DNAH17-AS1 promotes pancreatic carcinoma by increasing PPME1 expression via inhibition of miR-432-5p
Tao Xu, Ting Lei, Si-Qiao Li, Er-Hui Mai, Fei-Hu Ding, Bin Niu
Abstract BACKGROUND The incidence and mortality rates of pancreatic carcinoma (PC) are rapidly increasing worldwide. Long noncoding RNAs (lncRNAs) play critical roles during PC initiation and progression. Since the lncRNA DNAH17-AS1 is highly expressed in PC, the regulation of DNAH17-AS1 in PC was investigated in this study.AIM To investigate the expression and molecular action of lncRNA DNAH17-AS1 in PC cells.METHODS The PC expression data for the lncRNA DNAH17-AS1 was downloaded from The Cancer Genome Atlas database and used to examine its profile. Western blot and reverse transcription-quantitative PCR were employed to assess protein and mRNA expression. A subcellular fractionation assay was used to determine the location of DNAH17-AS1 in cells. In addition, the regulatory effects of DNAH17-AS1 on miR-432-5p, PPME1, and tumor activity were investigated using luciferase reporter assay, MTT viability analysis, flow cytometry, and transwell migration analysis.RESULTS DNAH17-AS1 was upregulated in PC cells and was associated with aggressive tumor behavior and poor prognosis for patients. Silencing DNAH17-AS1 promoted the apoptosis and reduced the viability, invasion, and migration of PC cells. In addition, DNAH17-AS1 served as a PC oncogene by downregulating miR-432-5p which normally directly targeted PPME1 to downregulate its expression.CONLUSION DNAH17-AS1 functions in PC as a tumor promoter by regulating the miR-432-5p/PPME1 axis. This finding may provide new insights for PC prognosis and therapy.
Key words: Long noncoding RNAs; DNAH17-AS1; Pancreatic carcinoma; MiR-432-5p;PPME1; Molecular mechanism
INTRODUCTION
Pancreatic carcinoma (PC) is a common digestive system malignancy with serious negative impacts on health, and the seventh leading cause of cancer-related death around the world[1]. Only 15%-20% patients present with resectable disease while the vast majority of patients have either locally advanced or metastatic disease unsuitable for surgical resection[2]. Nonspecific early symptoms, limited methods for detection and diagnosis, and the limited response of PC to chemotherapy and radiotherapy are the main reasons for the serious consequences of PC[3,4]: Overall five-year survival is about 8.3%[5]. It is thus critically important to explore new molecular markers for PC diagnosis and treatment monitoring.
With the increasing use of nuclei acid sequencing technology to understand the multifaceted human genome, more and more new classes of functional molecules have been found. Among these, long noncoding RNAs (lncRNAs) represent a novel and promising class of regulators for tuning tumorigenesis in several human cancers.For example, Changet al[6]recently reported that the lncRNA NEF restrains nonsmall-cell lung cancer cell proliferation, while Maet al[7]found that the lncRNA SPRY4-IT1 promotes proliferation and metastasis in hepatocellular carcinomaviaTNF signaling. In addition, some lncRNAs have already been implicated in the development of PC. For example, the lncRNAs MACC1-AS1 and ABHD11-AS1 accelerate the proliferation and metastasis of PC through PAX8/NOTCH1 signaling[8]and PI3K-AKT[9], respectively. In contrast, LINC01111[10]and LINC01197[11]suppress PC.
Numerous studies have revealed that lncRNAs exert their influence in cancer cells by acting as sponges for microRNAs (miRNAs), thereby indirectly regulating the mRNA expression by sequestering miRNAs. MiRNAs are short non-coding RNAs that act as post-transcriptional regulators. They are often involved in tumorigenesis,as they bind target mRNAs and thus reduce the stability and translation of their target genes[12]. LncRNAs and miRNAs and their overall competing endogenous RNA network are increasingly implicated in cancer. For example, the lncRNA CACNA1GAS1 facilitates hepatocellular carcinoma progressionviathe miR-2392/C1orf61 pathway[13]. sONE-lncRNA is downregulated in triple-negative breast cancer cells but can act as a tumor suppressor by repressing eNOS-induced NO production, affecting TP53 and c-Myc proteins levels and finally altering the levels of a panel of tumor suppressor miRNAs downstream[14]. There are also similar findings in PC, such as a study reporting that the lncRNA 00976 sponges miR-137 and affects the OTUD7B/EGFR/MAPK pathway to promote PC[15], as well as another demonstrating that lncRNA DLX6-AS1 modulates the miR-497-5p/FZD4/FZD6 axis and Wnt/βcatenin pathway to increase tumorigenesis in PC[16].
The lncRNA DNAH17-AS1 was recently investigated for its potential oncogenic role in colorectal cancer. DNAH17-AS1 was upregulated in colorectal cancer[10]. It has also been reported that lncRNA DLX6-AS1 can promote the progression of liver cancer by targeting miR-424-5p[17]. Interestingly, miR-424-5p also exerts a tumorsuppressive effect in PC[18], but the specific functions of DNAH17-AS1 in PC remain elusive. The interactions between lncRNA DNAH17-AS1 and miRNAs have not yet been investigated thoroughly in PC. Thus, the aim of this work was to investigate the expression of the lncRNA DNAH17-AS1 and its impact on the viability, metastasis,and apoptosis of PC cells, and to unravel its exact mechanistic role in PC by exploring its impact on regulatory proteins and their downstream miRNAs.
MATERIALS AND METHODS
Patients and tissue samples
Seventy-eight pairs of PC tissue and adjacent normal tissue were obtained from patients with PC at Luoyang Central Hospital Affiliated to Zhengzhou University.Written informed consent was obtained from all patients. The patients did not receive radiotherapy or chemotherapy prior to surgery. Permission for and ethical approval of this research were acquired from the Institutional Ethics Committee of Luoyang Central Hospital affiliated to Zhengzhou University.
Cell culture
Normal human pancreatic duct epithelial cells (HPDE6-C7) and PC cells (Hs766T and SW1990) were purchased from the American Type Culture Collection (ATCC,Manassas, VA, United States). Cells were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS) and kept at 37 °C with 5% CO2.
Cell transfection
Small interfering RNA (siRNA) oligonucleotides targeting human DNAH17-AS1 (si-DNAH17-AS1), negative control siRNA (si-NC), miR-432-5p mimic, miR-432-5p inhibitor, negative control miR (NC-mimic), the pcDNA3.1 vector targeting PPME1,and an empty vector were constructed by RiboBio (Guangzhou, China). Hs766T PC cells were transfected with the siRNAs (50 nmol), mimics (20 nmol), or inhibitors (20 nmol) using Lipofectamine 2000 (Invitrogen/Thermo Fisher Scientifc). The transfection efficiency was assessed by reverse transcription-quantitative PCR (RTqPCR).
RNA isolation and RT-qPCR
Total RNA was isolated using Trizol reagent (Invitrogen, United States). cDNA was synthesized using the PrimeScript RT reagent kit (Takara, Dalian, China). RT-qPCR was performed with real-time PCR mixture assays (Takara) according to the manufacturer’s instructions. Expression levels of DNAH17-AS1, miR-432-5p, PPME1,CCNH, and SNRPD2 were normalized to GAPDH and quantified using the 2−Cqmethod.
MTT assay
Transfected Hs766T PC cells (2000 cells/well) were incubated for 24, 48, 72, or 96 h in a 96-well plate. Then, 20 μL of MTT solution (5 mg/mL) was added to each well and then replaced with 150 μL DMSO solution after 4 h of incubation. The absorbance of each suspension at 490 nm was measured using a microplate reader.
Wound healing assay
Transfected Hs766T PC cells were added into 6-well plates. Artificial wounds for a live cell analysis were made on the cell monolayer using culture inserts. Migratory cells and wound healing were monitored at 0 and 24 h. Three artificial wounds were immediately photographed for each group at the indicated time points following the wound formation. Cell migration was assessed by measuring the difference in the wound area between groups.
Transwell assay
Cell invasion was detected in the upper chamber with 60 μL of diluted Matrigel. After 30 min, a Hs766T cell suspension (2 × 103cells/well) was added to the upper chamber of the transwell plate. RPMI-1640 medium (500 μL, 10% FBS) was then added to the lower chamber. After 24 h, 0.1% crystal violet was used to stain the cells that had invaded the lower chamber. Observations were performed with a light microscope.
Flow cytometry
Flow cytometry was used to detect apoptosis in PC. First, transfected Hs766T cells (3× 103cell/well) were seeded in 6-well plates. After 48 h, trypsin (EDTA-free) digestion was used to collect the cells. We then suspended the collected Hs766T cells in PBS at 4°C. After resuspension in binding buffer, the Annexin V-FITC probe and propidium iodide (Biovision, K101) were added and the cells were incubated at room temperature for 5 min in the dark, followed by detection using a flow cytometer (BD Biosciences).
Dual luciferase reporter assay
Reporter plasmids of DNAH17-AS1 (wt-DNAH17-AS1 and mut-DNAH17-AS1) and PPME1 (wt-PPME1 and mut-PPME1) were purchased from GenePharma (Shanghai,China). The reporter plasmids were transfected into Hs766T cells with the miR-432-5p mimic (RiboBio, Guangzhou, China) for 48 h. Luciferase activity was examined with a dual-luciferase reporter assay system (Promega, United States).
Subcellular fractionation assay
RNA was isolated from the nuclear or cytoplasmic fraction using the Nuclear/Cytosol Fractionation Kit (Biovision, San Francisco Bay, CA, United States) and was measured by qRT-PCR. U1 and GAPDH acted as the identifiers for the nuclear or cytoplasmic fractions, respectively. Nuclear and Cytoplasmic Extraction Reagents were purchased from Thermo Fisher Scientific.
Western blot analysis
RIPA lysis buffer was used to extract protein from the samples. Next, we used 10%SDS-PAGE to separate proteins. Protein was then transferred to PVDF membranes.After blocking with 5% skim milk for 2 h, the membranes were incubated with primary antibody at 4 °C overnight, using rabbit monoclonal anti-PPME1 (1/2000,ab205956, Abcam, Cambridge, United States), anti-CCNH (1/2000, ab92376, Abcam),anti- SNRPD2 (1/2000, ab251254, Abcam), or rabbit polyclonal anti-GAPDH antibody(1:500, Abcam). The membranes were then washed to remove the primary antibody and incubated with IgG H and L goat anti-rabbit secondary antibody (HRP, 1:1000;Abcam) at 37 °C for 1 h. Finally, ECL (ECL, Pierce) was used to visualize and measure protein expression levels.
Statistical analysis
Data are expressed as the mean ± SD and were analyzed using SPSS 17.0 or Graphpad Prism 6. The statistical methods employed included Student’st-test, one-way ANOVA, univariate Kaplan-Meier method with log-rank test, andχ2test. Differences were considered significant ifP< 0.05.
RESULTS
DNAH17-AS1 is upregulated in PC patients
DNAH17-AS1 is an lncRNA that is presumed to be overexpressed in PC patients and tissues, due to its inclusion in the top ten abundant lncRNAs as noted in The Cancer Genome Atlas dataset (Figure 1A and 1B). Indeed, RT-qPCR analysis revealed upregulation of DNAH17-AS1 in PC tissues compared with normal tissues (Figure 1C). Similarly, DNAH17-AS1 expression was higher in pancreatic adenocarcinoma cells (Hs766T and SW1990 cells) than in normal human pancreatic duct epithelial cells(HPDE6-C7; Figure 1D). Since Hs766T cells had the highest DNAH17-AS1 expression,Hs766T cells were selected to perform gain- and loss-of-function experiments.
High DNAH17-AS1 expression was related to aggressive PC tumor behavior,including TNM stage, lymph node metastasis, and differentiation (Table 1).Furthermore, PC patients with DNAH17-AS1 upregulation had shorter overall survival and disease-free survival (Figure 1E and 1F). Based on these results, we suspected that DNAH17-AS1 may be an important regulator of PC progression, and high levels of DNAH17-AS1 expression may play an oncogenic role.
DNAH17-AS1 regulates PC cell viability, metastasis, and apoptosis
Next, the functions of DNAH17-AS1 were investigated in Hs766T cells transfected with si-DNAH17-AS1 or si-NC. As expected, DNAH17-AS1 was downregulated after transfection with si-DNAH17-AS1 compared with si-NC (Figure 2A). DNAH17-AS1 silencing greatly inhibited Hs766T cell viability compared to control (Figure 2B), with an approximate 50% reduction in cellular viability. Flow cytometry showed that downregulation of DNAH17-AS1 (Figure 2C) promoted apoptosis in PC cells.Furthermore, wound healing and transwell assays indicated that the migration and invasion of Hs766T cells were restrained following DNAHS1-AS1 silencing (Figure 2D and 2E). Taken together, these results showed that DNAH17-AS1 silencing inhibits PC cell viability and metastasis and promotes apoptosis in PC cells.
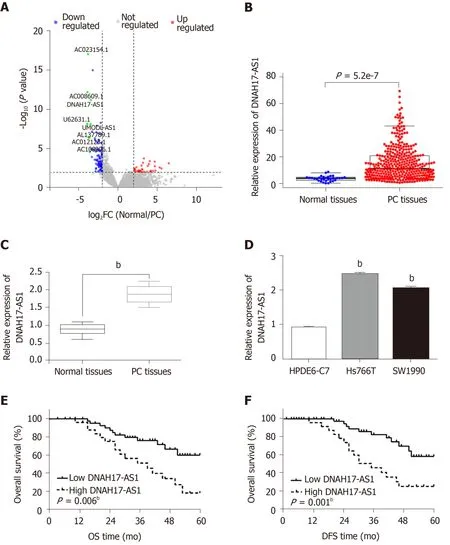
Figure 1 The expression of DNAH17-AS1 is upregulated in pancreatic carcinoma patients. A: Volcano plot of the TCGA_lncRNA profile. The top ten highly expressed lncRNAs are marked in green; B: The expression pattern of DNAH17-AS1 in pancreatic carcinoma (PC) patients and normal patients from The Cancer Genome Atlas dataset; C: DNAH17-AS1 expression in PC tissues collected by our hospital; D: DNAH17-AS1 expression in Hs766T, SW1990, and HPDE6-C7 cells;E: Dysregulation of DNAH17-AS1 is related to overall survival in PC patients; F: Dysregulation of DNAH17-AS1 is related to disease-free survival in PC patients. bP <0.01. DFS: Disease-free survival; OS: Overall survival.
DNAH17-AS1 promotes PC by targeting and inhibiting miR-432-5p
To determine the mechanism by which DNAH17-AS1 exerts its effects in PC, first we examined its cellular location. A subcellular fractionation assay showed that DNAH17-AS1 was predominantly located in the cytoplasm but also occasionally in the nucleus (Figure 3A). We therefore speculated that it is involved in competing endogenous RNA activity in the cytoplasm. A search of the downstream miRNA targets of DNAH17-AS1 in the starBase database (http://starbase.sysu.edu.cn/) and TargetScan database (http://www.targetscan.org) revealed that DNAH17-AS1 can bind to miR-432-5p (Figure 3B). In dual-luciferase reporter assays, miR-432-5p mimic decreased luciferase activity in the DNAH17-AS1-WT vector but had no effect on DNAH17-AS1-MUT (Figure 3C), indicating the specificity of the interaction between DNAH17-AS1 and miR-432-5p. Furthermore, RT-qPCR indicated that miR-432-5p was upregulated following downregulation of DNAH17-AS1 in transfected Hs766T cells(Figure 3D). In addition, two experiments in Hs766T cells showed that miR-432 mimic had similar effects to si-DNAH17-AS1 for regulating PC cell behavior (Figure 2B-E and 3E-H) and the observed consequences of decreased DNAH17-AS1 expression(Figure 2B-E) were rescued by a miR-432-5p inhibitor in cells transfected with si-DNAH17-AS1 (Figure 3E-H). Specifically, the inhibition of cell viability, migration,and invasion was resulted from miR-432-5p increasing and the effect of DNAH17-AS1 silencing on Hs766T cells was reversed by miR-432-5p inhibition (Figure 3E-G).Moreover, the promotion of apoptosis was induced by miR-432-5p mimic and the impact of si-DNAH17-AS1 was also weakened by downregulation of miR-432-5p(Figure 3H). These findings implied that DNAH17-AS1 functions as a tumor promoter in PC by binding to miR-432-5p and inhibiting its level in the cytoplasm.
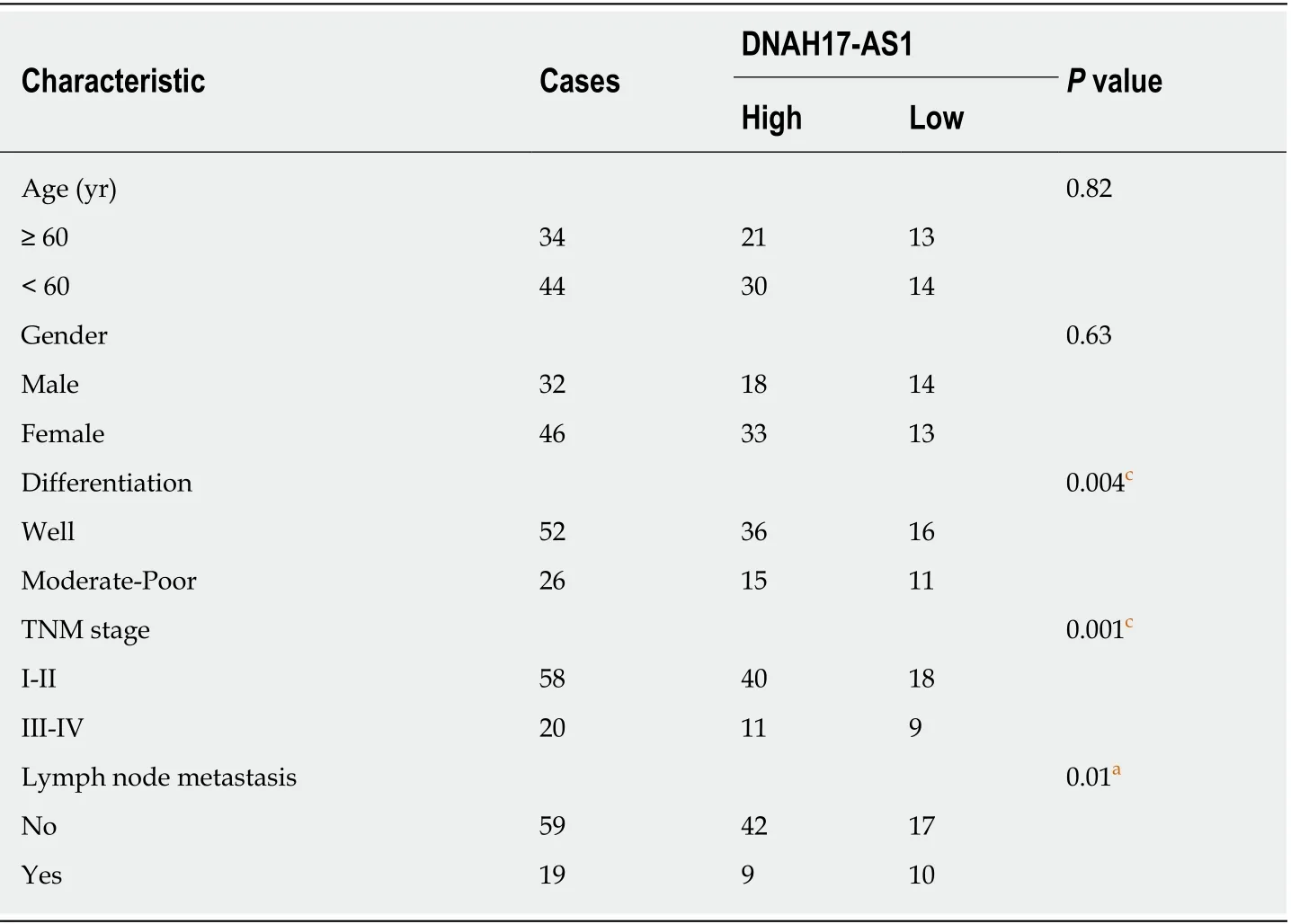
Table 1 Relationship between DNAH17-AS1 expression and clinicopathological characteristics of pancreatic carcinoma patients
MiR-432-5p directly targets and silences PPME1
We next investigated the potential downstream mRNAs affected by the interaction of DNAH17-AS1 and miR-432-5p. We used miRanda, miRmap, PITA, RNA22, and TargetScan to identify overlapping targets. A total of three mRNAs were identified as possible targets, including PPME1, CCNH, and SNRPD2 (Figure 4A). In Hs766T and SW1990 cells, however, miR-432-5p mimic reduced PPME1 mRNA and protein levels more than those of CCNH and SNRPD2 (Figure 4B-E). This showed that PPME1 is the most likely one in the three predicted mRNAs regulated by mir-490-3p. A search for targets of miR-432-5p in the TargetScan database revealed that miR-432-5p has a binding site in the 3’-untranslated region (UTR) of PPME1 (Figure 4F). While miR-432-5p mimic suppressed the luciferase activity of PPME1-WT in dual-luciferase reporter assays, PPME1-MUT luciferase activity was unaffected (Figure 4G).Consistent with these results, PPME1 expression was downregulated by overexpression of miR-432-5p and increased by miR-432-5p inhibitor (Figure 4H-I).PPME1 was also upregulated in PC tissues compared with normal tissues (Figure 4J),and miR-432-5p inversely correlated with PPME1 expression in PC tissues (Figure 4K). These results showed that miR-432-5p downregulates PPME1 expression by binding to its 3’-UTR in PC.
DNAH17-AS1 interacts with miR-432-5p to regulate PPME1 expression
Taking all the above results together, we suspected that DNAH17-AS1 could regulate PPME1 expression through interaction with miR-432-5p. Indeed, DNAH17-AS1 was positively correlated with PPME1 expression in PC (Figure 5A). Moreover, PPME1 expression was downregulated after transfection of si-DNAH17-AS1 vector in Hs766T cells and could be recovered by PPME1 vector (Figure 5B). Upregulation of PPME1 also inhibited the DNAH17-AS1 siRNA-mediated suppression of cell viability and metastasis in Hs766T cells (Figure 5C-E). Similarly, the PPME1 vector weakened the increase in apoptosis typically induced by DNAH17-AS1 siRNA (Figure 5F).Collectively, these data indicated that DNAH17-AS1 promotes the progression of PC through regulation of the miR-432-5p/PPME1 axis.
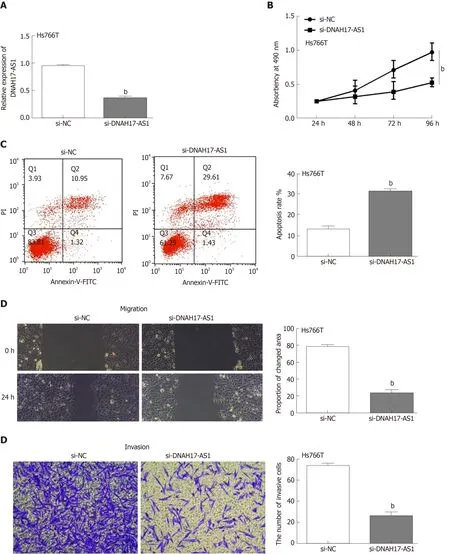
Figure 2 DNAH17-AS1 regulates viability, migration, and invasion of pancreatic carcinoma cells. A: DNAH17-AS1 expression in Hs766T cells with si-DNAH17-AS1 or si-NC; B and C: DNAH17-AS1 siRNA inhibited cell proliferation (B) and promoted apoptosis (C) in Hs766T cells; D and E: DNAH17-AS1 siRNA also inhibited Hs766T cell migration (D) and invasion (E). bP < 0.01.
DISCUSSION
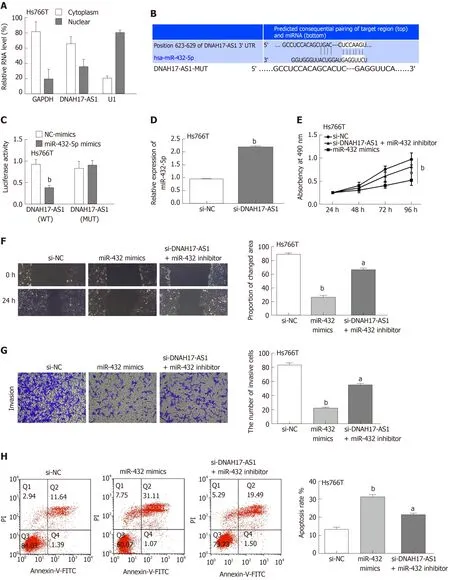
Figure 3 DNAH17-AS1 acts as a sponge for miR-432-5p in pancreatic carcinoma cells. A: Subcellular fractionation assay was performed to identify the location of DNAH17-AS1 in Hs766T cells; B: The binding sites between DNAH17-AS1 and miR-432-5p; C: Luciferase activity of miR-432-5p mimic with DNAH17-AS1-WT or DNAH17-AS1-MUT; D: MiR-432-5p expression is regulated by si-DNAH17-AS1 in Hs766T cells. E-H: Cell proliferation (E), migration (F), invasion (G), and apoptosis(H) in Hs766T cells treated with miR-432-5p mimic or si-DNAH17-AS1 and miR-432-5p inhibitor. aP < 0.05, bP < 0.01.
The incidence, mortality, and morbidity of PC are increasing every year around the world[19]. It is therefore urgent to understand the mechanisms underlying PC tumorigenesis and progression. In addition, the absence of reliable diagnostic and prognostic markers has recently fostered significant research efforts to identify molecular “drivers”. LncRNAs are a promising candidate, and participate in the development of many cancers including PC[10]. Therefore, this study began with mining The Cancer Genome Atlas lncRNA database, focused on a highly-expressed lncRNA DNAH17-AS1 in PC, and deciphered the role of this novel lncRNA in PC.
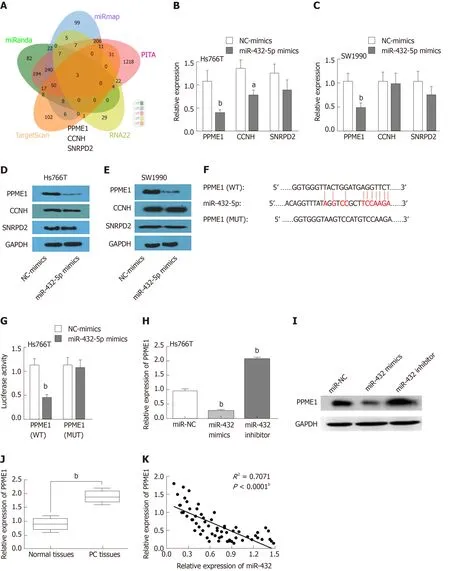
Figure 4 MiR-432-5p regulates PPME1 expression in pancreatic carcinoma. A: Three possible mRNAs regulated by miR-432-5p were selected and are presented in a Venn diagram; B and C: The expression levels of three target mRNAs were assessed after overexpression of miR-432-5p; D and E: The protein levels of three possible proteins were assessed after overexpression of miR-432-5p; F: The binding sites between PPME1 and miR-432-5p; G: Luciferase activity of miR-432-5p mimic with PPME1-WT or PPME1-MUT; H and I: PPME1 expression regulation by miR-432-5p mimic or inhibitor in Hs766T cells; J: PPME1 expression in pancreatic carcinoma tissues; K: MiR-432-5p inversely regulates PPME1 expression. aP < 0.05, bP < 0.01.
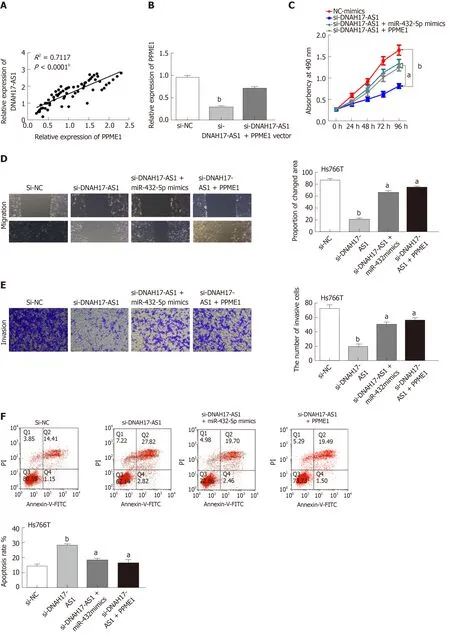
Figure 5 DNAH17-AS1 interacts with miR-432-5p to regulate PPME1 expression. A: PPME1 is positively correlated with DNAH17-AS1 expression; B: PPME1 expression in Hs766T cells containing si-DNAH17-AS1 and PPME1 vector; C-F: Cell proliferation (C), migration (D), invasion (E), and apoptosis (F) in Hs766T cells containing si-DNAH17-AS1, miR-432-5p mimic, and PPME1 vector. aP < 0.05, bP < 0.01.
To date, many lncRNAs have been discovered to be involved in PC tumorigenesis,such as the lncRNAs BANCR and LINC00339[20,21]. Previous reports have indicated that lncRNA DNAH17-AS1 promotes the progression of colorectal cancer[10], but whether DNAH17-AS1 is involved in the regulation of other cancers remains unclear.In this study, we first showed that DNAH17-AS1 is involved in PC progression through suppression of miR-432-5p. In addition, this study is the first to report that PPME1 is a target of miR-432-5p in PC cells. The results of this study will help to elucidate the regulatory mechanisms of DNAH17-AS1 and the miR-432-5p/PPME1 axis in PC.
Consistent with our results, previous studies have reported that DNAH17-AS1 is an lncRNA that is significantly associated with the overall survival of patients with PC[22]. Further, numerous studies have shown that DNAH17-AS1 may play an oncogenic role in both colorectal cancer[10]and head and neck squamous cell carcinoma[23]. All current hypotheses regarding DNAH17-AS1 as a proto-oncogene come from bioinformatic analyses, but to date, none have been verified experimentally. Here we found that DNAH17-AS1 serves as an oncogene in PC by downregulating miR-432-5p using bothin silicoanalysis and experimental data.
LncRNAs can exert various biological effects through both transcriptional and posttranscriptional regulation. The molecular mechanisms of lncRNAs/miRNAs are complicated and can form a complex regulatory network of lncRNA, miRNA, and genes. Consistent with our results, many lncRNAs can interact with miR-432-5p to regulate the occurrence and progression of cancers[24,25]. In addition, some circular RNAs also regulate miR-432-5p to affect the development of cancer[26,27]. These studies showed that ncRNA can promote tumor growth or progression and restrain the apoptosis of tumor stem cells by sponging miR-432-5p. In the current study, we found that miR-432-5p negatively regulated PPME1 expression in PC by binding to its 3’-UTR. Similarly, Huet al[24]reported that tumor-suppressive miR-432-5p can serve as a prognostic indicator in lung adenocarcinoma by mediating epithelial-mesenchymal transition. Furthermore, Wanget al[28]found that miR-432-5p expression is downregulated in PC. It is clear that miR-432-5p is an important regulator in cancer development and its downregulation predicts poorer prognoses for PC patients.
In this study, we showed that DNAH17-AS1 promoted PC progression by inhibiting miR-432-5p expression. Furthermore, miR-432-5p normally inhibited PPME1 expression to restrain PC progression. Consistent with these findings, the upregulation of PPME1 and resulting carcinogenesis have also been examined in gastric and lung cancer[29]. Hsiehet al[30]demonstrated that the endopeptidase catalytic subunit IMP1 facilitates choriocarcinoma cell migration and invasionviaPPME1 upregulation. Taken together, these studies demonstrated that PPME1 plays an important role in the development of cancers including PC and may be a biomarker for PC.
In conclusion, the current study has demonstrated that upregulation of DNAH17-AS1 is related to adverse clinical features and poor prognosis of PC patients.Moreover, DNAH17-AS1 promotes PC progression and serves as a competitive factor to upregulate PPME1 by sequestering miR-432-5p. This study implies that DNAH17-AS1, miR-432-5p, and PPME1 may serve as therapeutic targets and prognostic biomarkers for PC patients and may provide new strategies for PC prevention and treatment.
ARTICLE HIGHLIGHTS
Research background
Among common gastrointestinal malignancies, the annual incidence of pancreatic carcinoma(PC) has dramatically increased in recent years. Many studies have demonstrated that posttranscriptional regulation by long noncoding RNAs (lncRNAs) is important in PC progression.LncRNAs have emerged as pivotal molecules that participate in the initiation and progression of PC, including maintenance of cell growth, evasion of apoptosis, promotion of invasion and metastasis, maintenance of potency, and epithelial-mesenchymal transition.
Research motivation
To discover biomarkers for the diagnosis and treatment of PC.
Research objectives
To investigate the underlying mechanisms of the lncRNA DNAH17-AS1 in PC.
Research methods
LncRNA DNAH17-AS1 expression was analyzed by Western blot and reverse transcriptionquantitative PCR in PC tissue and cell lines, and the clinicopathological significance of DNAH17-AS1 expression in PC patients was also investigated. In vitro experiments were performed to explore the functions of lncRNA DNAH17-AS1 in PC cells. The regulatory effects of DNAH17-AS1/miR-432-5p/PPME1 were also investigated using luciferase reporter assay, MTT assay,flow cytometry, and transwell assay.
Research results
We found that the lncRNA D NAH17-AS1 was upregulated in PC tissues and cell lines and had a significant positive relationship with degree of tumor differentiation, TNM stage, and lymph node metastasis.In vitroexperiments showed that DNAH17-AS1 increased the proliferation and invasion capacity of PC cells but inhibited their apoptosis. Additionally, DNAH17-AS1 served as an oncogene in PC by downregulating miR-432-5p. Furthermore, miR-432-5p directly targeted PPME1 and normally downregulated its expression. Thus, DNAH17-AS1 increased PPME1 expression to promote PC progression.
Research conclusions
This study demonstrates that the lncRNA DNAH17-AS1 can significantly increase the growth,migration, and invasion of PC cells. DNAH17-AS1 also functions by sequestering miR-432-5p,which increases PPME1 expression and ultimately promotes PC progression. Taken together, our study provides the functional involvement of three new possible diagnostic biomarkers for PC.
Research perspectives
In the future, additional research will be carried out to further explore the important role of the lncRNA DNAH17-AS1 and whether it can be harnessed to enhance the sensitivity of PC detection and to develop novel anti-cancer treatments. The identification of the lncRNA DNAH17-AS1/miR-432-5p/PPME1 molecular axis may further provide new strategies for PC prevention and treatment.
 World Journal of Gastroenterology2020年15期
World Journal of Gastroenterology2020年15期
- World Journal of Gastroenterology的其它文章
- Determining the role for uric acid in non-alcoholic steatohepatitis development and the utility of urate metabolites in diagnosis: An opinion review
- Torque teno virus in liver diseases: On the way towards unity of view
- Blood-based biomarkers for early detection of esophageal squamous cell carcinoma
- Spontaneous porto-systemic shunts in liver cirrhosis: Clinical and therapeutical aspects
- Update on quinolone-containing rescue therapies for Helicobacter pylori infection
- PTEN-induced kinase 1-induced dynamin-related protein 1 Ser637 phosphorylation reduces mitochondrial fission and protects against intestinal ischemia reperfusion injury
