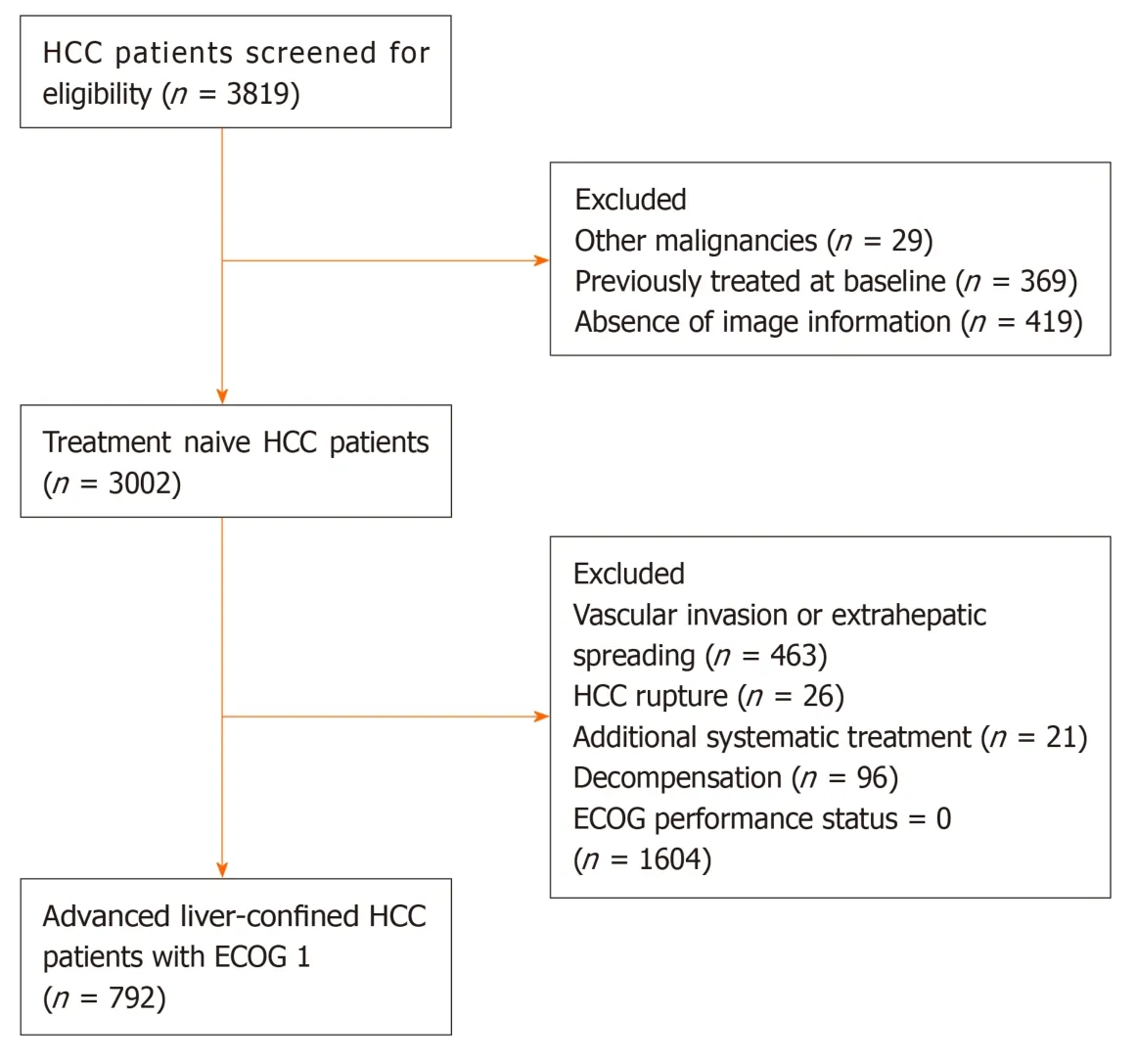
Figure 1 Flow chart for patient eligibility. HCC: Hepatocellular carcinoma; ECOG: Eastern Cooperative Oncology Group.
RESULTS
Patient characteristics
Baseline characteristics for the cohort consisting of 792 eligible patients are shown in Table 1. The mean age was 56.4 years, and hepatitis B virus infection was the main etiology of HCC. Liver function in 742 (93.7%) patients was classed as Child–Pugh class A while the remaining 50 (6.3%) were class B (only Child–Pugh score 7). At the same time, 323 (40.8%) patients were graded as ALBI 1, 458 (57.8%) as ALBI 2 and 11(1.4%) as ALBI 3. The median tumor size was 8.2 cm with an average of 8.6 cm.Median tumor number was 1 (IQR 1-3). During follow-up, the median number of TACE sessions for each patient reached 2 (IQR 1-4).
OS
With a median follow-up of 14.9 mo (IQR 7.0-27.1), 97 (12.2%) patients were lost to follow-up, 500 (63.1%) died and 195 (24.6%) survived. Median OS was 18.9 mo(95%CI: 16.9-21.0) for the whole cohort (Figure 2A). According to Child–Pugh classification, patients with class A had a median OS of 19.6 mo, which was better than 13.5 mo for class B (log-rankP= 0.046) (Figure 2B). As for ALBI grade, the median OS for patients with grade 1 was 20.5 mo, which was significantly longer than the 17.7 mo and 5.8 mo for patients with grade 2 and 3, respectively (log-rankP=0.001) (Figure 2C). The patients with lower α-fetoprotein (AFP; no more than 400 ng/mL) had a median OS of 22.7 mo, while those with higher AFP value had a shorter median OS of 13.5 mo (log-rankP< 0.001) (Figure 2D).
Independent prognostic factors
Univariate analyses for OS are shown in Table 2, suggesting that tumor size, tumor number, Child–Pugh class, ALBI grade, AFP level, white blood cells, platelets,aspartate aminotransferase and TBIL were all associated with survival (P< 0.05).Including all these predictive factors except ALBI grade and TBIL, the multivariate model 1 demonstrated that tumor size (HR = 1.10, 95%CI: 1.08-1.12,P< 0.001), tumor number (HR = 1.10, 95%CI: 1.07-1.14,P< 0.001) and AFP level (HR = 1.31, 95%CI:1.09-1.98,P= 0.047) were independent prognostic factors of OS (Table 3). However,the multivariate model 2 included the significant factor in univariate analysis except for Child–Pugh class and TBIL and found that tumor size (HR = 1.10, 95%CI: 1.08-1.12,P< 0.001), tumor number (HR = 1.10, 95%CI: 1.06-1.64,P< 0.001), AFP level (HR= 1.36, 95%CI: 1.13-1.63,P= 0.001) and ALBI grade (HR = 1.44, 95%CI: 1.20-1.72,P<0.001) were associated with OS. Finally, the multivariate model 3 revealed that tumor size (HR = 1.10, 95%CI: 1.07-1.12,P< 0.001), tumor number (HR = 1.11, 95%CI: 1.07-1.15,P< 0.001), AFP level (HR = 1.28, 95%CI: 1.06-1.53,P= 0.010) and TBIL (HR =1.01, 95%CI: 1.00-1.02,P= 0.025) predicted OS independently after including the predictors of univariate analysis, except for Child–Pugh class and ALBI grade.
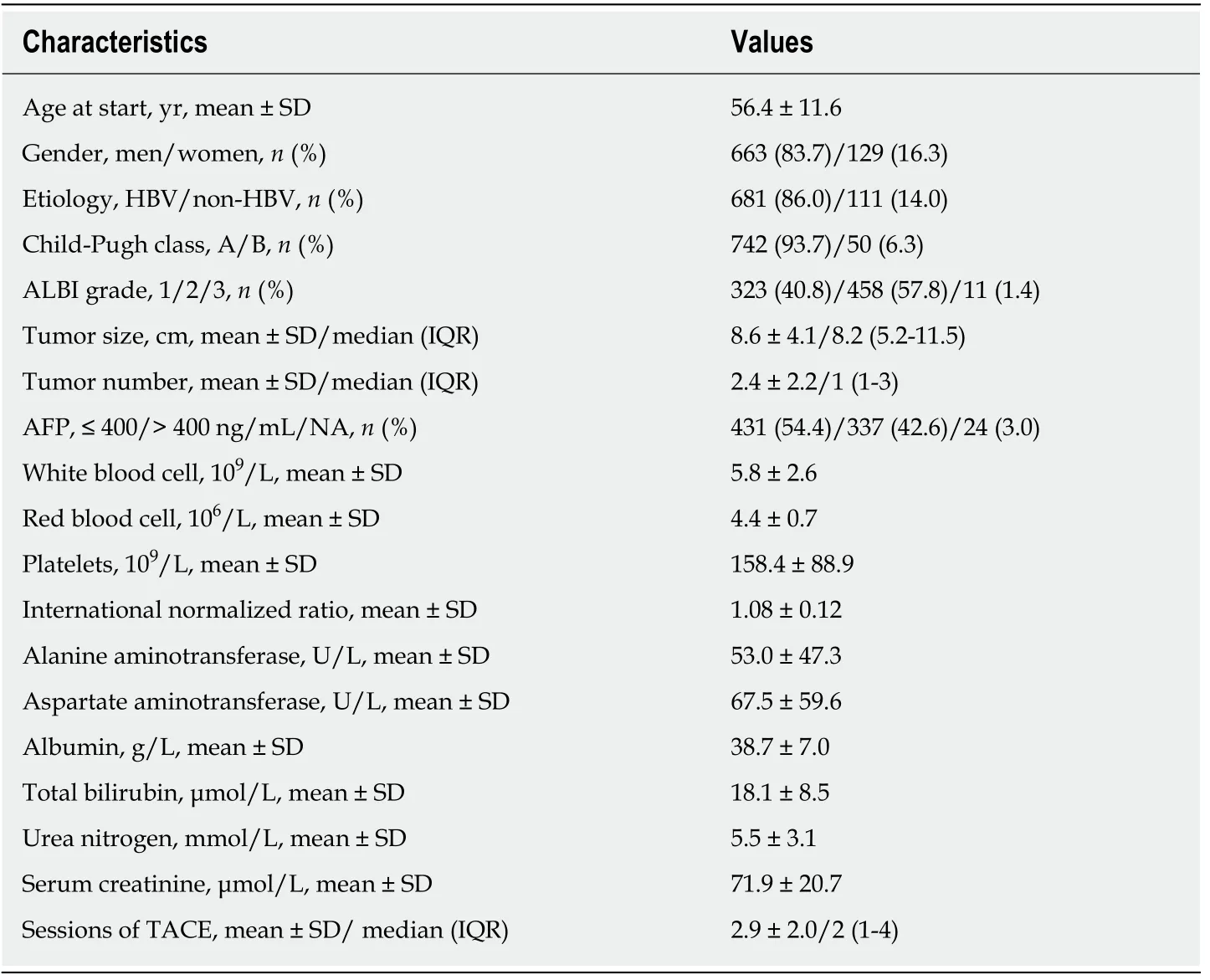
Table 1 Baseline characteristic for the cohort, n = 792
Development of the prognostic model
According to the three multivariate models, tumor size, tumor number and AFP level were reliable prognostic factors of OS, but the factors related to liver function performed differently in these models (Table 3). The regression coefficients for tumor size, tumor number and AFP level were 0.093, 0.097 and 0.266, respectively in model 1. In model 2, the regression coefficients for tumor size, tumor number, AFP level and ALBI grade were 0.095, 0.097, 0.304 and 0.361, respectively. In model 3, the regression coefficients for tumor size, tumor number, AFP level and TBIL were 0.092, 0.102, 0.243 and 0.012, respectively. For ease of use, 1 divided by the regression coefficients for tumor size was the constant of each formula. Then, the regression coefficients for other predictors were respectively multiplied by the constant to achieve their coefficients in the formulas. Additionally, considering the robust prognostic value of tumor size and tumor number, our previously proposed six-and-twelve (6&12)criteria was also evaluated[16]. This prognostic model was “linear predictor = largest tumor diameter (cm) + tumor number” and could divide patients enrolled into three risk stratifications with the cut-off values “6” and “12”, which may provide an easyto-use tool (a nomogram developed based on statistical results) for classification and individual survival prediction. Finally, the formulas as shown in Table 4 were used for calculating the linear predictor of each proposed model. Comparisons among them demonstrated that model 3 and 6&12 criteria model exhibited an advantage over models 1 and 2 in predicting performance and discriminating ability, while no significant differences were seen between model 3 and the 6&12 criteria model (Figure 3). It can be seen from above that the 6&12 criteria model was still the first choice as an easy-to-use clinical tool. In particular, the 6&12 criteria could predict OS regardless of gender, age, AFP level, Child–Pugh score, ALBI grade and etiology (Figure 4).
Individual survival prediction and risk stratification
Based on these findings, the relationship between tumor size and tumor number, as well as 3-year survival probability was depicted in a contour plot (Figure 5A). In addition, a nomogram was created for individual survival prediction of patients; the 1-year, 2-year and 3-year survival rates of individual patients can be predicted by the sum of tumor size and number (6&12 criteria) prior to TACE (Figure 5B). For risk stratification of patients, the cut-off values of the 6&12 criteria were used for the patient classification (Figure 5C). For patients with low tumor burden, the median OS reached 39.8 mo (95%CI: 23.9-55.7), which was better than that of 21.1 mo (95%CI:18.4-23.8) in moderate tumor burden and 9.8 mo (95%CI: 8.3-11.3) in high tumor burden (P< 0.001).
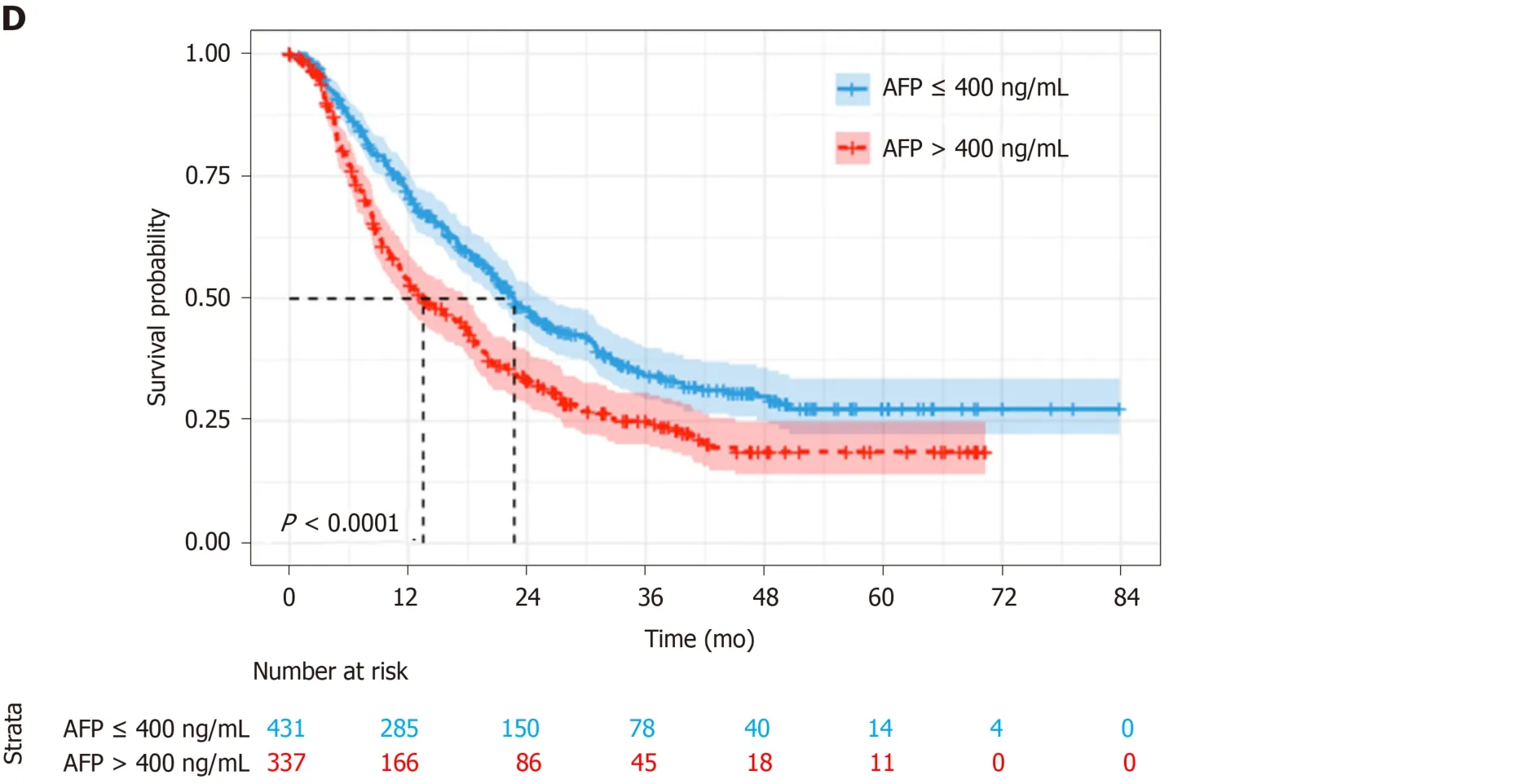
Figure 2 Kaplan–Meier curves for survival analysis and comparisons. A: Survival analysis of the whole cohort; B: Comparisons among patients with different Child-Pugh class; C: Comparisons among patients with different albumin-bilirubin grade; D: Comparisons among patients with different alpha-fetoprotein level. ALBI:Albumin-bilirubin; AFP: Alpha-fetoprotein.
DISCUSSION
In this nationwide multicenter study, we retrospectively explored the treatment outcomes of TACE in advanced HCC patients with ECOG 1 alone and investigated the prognostic factors for OS. Overall, the median OS for all patients reached 18.9 mo.Remarkably, it was demonstrated that tumor size and number were robust prognostic factors of OS, based on which the 6&12 criteria showed consistent discriminatory ability. In addition, the 6&12 criteria could stratify these patients into three subgroups with significantly different OS after TACE.
According to the current treatment guidelines, TACE is recommended for patients with intermediate HCC[2,3]. However, many advanced HCC patients receive TACE in clinical practice especially those with tumors confined to the liver[4,17]. The median OS of 18.9 mo in our study was consistent with previous reports in real-world conditions with a median OS of 19.2 mo in which the patients with unresectable early or intermediate HCC were included[18]. Therefore, the treatment outcomes of the current study indicated that those advanced HCC patients with ECOG 1 would be suitable for TACE and could be included in the intermediate stage. In the Hong Kong Liver Cancer staging system, ECOG 1 was regarded the same as ECOG 0, and those patients should be treated with TACE or curative therapies[11]. In the BCLC system, inclusion of patients with ECOG 1 but without MVI/EHS in BCLC B stage improved the discrimination of the staging system[9]. However, according to the substratification of BCLC B stage, advanced HCC patients with ECOG 1 were recognized as B4 stage,who had a worse prognosis compared with others in B stage[19,20]. In addition, best supportive care was initially recommended to them rather than TACE and even systemic therapies[10]. Consequently, the risk stratification and treatment arrangement of these patients remain controversial.
As a highly complex technical procedure, TACE is operator-dependent and heterogeneity exists in the techniques and agents used, which might explain variations in outcomes in patients with HCC[21]. However, there is no consensus on the optimal chemotherapeutic agent to use in TACE. Worldwide, the most popular anticancer drug injected is doxorubicin[22]. In addition, a recent randomized controlled trial comparing TACE with transarterial embolization found no dierences in terms of tumor response and OS, which questioned the effects of chemotherapy agents[12].Considering these points, using the mixture of doxorubicin, cisplatin, epirubicin or oxaliplatin might have little effect on our current analysis. Although TACE was the most commonly used treatment for patients with unresectable HCC, sorafenib was the recommended systemic therapy for advanced disease and has been effective worldwide[4,6,23,24]. Furthermore, combining TACE and sorafenib might be a “good marriage” for unresectable HCC[25,26]. In addition, systemic chemotherapy with doxorubicin or FOLFOX did not demonstrate survival benefits[27,28]. Major emphasis has been focused on the efficacy of transarterial radioembolization. In cohort studies,transarterial radioembolization showed tumor response rates between 40% and 90%,and survival was comparable to that obtained with TACE and sorafenib[29,30].However, randomized controlled trials failed to demonstrate a survival benefit from transarterial radioembolization compared with sorafenib[31]. Recently, lenvatinib was found to be non-inferior to sorafenib, offering another treatment option for patients with advanced HCC[32]; however, large multicenter real-world studies are highly needed.
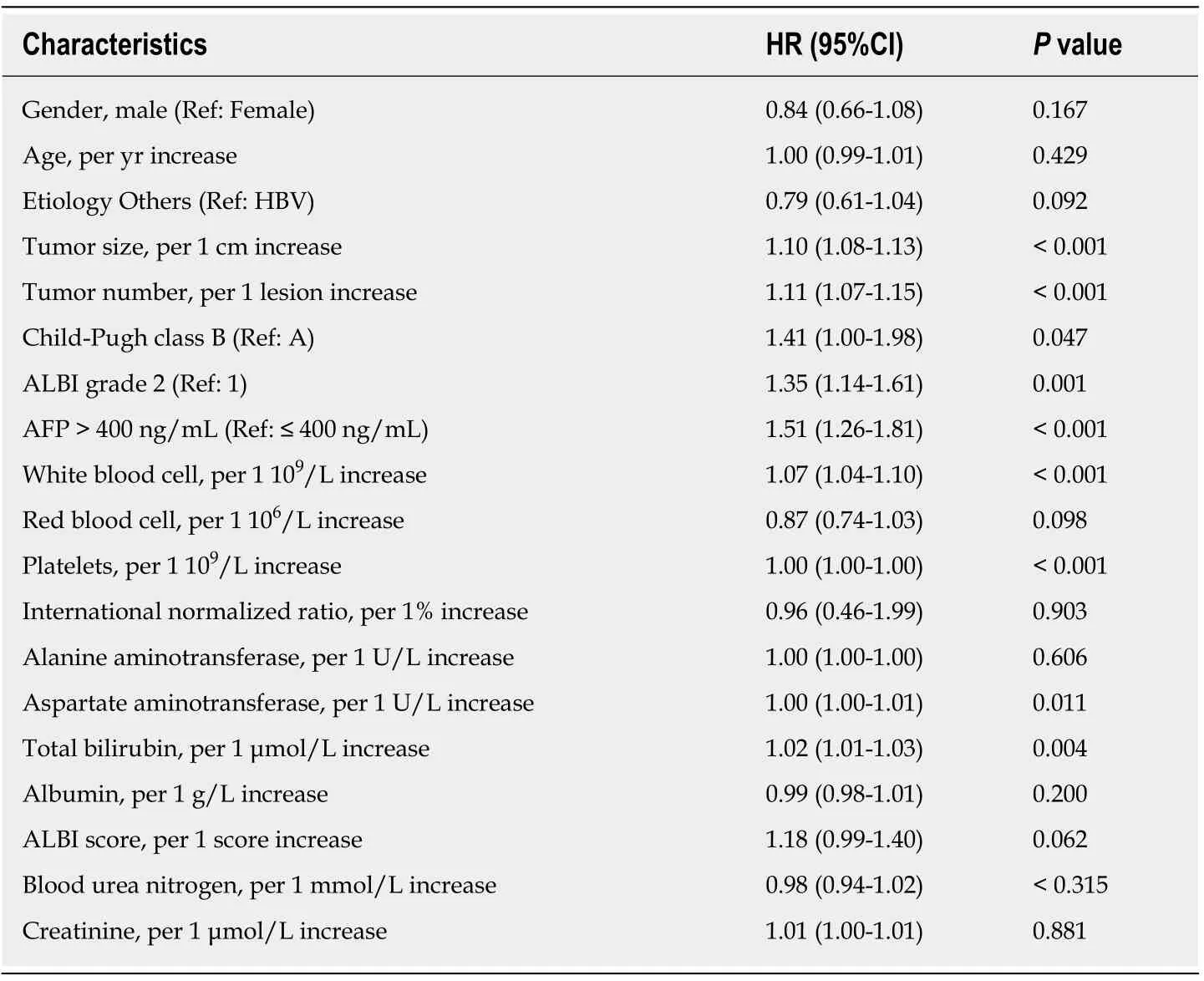
Table 2 Univariate analyses for overall survival
In the current study, we performed in-depth investigation into this population and stratified the patients according to tumor burden. For patients with low tumor burden based on 6&12 criteria, the median OS was 39.8 mo, which was comparable to that of the patients with intermediate HCC[16]. It might be inferred that this group of patients would be suitable for TACE alone and should be preferably regarded as intermediate stage. As for patients with high tumor burden, they had a median OS of 9.8 mo.Compared with the advanced HCC patients receiving systemic therapy, TACE might be unacceptable[32-35]. Because of the potential adverse effect of TACE on liver function,HCC tumors > 10 cm are commonly considered to be a contraindication for TACE[21].In addition, the patients with moderate tumor burden had a median OS of 21.1 mo.For them, combining TACE with systemic treatment might make a difference.Consequently, according to the patient stratification with 6&12 criteria, these findings might not only facilitate the outcome prediction but also provide a reference for stratified staging of these patients and help the decision-making.
The current study, for the first time, focused on advanced HCC patients with ECOG 1 alone, investigated the treatment outcomes of TACE and proposed a specific prognostic system for patient stratification and survival prediction, which could better guide treatment selection in this controversial population. There were several limitations to this retrospective cohort study. Firstly, the potential patient selection bias was unavoidable for this retrospective observational study. Nevertheless, this bias might be reduced by including a large cohort of consecutive patients. Secondly,TACE is not recommended by the guidelines for treatment of advanced HCC[2,3].
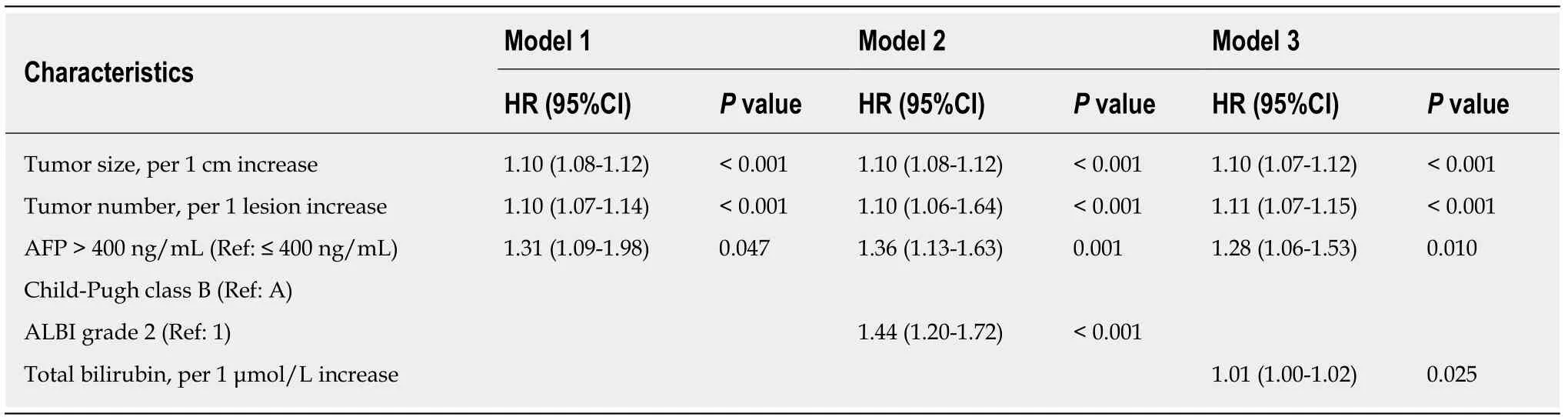
Table 3 Multivariate analyses for overall survival
Although systemic therapies are recommended for advanced HCC, they mainly refer to patients with MVI/EHS, and it is inconclusive whether advanced HCC patients with ECOG 1 alone should be placed in intermediate or advanced stages. Finally, with the absence of external validation, the generalization and extrapolation of the current findings should be made with caution. However, the results of this study came from a multicenter cohort from 24 tertiary Chinese hospitals, and subgroup analysis demonstrated the reliable and consistent predictive factors of the proposed 6&12 criteria.
In summary, our study demonstrated that TACE was safe and effective for advanced HCC patients with ECOG 1 alone; tumor size and tumor number, rather than the liver function or AFP level, were robust prognostic factors of OS after TACE.Based on these findings, the 6&12 criteria could predict prognosis regardless of baseline characteristics. Future studies focusing on these patients with other treatment modalities are still required.
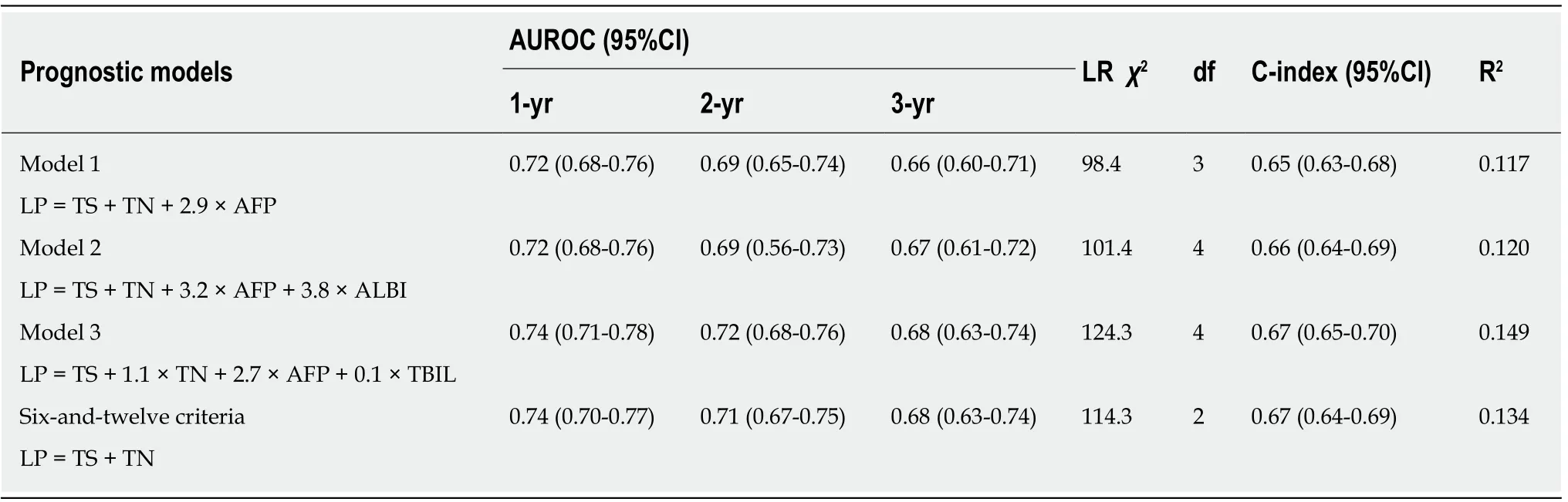
Table 4 Comparison of the performance and discriminating abilities of the proposed models and six-and-twelve criteria
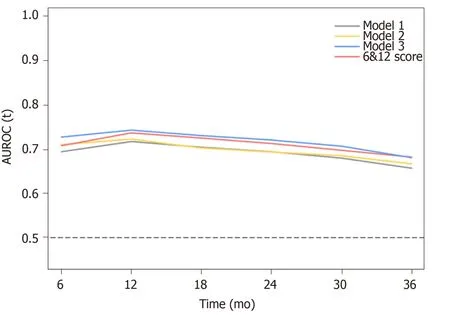
Figure 3 Time-dependent receiver operating characteristic analysis for the proposed prognostic models and six-and-twelve criteria. AUROC: Area under receiver operating characteristic. 6&12: Six-and-twelve.
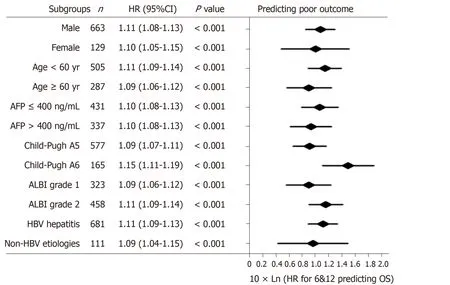
Figure 4 Subgroup analysis for evaluating the prognostic values of six-and-twelve criteria. HR: Hazard ratio; CI: Confidence interval; ALBI: Albumin-bilirubin;AFP: Alpha-fetoprotein; HBV: Hepatitis B virus. 6&12: Six-and-twelve.
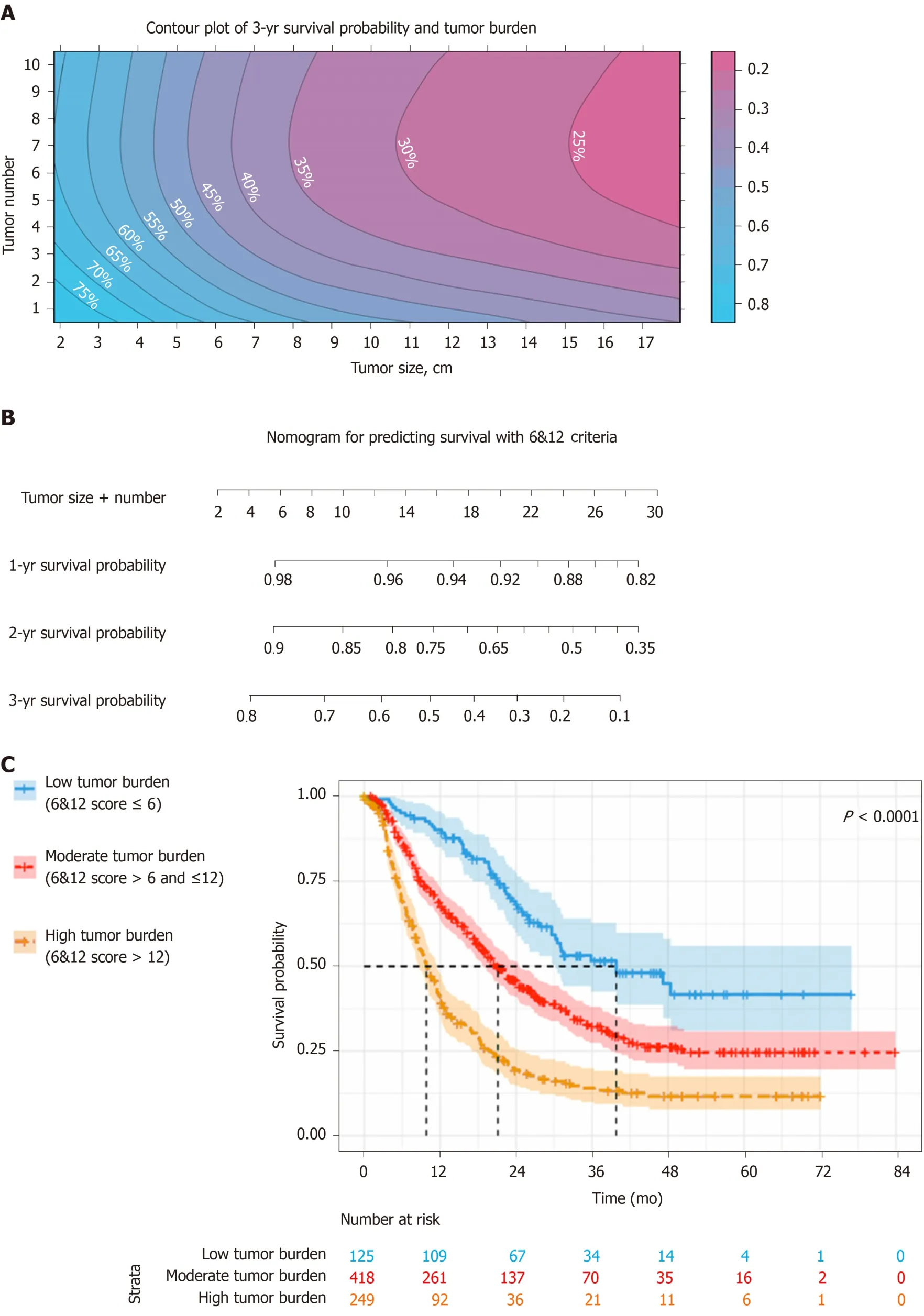
Figure 5 Six-and-twelve criteria tools for clinical use in advanced hepatocellular carcinoma patients with Eastern Cooperative Oncology Group performance status score 1 alone. A: Survival prediction for six-and-twelve (6&12) criteria using contour plot of 3-year survival probability; B: Nomogram based on the cut-off value of 6&12 criteria; C: Patient stratification based on the cut-off value of 6&12 criteria.
ARTICLE HIGHLIGHTS
Research background
According to the international guidelines, the advanced stage of hepatocellular carcinoma (HCC)covers patients with liver-confined HCC and Eastern Cooperative Oncology Group (ECOG)performance status score 1. Despite the recommended standard treatment of systemic therapy,these patients are frequently treated with transarterial chemoembolization (TACE) in real world clinical practice.
Research motivation
Previously, some studies demonstrated different prognoses between advanced HCC patients with ECOG 1 alone and others with macrovascular invasion or extrahepatic spread. Whether such patients should be classified into intermediate stage and treated with TACE still remains unknown. Specific studies focusing on the survival is necessary.
Research objectives
This nationwide multicenter study aimed to investigate treatment outcomes of TACE in advanced HCC patients with ECOG 1 alone and propose a specific prognostic model.
Research methods
Several potential prognostic models were developed based on univariate analyses and multivariate Cox regression analyses. Then, the discriminatory ability of them were compared with six-and-twelve (6&12) criteria, defined as the algebraic sum of tumor size (cm) and tumor number, in 792 patients and their subgroups. Contour plot of 3-year survival probability and nomogram were used to illustrate the individual survival prediction of 6&12 criteria in advanced HCC patients with ECOG 1 alone receiving TACE.
Research results
The analyses showed that tumor size, tumor number, α-fetoprotein level, albumin–bilirubin grade and total bilirubin were prognostic factors of overall survival (OS). In the comparisons between 6&12 criteria and three newly proposed models containing different prognostic factors,the 6&12 criteria retained the highest predictive ability and was the easiest to use. Additionally,the 6&12 criteria was correlated with OS in various subgroups of patients and could stratify patients into three risk strata with cut-off values “6” and “12”.
Research conclusions
The results from this study suggest that TACE is effective for advanced HCC patients with ECOG 1 alone. The 6&12 criteria including two robust prognostic factors (tumor size and tumor number) of OS could be applied in risk stratification and individual prediction, which might help with clinical decision-making.
Research perspectives
This study explored the applicability of TACE for advanced HCC patients with ECOG 1 alone and proposed a predictive score for OS. Also, other possible treatment approaches used in clinical practice exist. Future studies should investigate the outcomes of different treatments and compare them with TACE to further manage these patients with the most appropriate therapy.
ACKNOWLEDGEMENTS
We gratefully acknowledge the following investigators for their dedication in performing TACE procedures and follow-up of patients: Professor Junhui Sun,Department of Hepatobiliary and Pancreatic Interventional Cancer, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China;Professor Ming Huang, Department of Minimally Invasive International Therapy, The Third Affiliated Hospital of Kunming University, Tumor Hospital of Yunnan Province, Kunming, China; Professor Wei Mu, Department of Radiology, The Southwest Hospital, Third Military Medical University, Chongqing, China; Professor Guowen Yin, Department of Interventional Radiology, Jiangsu Provincial Cancer Hospital, The Affiliated Cancer Hospital of Nanjing Medical University, Nanjing,China; Professor Hailiang Li, Department of Interventional Radiology, Henan Cancer Hospital, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou,China; Professor Hui Zhao, Department of Interventional Radiology, The Affiliated Hospital of Nantong University, Nantong, China; Professor Jing Li , Department of Hepatobiliary Surgery, Xinqiao Hospital, Third Military Medical University,Chongqing, China; Professor Chunqing Zhang, Department of Gastroenterology and Hepatology, Shandong Province Hospital Affiliated to Shandong University, Jinan,China; Professor Xiaoli Zhu, Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou, China; Professor Jianbing Wu,Department of Oncology, The Second Affiliated Hospital of Nanchang University,Nanchang, China; Professor Jiaping Li, Department of Interventional Radiology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China; Professor Weidong Gong, Department of Interventional Radiology, Tangdu Hospital, Fourth Military Medical University, Xi’an, China; Professor Zixiang Li, Interventional Medical Centre of the Affiliated Hospital of Qingdao University, Qingdao, China;Professor Zhengyu Lin, Department of Interventional Radiology, First Affiliated Hospital of Fujian Medical University, Fuzhou, China; Professor Xingnan Pan,Clinical Liver Diseases Research Centre, Nanjing Military Command, 180th Hospital of PLA, Quanzhou, China; Professor Haibin Shi, Department of Interventional Radiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing,China; Professor Guoliang Shao, Department of Radiology, Zhejiang Cancer Hospital,Hangzhou, China; Professor Jueshi Liu, Department of Interventional Radiology and Vascular Surgery, Hunan Provincial People’s Hospital, Changsha, China; Professor Shufa Yang, Department of Interventional Radiology, The Affiliated Tumor Hospital of Xinjiang Medical University, Urumqi, China; Professor Yanbo Zheng, Department of Interventional Radiology, Yantai Yuhuangding Hospital, Yantai, China; Professor Jian Xu, Department of Medical Imaging, Nanjing General Hospital of the Nanjing Military Command, Nanjing, China; Professor Jinlong Song, Department of Interventional Therapy, Shandong Tumor Hospital, Jinan, China; Professor Wenhui Wang, Department of Interventional Medicine, The First Affiliated Hospital of Lanzhou University, Lanzhou, China.
 World Journal of Gastroenterology2020年15期
World Journal of Gastroenterology2020年15期
- World Journal of Gastroenterology的其它文章
- Determining the role for uric acid in non-alcoholic steatohepatitis development and the utility of urate metabolites in diagnosis: An opinion review
- Torque teno virus in liver diseases: On the way towards unity of view
- Blood-based biomarkers for early detection of esophageal squamous cell carcinoma
- Spontaneous porto-systemic shunts in liver cirrhosis: Clinical and therapeutical aspects
- Update on quinolone-containing rescue therapies for Helicobacter pylori infection
- DNAH17-AS1 promotes pancreatic carcinoma by increasing PPME1 expression via inhibition of miR-432-5p
