Optimal hang time of enteral formula at standard room temperature and high temperature
Narisorn Lakananurak,Nutbordee Nalinthassanai,Wanlapa Suansawang,Palakorn Panarat
Narisorn Lakananurak,Nutbordee Nalinthassanai,Department of Medicine,Faculty of Medicine,Chulalongkorn University,Bangkok 10330,Thailand
Wanlapa Suansawang,Department of Dietetic and Diet Therapy,King Chulalongkorn Memorial Hospital,Bangkok 10330,Thailand
Palakorn Panarat,Department of Medicine,Queen Savang Vadhana Memorial Hospital,Chon Buri 20110,Thailand
Abstract BACKGROUND Despite high risk of bacterial contamination,yet there are no studies that have evaluated the optimal hang time of blenderized and reconstituted powdered formulas at standard room temperature and high temperature.AIM To investigate the optimal hang time of both types of formulas at standard room temperature and high temperature.METHODS Ten specimens of blenderized formula and 10 specimens of reconstituted powdered formula were prepared using aseptic techniques.Five specimens of each formula were administered at 25 °C and 32 °C.Simulated administration was done in an incubator.The samples were collected at 0,2,4,6 h and aerobic culture was performed.Food and drug administration criteria were used to determine the unacceptable levels of bacterial contamination.RESULTS Unacceptable contamination for blenderized formula began at 4 h at 25 °C and at 2 h at 32 °C.As for the reconstituted powdered formula,there was no bacterial growth in all specimens up to 6 h at both temperatures.CONCLUSION The optimal hang time to avoid significant bacterial contamination of the blenderized formula should be limited to 2 h at standard room temperature and be administered by bolus method at high temperature,while a reconstituted powdered formula may hang up to 6 h at both temperatures.
Key Words:Enteral nutrition;Blenderized diet;Powdered diet;Contamination;Hang time;Infectious diarrhea
INTRODUCTION
Enteral nutrition(EN)is an important form for nutritional support.It has many benefits,including maintenance of the gut integrity and prevention of infectious complication[1].EN may be contaminated with microorganisms and result in serious complications such as diarrhea,septicemia,and death[2].The Food and Drug Administration(FDA)proposed the standard for unacceptable levels of contamination for enteral formulas.The criteria were:any aerobic agar plate growing >104colonyforming unit(CFU)/mL,three or more samples >103CFU/mL,or any pure culture of
Bacillus cerus,Listeria monocytogenes,Staphlococcus aureusorcoliforms[3].Hang time is one of the important factors that can contribute to bacterial contamination in EN.It is recommended that blenderized formula,reconstituted powdered formula,sterile in open system,and sterile in closed system formulas should hang no more than 2 h,4 h,8-12 h,and 24-48 h,respectively[4].Nevertheless,most of the evidence was extrapolated from reports of EN contamination in patients with diarrhea and prolonged hang time[5].
Blenderized and reconstituted powdered formulas are still frequently used in several countries such as Cambodia,Myanmar,Thailand,and Brazil[6].Despite high risk of contamination,studies that directly evaluated the optimal hang time of these formulas are scarce.Additionally,no previous study has investigated the hang time between standard room temperature and high temperature for these formulas,which is crucial for food safety of EN administration during the summer period or in tropical regions.The aim of this study was to investigate the optimal hang time of the blenderized formula and reconstituted powered formula at standard room temperature and high temperature.
MATERIALS AND METHODS
Enteral formulas
Blenderized and reconstituted powdered formulas were prepared by a trained dietitian using aseptic techniques.The blenderized diet was made from cooked ingredients(rice,chicken,pumpkin,eggs,and vegetable oil).The dietitian prepared,mixed,and blended all ingredients in sterile containers.Sterile water was used to dilute the formula to achieve caloric density of 1 calorie per 1 mL.The caloric ratio for carbohydrate:protein:fat for the standard blenderized diet at the King Chulalongkorn Memorial Hospital was 55%:15%:30%.
A polymeric formula(Neomune®)and sterile water were used to make the reconstituted powdered formula.The same dietitian prepared and mixed the reconstituted powdered formula in a sterile container.Caloric density of the formula was also 1 calorie per 1 mL,and the caloric distribution for carbohydrate:protein:fat of the reconstituted powdered formula was 50%:25%:25%.
Both formulas were put into a 500 mL sterile feeding bag(Nutri-Bag®)and were immediately transferred and used after preparation.
Enteral nutrition administration
In order to precisely control the temperature,simulated administration was done in an incubator.The feeding bag was connected to a feeding tube and an infusion pump with aseptic techniques to mimic EN administration in patients.Standard enteral feeding pump and infusion tubing(Kangaroo®)were used to deliver both formulas.The pump does not have heat preservation function which might have an effect on the temperature control.Standard room temperature was set at 25 °C,and high temperature was set at 32 °C,based on the average temperature in Thailand.Both formulas were delivered at the rate of 80 mL/h to mimic standard continuous feeding rate in most patients.A sterile container was used to receive enteral formulas,and there was no contact between the feeding tube and the container.
Sample collection and culture
Five milliliters of formula were collected from the tip of the feeding tube at 0,2,4,and 6 h.All samples were sent for aerobic culture using blood agar and MacConkey agar.A colony count and bacterial identification were done by a microbiologist,who was blinded from the experiment.
FDA criteria were used to determine unacceptable levels of contamination.Given that no previous study has investigated this issue before and at least 3 specimens were needed according to the FDA criteria,we decided to conduct a pilot study by evaluating 5 specimens of each formula at 25 °C and 32 °C,resulting in a total of 20 specimens(Table 1).The protocol for this study was approved by the Institutional Review Board of the Faculty of Medicine,Chulalongkorn University,Bangkok,Thailand(Institutional Review Board number 645/59).
Statistical analysis
Descriptive statistics were used to describe the optimal hang time,type and quantity of bacterial growth in each culture positive specimen.Optimal hang time was described as number of hours without unacceptable bacterial contamination of each formula at both temperatures as per the FDA criteria.Bacterial growth was described as CFU and specific types of bacteria.The number and percentage of specimens with unacceptable levels of bacterial contamination for each enteral formula at standard room temperature and high temperature were also described.
RESULTS
Blenderized formula
At 25 °C,the bacterial growth did not exceed FDA criteria at 0 and 2 h.Unacceptable bacterial contamination occurred at 4 h in 2 specimens(40%).At 6 h,4 specimens(80%)had unacceptable bacterial growth.
At 32 °C,although the number of bacteria was at acceptable level at 0 h,all 5 specimens had positive culture with more growth than specimens at 25 °C.Unacceptable contamination began at 2 h in 1 specimen(20%).At 4 and 6 h,all specimens had unacceptable contamination.Mixed organisms were identified in all culture positive samples(Table 2).
Reconstituted powered formula
There was no bacterial growth at 0,2,4,and 6 h at both 25 and 32 °C(Table 2).
DISCUSSION
The American Society of Parenteral and Enteral Nutrition recommends to limit the hang time of blenderized formula to 2 h at room temperature[4,7].However,this recommendation was extrapolated from case reports of patients with diarrhea and prolonged hang time[5].In our study,the maximum hang time with acceptablebacterial levels at standard room temperature was 2 h.Therefore,our study helps to confirm this recommendation.At high temperature,blenderized formula had more than acceptable levels of bacterial growth as early as 2 h.Consequently,patients receiving this formula in hot climates exceeding 32 °C should consume the formula as quickly as possible by bolus regimen.
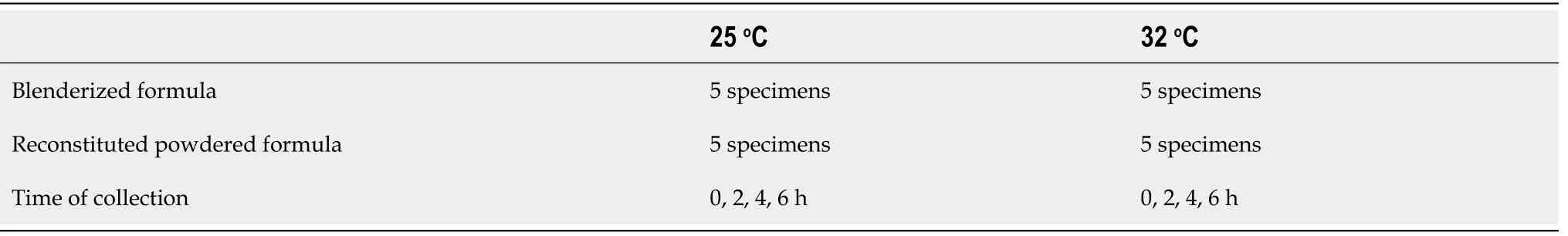
Table 1 Number of specimens and time of collection for blenderized and reconstituted powdered formulas
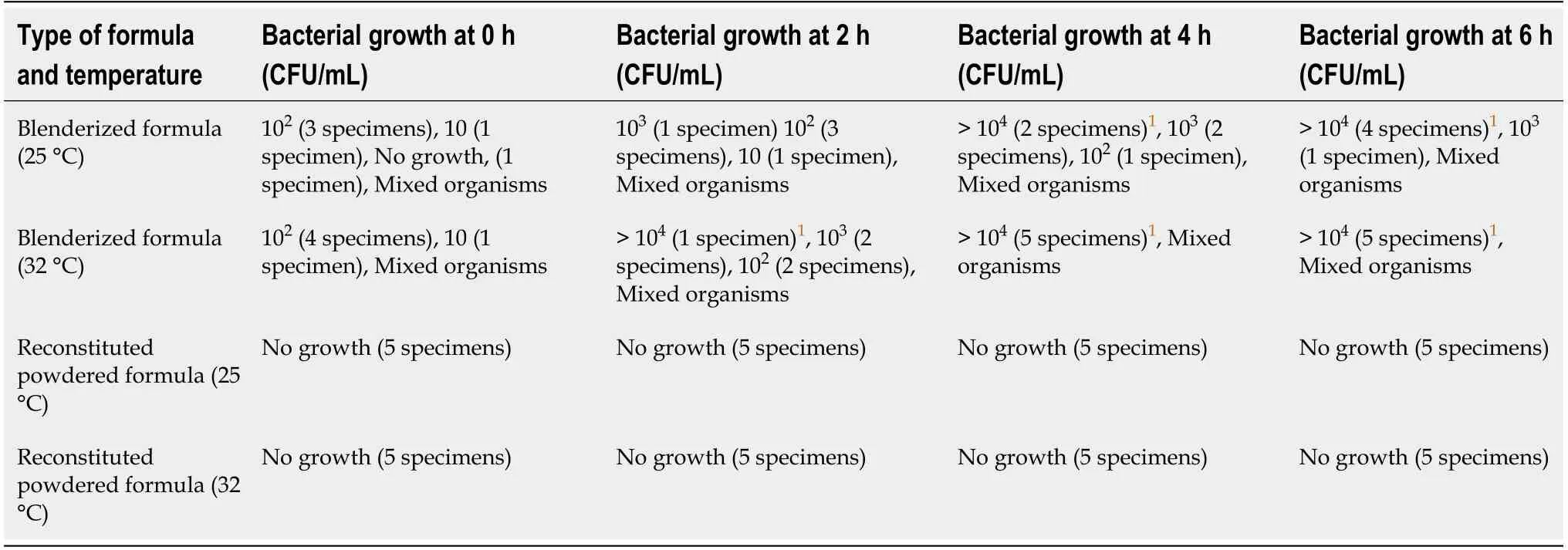
Table 2 Bacterial growth and type of bacteria in found in blenderized and reconstituted powdered formulas at 25 oC and 32 oC
The hang time of reconstituted powdered formula is limited to 4 h as recommended by American Society of Parenteral and Enteral Nutrition[4,5].A previous study demonstrated that the hang time up to 8 h for powdered formula was associated withEnterobacter sakazakiiinfections in 9 infants.After reducing the hang time to less than 4 h,there was no new episode of infection[8].In contrast,our study showed that reconstituted powdered formula can hang up to 6 h with no bacterial growth at both temperatures.The previous study did not directly investigate the hang time and did not evaluate other possible sources of bacterial infection.Moreover,it is possible that acceptable bacterial levels in immunocompromised neonates might be different from adults.
In our study,the effect of high temperature on bacterial growth was shown in only the blenderized formula.High temperature may contribute to quicker and greater bacterial growth in formula that has a high possibility of contamination.This result was similar to data from a previous study,which reported that the risk of bacterial contamination to be very low in prefilled close enteral formula even at high temperature[9].
To the best of our knowledge,this is the first study that directly evaluated the hang time for both blenderized and reconstituted powdered formulas at standard room and high temperatures.This study also has some mentionable limitations.First,the bacterial growth might be lower than in real life practice since there was no risk of retrograde microorganism from the stomach or lungs[10].Second,the maximum hang time of the reconstituted formula could not be concluded because for this study,our cut-off was at 6 h.Finally,even though the sample size is adequate to evaluate the unacceptable bacterial contamination in enteral formula according to the FDA recommendation,this study has a small sample size that can result in the bias results.
CONCLUSION
The optimal hang time to prevent significant bacterial contamination of the blenderized formula should be limited to 2 h at standard room temperature and be administered by bolus method at high temperature,while reconstituted powdered formula may hang up to 6 h at both temperatures.Safety implications of hang time should be confirmed in further study that is correlated with the clinical outcomes.
ARTICLE HIGHLIGHTS
Research background
Optimal hang time is one of important factors to prevent bacterial contamination during enteral nutrition administration.However,the recommendation for optimal hang time was extrapolated from reports of enteral nutrition contamination in patients with diarrhea and prolonged hang time.No previous studies have directly investigated optimal hang time of blenderized and reconstituted powered formulas at standard room temperature and high temperature.
Research motivation
Despite high risk of contamination,studies that directly evaluated the optimal hang time of blenderized and reconstituted powered formulas at standard room temperature and high temperature are scarce.The investigation of optimal hang time will help prevent bacterial contamination in both hospital and home-based enteral nutrition support.
Research objectives
This study investigated the optimal hang time of both types of formulas at standard room temperature and high temperature.
Research methods
Ten specimens of blenderized formula and 10 specimens of reconstituted powdered formula were prepared using aseptic techniques.Five specimens of each formula were administered at 25 °C and 32 °C.Simulated administration was done in an incubator.The samples were collected at 0,2,4,6 h and aerobic culture was performed.Food and drug administration criteria were used to determine the unacceptable levels of bacterial contamination.
Research results
Unacceptable contamination for blenderized formula began at 4 h at 25 °C and at 2 h at 32 °C.As for the reconstituted powdered formula,there was no bacterial growth in all specimens up to 6 h at both temperatures.
Research conclusions
The optimal hang time of the blenderized formula should be limited to 2 h at standard room temperature and be administered by bolus method at high temperature,while a reconstituted powdered formula may hang up to 6 h at both temperatures.
Research perspectives
The findings from the current study helps establish and provide the groundwork for further large-scale studies to evaluate optimal hang time for blenderized and reconstituted powered formulas.
 World Journal of Clinical Cases2020年19期
World Journal of Clinical Cases2020年19期
- World Journal of Clinical Cases的其它文章
- Role of monoclonal antibody drugs in the treatment of COVID-19
- Review of simulation model for education of point-of-care ultrasound using easy-to-make tools
- Liver injury in COVID-19:A minireview
- Transanal minimally invasive surgery vs endoscopic mucosal resection for rectal benign tumors and rectal carcinoids:A retrospective analysis
- Impact of mTOR gene polymorphisms and gene-tea interaction on susceptibility to tuberculosis
- Establishment and validation of a nomogram to predict the risk of ovarian metastasis in gastric cancer:Based on a large cohort
