Oncogenic role of Tc17 cells in cervical cancer development
Zun-Sheng Zhang,Ying Gu,Bing-Gang Liu,Hong Tang,Yu Hua,Jun Wang
Zun-Sheng Zhang,Ying Gu,Bing-Gang Liu,Hong Tang,Yu Hua,Jun Wang,Department of Obstetrics and Gynecology,Shanghai Seventh People's Hospital,Shanghai 200120,China
Abstract
Key words: Cervical cancer;Tc17 cells;Interleukin-17;Cancer development;Biological function;Oncogenic role
INTRODUCTION
As the fourth most common malignant tumor worldwide,the incidence of new cervical cancer is approximately 130000,accounting for 28% of the total number of cases globally.About 20000 women die of cervical cancer each year[1,2].Tumor progression has been recognized to be the product of evolving crosstalk between immune cells and tumor cells.By affecting immune cell activation or differentiation,cancer cells escape host immune attack and enhance tumor resistance to immunotherapies[3,4].
As the primary component of tumor-infiltrating lymphocytes,T cells elicit a crucial effector function in cancer eradication.Recent studies showed that T cells that produce interleukin (IL)-17 are detected in human tumors,which have a certain proinflammatory effect[3].On the one hand,Th17 cell polarizing factor may induce differentiation and proliferation of Tc17 cells,despite exhibiting reduced cytotoxic activity in CD8+T cells,thereby interrupting host immune surveillance[5,6].Moreover,in a monkey immunodeficiency virus infection model with mammals such as macaques and black-and-white marmosets,Tc17 cells play a pathological role in promoting disease progression[7,8].However,the role of Tc17 cells in human cervical cancer development remains unclear.
A large number of Tc17 cells were found in black and white monkey tumor tissues,which were induced by the cytokine IL-23[8].At the same time,other studies have shown that IL-6 may also be crucial for Tc17 cell differentiation[9].However,it is unclear whether other cytokines can induce Tc17 cell differentiation and how Tc17 cells affect cancer development is still poorly understood.
In this study,we showed that Tc17 cells are highly enriched within cervical cancer tissue.Cervical cancer cells produced a large amount of IL-6,IL-1β,and IL-23,which induced Tc17 cell polarization.Increased levels of IL-17 induced by Tc17 cells led to CXCL12 upregulation in tumor cells,resulting in tumor cell proliferation and migration.Moreover,the percentage of Tc17 cells was associated with tumor progression and clinical outcome in patients with cervical cancer.Our data demonstrate the oncogenic role of Tc17 cells in cancer development and provide a theoretical basis for the clinical treatment of cervical cancer.
MATERIALS AND METHODS
Patients and tissue specimens
Fresh blood,tumor,peritumoral,or matched adjacent tissues (at least 5 cm distant from the tumor site) were obtained from patients with cervical cancer who underwent surgical resection at the Shanghai Seventh People's Hospital.None of these patients had received chemotherapy or radiotherapy before sampling.Individuals with autoimmune disease,infectious diseases,and multi-primary cancers were excluded.Blood from healthy donors was used for control experiments.The clinical stages of tumors were determined according to the TNM classification system of the International Union Against Cancer.
Isolation and stimulation of tumor cells
Fresh tissues were washed 3 times with Hank's solution containing 1% fetal calf serum (FCS) before being cut into small pieces.The specimens were then placed in RPMI 1640 medium containing 1 mg/mL collagenase IV and 10 mg/mL deoxyribonuclease I and mechanically dissociated using a gentle MACS Dissociator(Miltenyi Biotec,Bergisch Gladbach,Germany).Dissociated cell suspensions were further incubated for 1 h at 37 °C under continuous rotation.The cell suspensions were then filtered through a 70 μm cell strainer (BD Labware,Bedford,MA,United States).Then,part of cells was used for flow cytometry to detect the number of Tc17 cells,and the remaining cells were cultured with IL-17 (0-10 ng/mL),IL-22 (0-10 ng/mL),interferon (IFN)-γ (0-10 ng/mL),IL-17 plus IL-22,or 100% Tc17 cellpolarizing culture for 48 h.The culture supernatants were harvested for ELISA.
In vitro monocyte-T-cell co-culture system
Peripheral blood mononuclear cells from cervical cancer patients were isolated by Ficoll density gradient centrifugation.Fresh peripheral blood CD8+T cells were selected using positive isolation and negative isolation kits,respectively.In a 5-d incubation period,bead-puriWed peripheral CD8+T cells were co-cultured with autologous blood monocytes at a 2:1 ratio in the presence or absence of recombinant human IL-6 (10 ng/mL),IL-1β (10 ng/mL) and IL-23 (10 ng/mL) in 200 μL RPMI 1640 medium supplemented with 10% FCS.After 5-d incubation,the supernatants were harvested for ELISA and the cells for intracellular cytokine staining.
Flow cytometry analysis
For detection of intracellular molecules,T lymphocytes were stimulated for 5 h with phorbol myristate acetate (50 ng/mL) plus ionomycin (1 μg/mL) in the presence of GolgiStop (BD Pharmingen,San Diego,CA,United States).Intracellular cytokine staining was performed after fixation and permeabilization using Perm/Wash solution (BD Pharmingen).The lymphocytes were analyzed by multicolor flow cytometry with FACSCanto II (BD Biosciences).Data were analyzed with FlowJo software (TreeStar,Ashland,OR,United States) or FACSDiva software (BD Biosciences).
Immunohistochemistry assay
Paraformaldehyde-fixed and paraffin-embedded samples were cut into 5 μm sections,which were incubated with rabbit anti-IL-17 antibody and stained by horseradish peroxidase anti-rabbit immunoglobulin G followed by diaminobenzidine.The sections were then incubated with mouse anti-CD8 antibody and stained using EnVision G2 System/AP Rabbit/Mouse (Permanent Red) (Dako,Glostrup,Denmark)and subsequently counterstained with hematoxylin.Slides were examined using an Olympus 71 inverted fluorescence microscope.
Immunofluorescence
Immunofluorescence was performed as previously described with minor modifications[10].In detail,Paraformaldehyde-Waxed cryostat sections of tumor tissues were washed in PBS and blocked for 30 min with 20% rabbit serum in PBS.Sections were incubated with goat anti-human IL-17 antibody (Ab) diluted in 5%rabbit serum.The bound Ab was detected with FITC-conjugated rabbit anti-goat Ab.After washing with PBS,the sections were blocked for 30 min with 20% goat serum in PBS and incubated with mouse anti-human CD8 Ab diluted in 5% goat serum.The bound Ab was detected with TRITC-conjugated goat anti-mouse Ab.After washing with PBS,the slides were examined with an Olympus 71 inverted fluorescence microscope.
ELISA
Cervical cancer tissues or their matched adjacent normal tissues were homogenized in 1 mL Protein Extraction Reagent (Rockford,IL,United States).Concentrations of IL-6,IL-1β,and IL-23 in the tissue supernatants;concentrations of CXCL12 in the co-culture supernatants or tissue supernatants;and concentrations of IL-17 in the co-culture supernatants were determined using ELISA kits according to the manufacturer's instructions.
Statistical analysis
Comparisons between data groups were performed as stated in each figure legend with the use of GraphPad Prism ver.6.A value ofP< 0.05 was considered statistically significant.The resulting data were presented as mean ± SE.
RESULTS
Tc17 cells specifically accumulate in tumor tissues of cervical cancer patients
To assess the status of Tc17 cells in human cervical cancer tissues,we isolated immune cells from cancer tissues,matched adjacent normal tissues as well as peripheral blood.Compared with healthy donors,the percentage of Tc17 cells in the peripheral blood from patients with cervical cancers was identical (Figure 1A).Of note,we found that Tc17 cells were selectively induced in tumors compared to their matched adjacent normal tissues (Figure 1A).To further confirm this result,we analyzed the distribution of Tc17 cells in the paracancerous stroma,carcinoma nets,and intratumor sites.The results showed that Tc17 cells accumulated in all these sites,especially in carcinoma nets and intra-tumor sites (Figure 1B-D).Thus,these data show that Tc17 cells have an oncogenic role in cervical cancer development.
Cervical cancer cells produce a greater amount of Tc17-polarizing cytokines
Previous studies revealed that IL-6,IL-1β,and IL-23 are essential for Tc17 cell differentiation[11,12].To investigate the mechanism of cervical cancer following modulation of Tc17 cell development,we assessed the levels of Tc17-polarizing cytokines in cancer-associated tissues.As expected,the concentrations of IL-6,IL-1β,and IL-23 were significantly increased in peritumoral and intra-tumor tissue relative to their matched normal tissues or peripheral blood (Figure 2A-C).Thus,these data indicate that tumor-derived cytokines play a stimulatory role in the modulation of Tc17 polarization.
IL-6,IL-1β,and IL-23 acts synergistically to enhance Tc17 cell differentiation
To evaluate the potential role of these cytokines in Tc17 cell differentiation,we isolated peripheral blood CD8+T cells and autologous blood monocytes of cervical cancer patients.After 5-day co-culture incubation,the supernatants were harvested for ELISA and the cells for intracellular cytokine staining.The results showed that the addition of exogenous IL-6,IL-1β,and IL-23 significantly increased the frequency of Tc17 cells either alone or in combination compared with co-culture without any cytokines added (Figure 3A and B).In addition,the ELISA results showed that IL-17 production was consistent with the percentage of Tc17 cells (Figure 3C).These findings showed that IL-6,IL-1β,and IL-23 act synergistically to induce Tc17 cell polarizationin vitroand suggest that a similar process might operatein vivo.
Tc17 cell-derived IL-17 promotes CXCL12 expression in tumor cells
To investigate the biological function of Tc17 cells in cervical cancer development,we isolated primary tumor cells and stimulated them with various concentrations of IL-17,IL-22,and IFN-γ,and the production of CXC12 was detected in the culture supernatants by ELISA.As shown in Figure 4A,CXCL12 expression in cancer cells was increased upon IL-17 stimulation,while IL-22 or IFN-γ exhibited little effects on CXCL12 upregulation in cancer cells (Figure 4A).Moreover,unlike the co-culture supernatants from blood or non-tumor tissue monocytes,TAM-derived Tc17 cell culture supernatants selectively induced CXCL12 production in cancer cells (Figure 4B).The effects of CXCL12 upregulation were attenuated by anti-IL-17 neutralizing antibody,but not anti-IL-22 neutralizing antibody (Figure 4B).These findings suggested that Tc17 cell-derived IL-17 induced chemokine CXCL12 production by tumor cells.
Tumor-infiltrating Tc17 cells are related to the poor outcome of patients with cervical cancer
As CXCL12 upregulation drives cancer development,we assessed the relationship between the number of Tc17 cells and the survival rate of patients with cervical cancer.We first analyzed the number of Tc17 cells per million total cells in each tissue from patients in different TNM stages.The results showed that as cancer progressed,the number of Tc17 cells significantly increased in each of the tested tissues (Figure 5A).Conversely,patient survival was significantly reduced (Figure 5A and B).Simultaneously,we analyzed Tc17 cell percentage within the total CD8+T cells in the same samples,and as cancer progressed,we found that the percentage of Tc17 cells significantly increased in the tested tissues,and was negatively associated with patient survival (Figure 5C and D).These findings suggested that the number of Tc17 cells was related to the survival rate of patients,thereby predicting the clinical outcome of patients with cervical cancer.
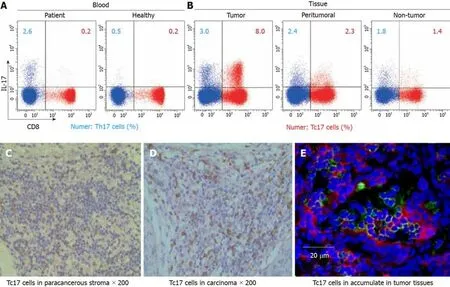
Figure1 Tc17 cells accumulate in tumor tissues of cervical cancer patients.
DISCUSSION
Although Tc17 cell-mediated immune regulation has been studied in tumor progression in both mice and black-and-white monkeys[13],the mechanism of Tc17 cells in human cervical cancer has been rarely reported.In this study,we found that Tc17 cells play a stimulatory role in the progression of cervical cancer.Compared with normal tissues,cervical cancer cells produced a greater amount of IL-6,IL-1β,and IL-23A,which are essential for Tc17 cell differentiation[11,12].Tc17 induction in turn augmented CXCL12 expression in tumor cellsviaactivation of IL-17 signaling.Furthermore,the increased ratio of Tc17 cells in tumors predicted a poor prognosis in patients with cervical cancers.Thus,these data demonstrate that Tc17 cells have an oncogenic role in cervical cancer development.
Recent studies have shown that persistent antigenic stimulation leads to CD8+T cell exhaustion[14,15].Blockade of the PD-1/PD-L1 strategy is promising for the treatment of cancer[16].However,an increasing number of studies have revealed that tumor-elicited inflammation enhances resistance to cancer immunotherapy,which suggests that cancer cells adopt other strategies to escape immune attack.In the present study,we found that Tc17 cells specifically accumulated in cervical cancer tissue.Unlike the necessary role of IL-6 and IFN-γ in stimulation of Tc17 cell expansion in colorectal or liver cancers[14,15],cervical cancer cells secrete abundant Tc17-polarizing cytokines including IL-6,IL-1β,and IL-23,suggesting the enhanced effects on Tc17 cell development is attributed to the cancer microenvironment.
Murine tumor models have shown that Tc17 cells impair immune surveillance and promotede novocarcinogenesis and neovascularization of tumors[16,17].However,other groups have identified anti-tumor activity of Tc17 cells in mice[18,19].Therefore,the precise underlying mechanism is still unclear.Our data revealed that increased IL-17 derived from Tc17 cells drives CXCL12 expression in tumor cells,which has been reported to enhance cell proliferation and migration,thereby exacerbating malignant formation.Finally,we assessed the relationship between the number of Tc17 cells and the survival rate of patients with cervical cancer.Similar studies have been conducted in mice and gastric cancer patients[20].The results showed that the increased ratio of Tc17 in tumors is highly correlated with poor outcome in patients with cervical cancer.
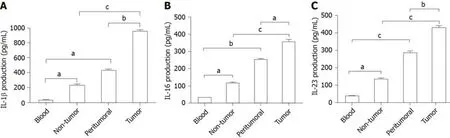
Figure2 Interleukin-6,interleukin-1β,and interleukin-23 accumulated in tumor tissues of cervical cancer patients.
In conclusion,our data demonstrate that Tc17 cells are specifically induced by the cervical cancer microenvironment.Tc17 cells promote tumor developmentviaactivation of IL-17 signaling and are associated with the prognosis of patients with cervical cancer.Thus,we propose that Tc17 cells can be used as a useful clinical index in the prognosis of cervical cancer.
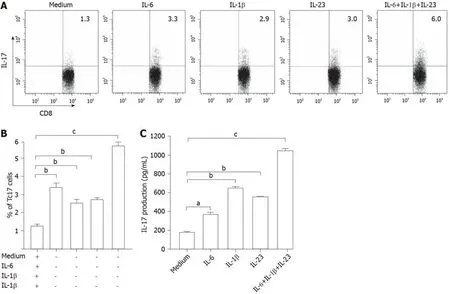
Figure3 Interleukin-6,interleukin-1β,and interleukin-23 induced differentiation of Tc17 cells.
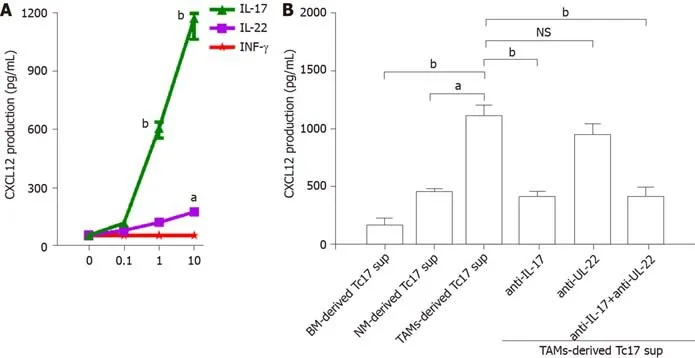
Figure4 Tc17 cell-derived interleukin-17 induces tumor cells to produce CXCL12.
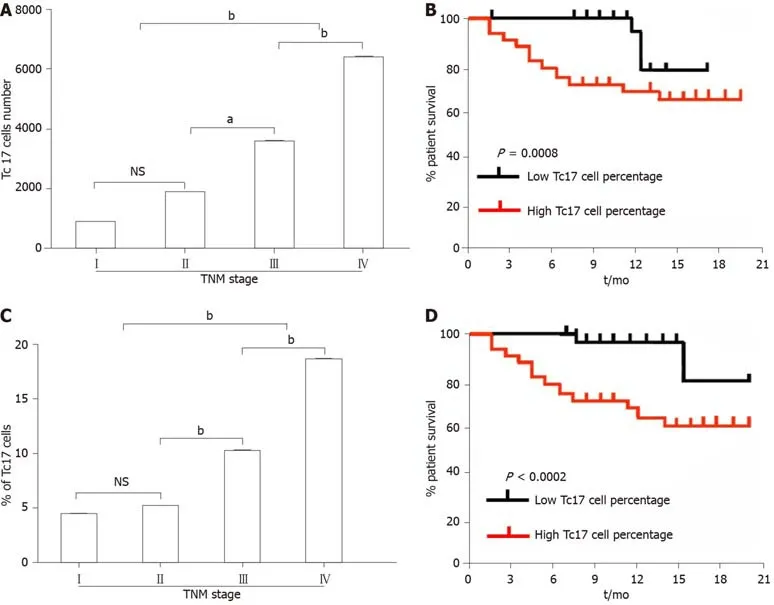
Figure5 Analysis of the correlation between the percentage of Tc17 cells and the survival rate of cervical cancer patients.
ARTICLE HIGHLIGHTS
Research background
The existence of Tc17 cells was recently shown in several types of inflammatory diseases.
Research motivation
The distribution and functions of Tc17 cells in cervical cancer have not been fully elucidated.
Research objectives
To investigate the role of Tc17 cells in the pathogenesis of cervical cancer.
Research methods
The frequency of Tc17 cells in blood and tumor samples from patients with cervical cancer was determined by flow cytometry.In addition,the levels and phenotype of Tc17 cells in tissue samples from cervical cancer patients were assessed by immunohistochemistry staining.
Research results
Tc17 cells specifically accumulate in the tumor tissues of cervical cancer patients.Cervical cancer-elicited inflammation increases Tc17-polarizing cytokine production,which attenuates cytotoxic CD8+T cell development.High interleukin-17 production by Tc17 cells leads to CXCL12 upregulation and cancer cell migration.
Research conclusions
Consistent with the oncogenic role of Tc17 cells in cancer development,the ratio of cancerinfiltrating Tc17 cells is highly associated with poor prognosis in patients with cervical cancer.
Research perspectives
This study indicates that Tc17 cells in cervical cancer and their regulatory mechanisms are associated with cancer progression.
 World Journal of Clinical Cases2020年1期
World Journal of Clinical Cases2020年1期
- World Journal of Clinical Cases的其它文章
- Role of oxysterol-binding protein-related proteins in malignant human tumours
- Acute distal common bile duct angle is risk factor for postendoscopic retrograde cholangiopancreatography pancreatitis in beginner endoscopist
- Three-dimensional computed tomography mapping of posterior malleolar fractures
- Application of a modified surgical position in anterior approach for total cervical artificial disc replacement
- Potential role of the compound Eucommia bone tonic granules in patients with osteoarthritis and osteonecrosis:A retrospective study
- Prognostic factors for overall survival in prostate cancer patients with different site-specific visceral metastases:A study of 1358 patients
