An Anisotropic Diluted Magnetic Hybrid Perovskite Series of[CH3NH3][CoxZn1-x(HCOO)3]
Sa Chen, Ran Shang, Bingwu Wang, Zheming Wang, Song Gao
Beijing National Laboratory for Molecular Sciences, Beijing Key Laboratory of Magnetoelectric Materials and Devices,College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, P.R.China.
Abstract:Inorganic-organic or hybrid perovskite materials, which are the complementary counterparts of pure inorganic perovskites, can provide many new opportunities in the researches of phase transitions, critical phenomena, and relevant properties, as they combine the characteristics of inorganic and organic components.Therefore, the hybrid perovskites of ammonium metal formate framework are very promising, and their properties have been found to be strongly dependent on the characteristics of the constituent metal ions and/or ammonium ions.Herein, we used solid solution strategies, borrowed from solid state chemistry, to investigate the anisotropic diluted magnetic hybrid perovskite system of [CH3NH3][CoxZn1-x(HCOO)3],wherein the B-sites are occupied by the mixed metal ions of Co2+ and Zn2+.The solid solution compounds of this series in the range x = 0-1 (or the molar percent Co% = 0-100%) were successfully prepared using conventional solution chemistry methods.The resulting compounds were demonstrated to be iso-structural by using both single-crystal and powder X-ray diffraction analyses.The solid solution crystals belong to the orthorhombic space group Pnma, with the cell parameters being a = 8.3015(2)-8.3207(3) Å, b =11.6574(4)-11.6811(5) Å, c = 8.1315(3)-8.1427(4) Å, and V = 787.89(5)-790.98(7) Å3.The perovskite structure consists of a simple cubic anionic metal-formate framework and CH3NH3+ cations which are located in the framework cavities, with N―H···O hydrogen bonds formed between the framework and the cation.The members of this series showed negligible changes (< 0.4%) in their respective lattice and structural parameters.Thus, the prepared solid solution compounds constitute good molecule-based examples for the study of magnetic dilution under almost the same structural parameters and molecular geometries.Upon dilution, the magnetization per mole of Co at low temperatures and low fields was suppressed by the magnetic anisotropy of Co2+ and gradual destruction of the large spin canting between coupled Co2+ions, in contrast to the magnetization enhancement observed in the isotropic diluted system of [CH3NH3][MnxZn1-x(HCOO)3]with the same perovskite structure.The percolation limit was estimated as (Co%)P = 27(1)% (or xP = 0.27(1)) from the magnetic data, which was slightly lower than that predicted by the percolation theory for a simple cubic lattice (31%); this trend was due to the strong magnetic anisotropy of the present system.In addition, rare incommensurate phase transitions were primarily detected below ~120 K for the pure Co and Zn members, which may also affect the magnetic properties of the materials.
Key Words:Hybrid perovskite;Ammonium metal formate framework;Magnetic dilution;Structure;Magnetism
1 Introduction
The conventional perovskites (such as oxides) are of great importance in material sciences because such materials can show a very wide range of physical properties and practical applications1.Being complementary to the pure inorganic perovskites, the inorganic-organic or hybrid perovskite materials have caught great attractions2.By combing the characteristics of inorganic and organic components, the hybrid perovskites can provide many new possibilities and opportunities for the researches of phase transitions, critical phenomena and the relevant properties.A special class of hybrid perovskites of ammonium metal formate framework (AMFF) has been proven to be very promising along this line3-9.The AMFF perovskites are formulated as [AH][M(HCOO)3] (in ABX3, AH = A-site mono-ammonium, B = B-site octahedral M ions, and X =HCOO-), in which the simple cubic anionic metal-formate frameworks are of (412·63) topology, with the cubic framework cavities occupied by mono-ammoniums of 2 to 5 non-H atoms.In most compounds, formate ligand adaptsanti-antibridging mode.More than 10 kinds of both mono-ammoniums and metal ions have been employed in the studies, thus more than 100 AMFF perovskite materials have been prepared and characterized up to date, and these materials revealed abundant phase transitions and the relevant magnetic, electric and mechanical properties, and so on, which strongly depended on the characteristics of the constituent ammoniums and metal ions.Furthermore, the solid solution methods, borrowed from solid state chemistry, have been employed, resulting in the interesting mixed-ammonium (at A-site) and mixed-metal (at B-site) AMFF pervoskite systems8-10.Several [A1H-A2H][M(HCOO)3]perovskites exhibited phase transitions and dielectric properties modulated by mixed-ammonium components at A-site8.For B-site mixed-metal, solid solution perovskite systems of[Gua][CuxCd1-x(HCOO)3]9a(Gua = guanedinium) and[CH3NH3][MnxZn1-x(HCOO)3]9bwere reported.In the former an orbital disorder/order transition and a multipolar reorientation transition were observed and attributed to the combination of size, charge distribution, and percolation effects owing to the change of metal compositions.The later is a typical isotropic Heisenberg diluted magnetic system in molecule-based magnets.We have also explored several other mixed-metal perovskite systems showing interesting, metal-dependent magnetic properties10.All these results revealed promising potentials to modulate magnetic and electric properties and functionalities of AMFF perovskites by the solid solution strategies.In this work,we reported an anisotropic diluted magnetic system of[CH3NH3][CoxZn1-x(HCOO)3].Through the series, the isostructural members show almost no change in the lattice parameters and respective molecular geometries.On dilution,the magnetic anisotropy of Co2+ion and the destruction of large spin-canting by breaking of magnetic coupling suppressed the magnetizations in low temperature (LT) and low field region, in contrast to the magnetization enhancement in the isotropic,magnetically diluted system of [CH3NH3][MnxZn1-x(HCOO)3]9b.The percolation limit was estimated (Co%)P= 27(1)% (here Co% is the molar percent of Co content) by magnetic data,slightly lower than 31% by percolation theory11for the simple cubic lattice.In addition, incommensurate phases have been detected below 120 K for the pure Co and Zn members.
2 Experimental
2.1 Synthesis
All starting materials were commercially available and analytical pure, and were used without further purification.The solid solution compounds of [CH3NH3][CoxZn1-x(HCOO)3]were synthesized by using the mild solution chemistry methods we developed3.Typically for [CH3NH3][Co0.10Zn0.90(HCOO)3](Co10): Co(ClO4)2·6H2O 36.8 mg (0.10 mmol) and Zn(ClO4)2·6H2O 334.8 mg (0.90 mmol) were dissolved in 5.0 mL methanol.This solution was gently mixed with a 10 mL methanol solution containing 0.40 mol·L-1CH3NH2and 0.80 mol·L-1HCOOH.The mixed solution was sealed and kept undisturbed.Block-shaped pink crystals were harvested after one day, washed with ethanol and dried in air.The yield was 95%based on the metal salts.Other compounds of this series,including the pure Zn and Co ones, could be easily prepared by similar routes, in yields of about 90%.The products were all block-shaped crystals, showing homogeneous in color and morphology.The Co/Zn molar ratios of the products, determined by ICP (Inductively Coupled Plasma) analyses, were within 2%to the starting Co/Zn molar ratio (Table S1 in Supporting Information (SI), together with the elemental analysis results),showing slight enrichment of Zn in the products under the synthetic conditions.The compounds are named according to the molar percent, Co% (orxin formula [CH3NH3][CoxZn1-x(HCOO)3]).For example, Co10 was assigned to the compound of[CH3NH3][Co0.10Zn0.90(HCOO)3] with Co% = 10.0%, and the pure Zn and Co compounds are named as Co0 and Co100, see Table S1 (SI).
CAUTION!Perchlorate compounds are potentially explosive.They should be treated in small quantities and handled with care.
2.2 X-ray crystallography
The crystallographic data for the 11 compounds were collected at 180K on a Rigaku Oxford SuperNova Dual Atlas CCD diffractometer (Japan)12using mirror monochromated MoKαradiation (λ= 0.71073 Å, 1 Å = 0.1 nm) and an Oxford temperature control system (UK).For intensity data the numerical absorption corrections were applied by Gaussian methods based on the face indexing of the crystals.The previously published structure of [CH3NH3][Zn(HCOO)3]9b(Co0) was used as the starting structure model, and all structures were refined by full-matrix least-squares onF2using the SHELX program13.The ratios of Co and Zn at the unique metal site in the structures from Co10 to Co88 were set according to the Co/Zn molar ratios obtained by ICP analyses (Table S1 (SI)).Hydrogen atoms could be located from the difference Fourier synthesis, and refined with the constraints for ideal geometries.CCDC-1937173 to CCDC-1937183 contain the supplementary crystallographic data in the sequence of Co0, Co10, … , Co88 and Co100 in this paper.These data can be obtained free of chargeviawww.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road,Cambridge CB2 1EZ, UK; Fax: (+44) 1223-336-033; or Email:deposit@ccdc.cam.ac.uk).The variable temperature oscillation images for Co100 and Co0 in LT region were collected on the same CCD diffractometer by using long exposure time in order to reveal weak satellite spots.Powder X-ray diffraction (PXRD)patterns for the compounds were obtained in the range of 10° <2θ< 60° at room temperature against the bulk samples on a Rigaku Dmax 2000 diffractometer (Japan) with CuKα radiation in a flat plate geometry.
2.3 Physical measurements
Metal contents of the solid solution compounds were determined by ICP analyses on a Profile inductively coupled plasma-atomic emission spectrometer (ICP-AES, USA).Element analyses of C, H, and N were performed on an Elementar Vario MICRO CUBE analyzer (Germany).Fourier transform infrared (FTIR) spectra were recorded against pure samples in the range of 4000 to 650 cm-1on a NICOLET iN10 MX spectrometer (USA).The simultaneous differential scanning calorimeter-thermal gravimetric analysis (DSC-TGA)analyses were performed on a TA SDT Q600 instrument (USA)at the rate of 5 °C·min-1in air flow.Magnetic measurements were carried out for polycrystalline samples of Co10 to Co100 on a Quantum Design MPMS-XL5 SQUID (superconducting quantum interference device) system (USA).Diamagnetic corrections were estimated using Pascal constants14(-95 × 10-6--92 × 10-6cm3·mol-1) and background correction by experimental measurement on sample holders.Magnetization and susceptibility data per mole Co were calculated based on the molar percent Co% by the ICP analyses.
3 Results and discussion
3.1 Synthesis and basic characterizations
The solid solution compounds of [CH3NH3][CoxZn1-x(HCOO)3]in the full range ofx= 0-1 have been successfully synthesized in high yields by mild solution chemistry methods3,4a,4c,5a,7a,8a,9b,10,which are much more convenient than other methods such as solvent-thermal methods4b,6,7b-e.The present series added to the known solid solution AMFF family8-10.The phase purity of the bulk samples and isomorphism for the present compounds were confirmed by PXRD (Fig.S1a (SI)).Unlike the PXRD patterns of the previous mixed Mn-Zn system,[CH3NH3][MnxZn1-x(HCOO)3]9b, the PXRD peaks of the present Co-Zn system showed no systematic and metal-content dependent shifts, but very small, random ones.This indicates almost no change in the lattice size owing to the same ionic radius of Co2+and Zn2+(both 0.88 Å), and agrees with the results from the single-crystal structure determination (Tables 1 and 2,and Table S2 (SI), see later).The IR spectra of the present compounds are simple and similar one to another (Fig.S1b (SI)),indicating the iso-structural characters of the compounds.The IR absorption bands characterize CH3NH3+and HCOO-groups15,16,and these bands are coincident with those of the relevant compounds previously reported4,5a,8a,9b.Through the series all IR bands shift very little.In the combined TGA-DSC runs (Fig.S1c, d (SI)).The materials all showed two well-defined steps of weight loss, corresponding to the departure of one CH3NH2·HCOOH per formula at first step, then the further pyrolysis of the intermediate phases CoxZn1-x(HCOO)2.The thermal decomposition behaviors were also quite similar to the mixed Mn-Zn compounds9b, as well as other AMFFs3.
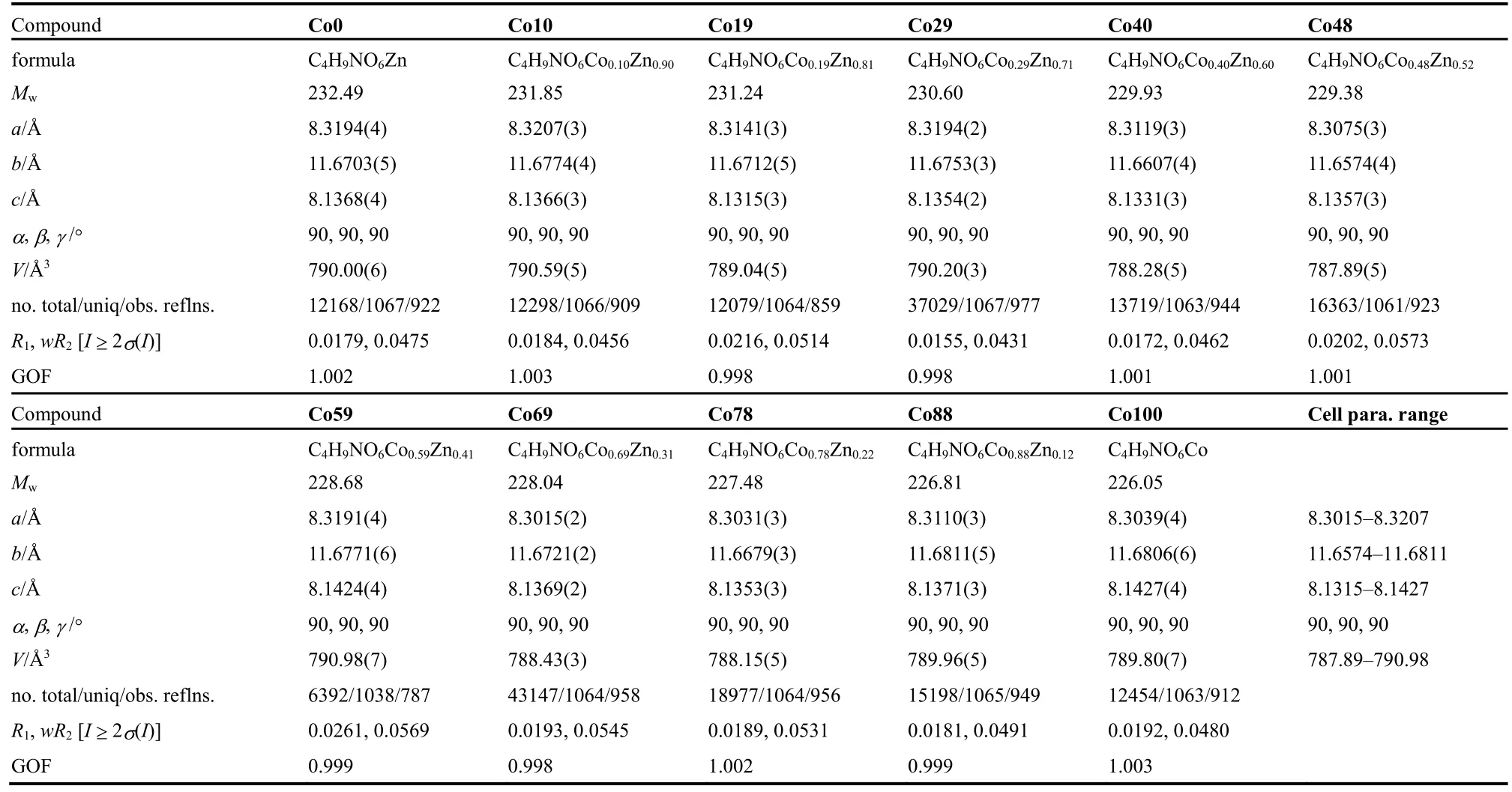
Table 1 The brief crystallographic data for Co0, Co10, …, Co88, and Co100, all at 180 K, and in orthorhombic space group Pnma.In the last column the ranges for respective cell parameters are given.
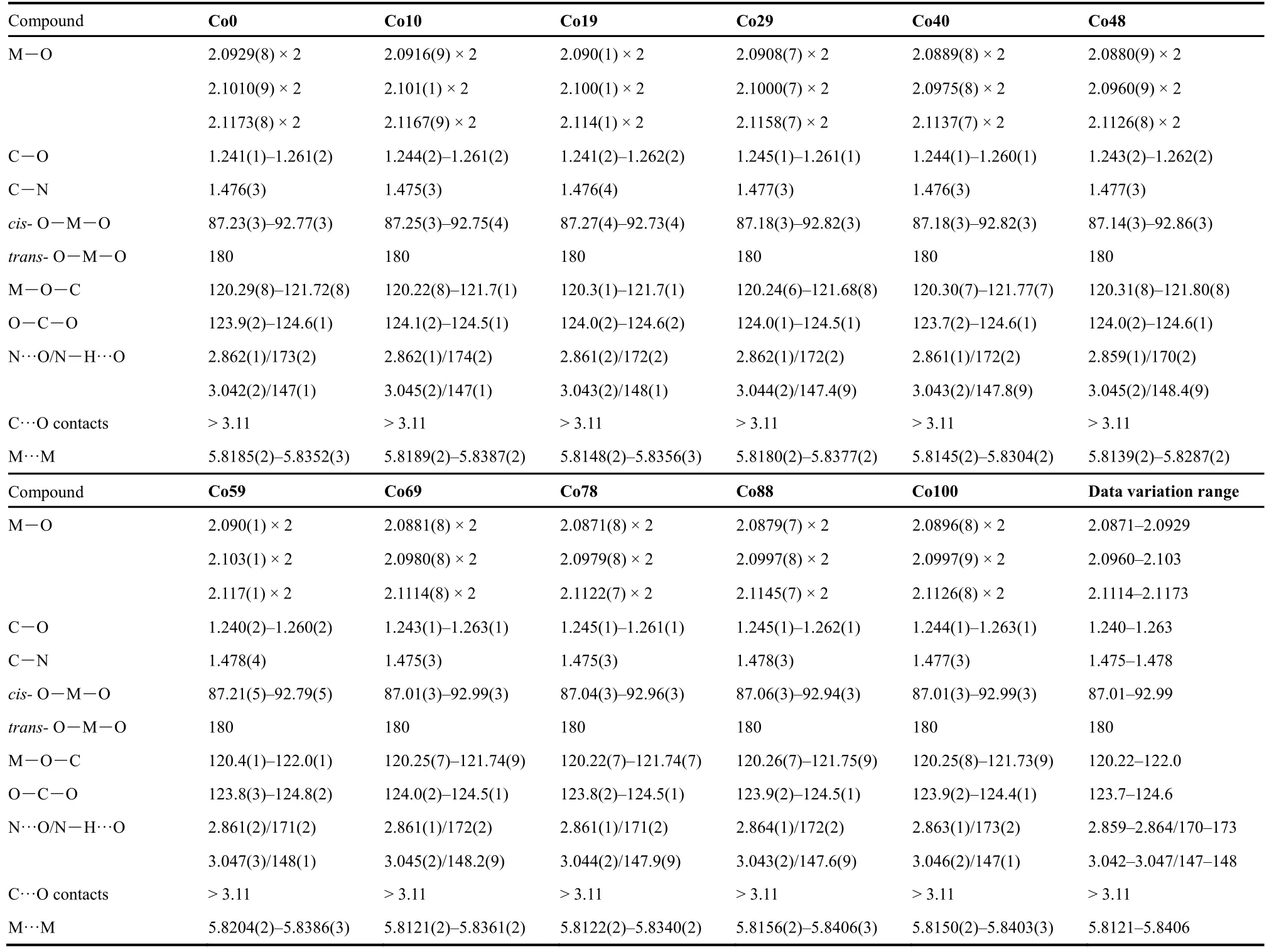
Table 2 Selected molecular geometries, bond distances (Å) and bond angles (°), N―H···O hydrogen bonds (N···O distances, Å, and N―H···O angles, °) between the CH3NH3+ cation and the anionic framework, shortest C···O contacts (Å), and the M···M distances (Å) in the structures of Co0, Co10, …, Co88, and Co100.The variation ranges for all respective molecular geometries are summarized in the last column.
3.2 Crystal structures
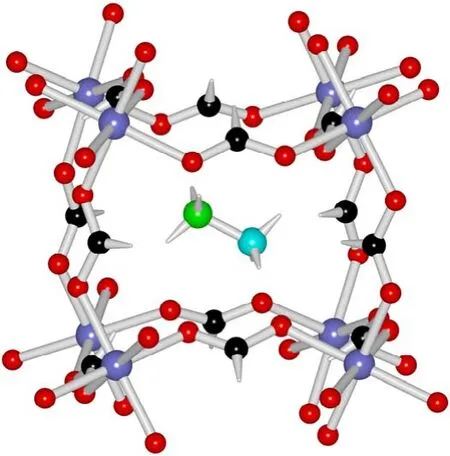
As expected, the 11 compounds of [CH3NH3]-[CoxZn1-x(HCOO)3] series are iso-structural (Tables 1 and 2,Table S2 (SI), and Fig.1) with the reported homometallic[CH3NH3][M(HCOO)3] (M = divalent Mn to Zn)9b,10a.They belong to the space groupPnma, and feature perovskite ABX3 structures in which A = CH3NH3+, B = M2+(M = Co and/or Zn),and X = HCOO-.The structure possesses the simple cubic anionic framework of [M(HCOO)3-] and the CH3NH3+cations inside the cubic framework cavities (Fig.1).In the perovskite structure, the unique, octahedral M2+ion lies at the inversion center of (0, 1/2, 0) (Wyckoff sitea).Each M2+ion is connected to its six neighboring M2+ionsviasixanti-antibridging formate ligands.The connections result in a (412·63) metal-formate network, in which the Co2+and Zn2+ions are statistically or randomly arranged for the mixed metal members.M―O bond distances are 2.0871-2.1173 Å, and the framework cube has M―O―CH―O―M edges of 5.8121-5.8406 Å.The A-site CH3NH3+cations in the cavities form N―H···O hydrogen bonds to the oxygen atoms of the framework, with the hydrogen bond geometries of N···O distances/N―H···O angles in two sets,2.859-2.864 Å/170-173° and 3.042-3.047 Å/147-148° (Table 2).These molecular geometries are all comparable with those in other AMFFs3-9.It is noted that, along the series from Co0 to Co100, the respective molecular geometries, as well as the unit cell parameters, almost remain unchanged, or the changes are less than 0.4% (Tables 1 and 2), in agreement with the PXRD studies.These very small structural changes are different from the Mn-Zn solid solution system9bshowing the systematic changes up to 3% upon the increase molar percent Mn%.Clearly,the same ionic radius (0.88 Å) of Co2+and Zn2+ions in the present Co-Zn systemversussignificant different ionic radius of Mn2+and Zn2+ions (0.97 and 0.88 Å, respectively) in the Mn-Zn system resulted in the different structural changes.The present Co-Zn system provides a good example for studying magnetic dilution under nearly unchanged structural parameters and molecular geometries11a.
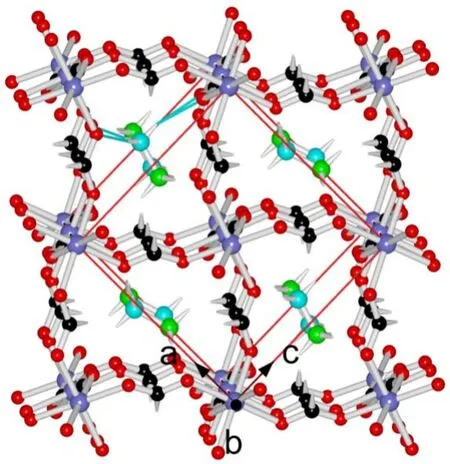
Fig.1 The perovskite structure of Co100, [CH3NH3][Co(HCOO)3],at 180 K, viewed down the b axis.One cavity (top-left-front) with the CH3NH3+ cation showing N―H···O hydrogen bonds in cyan thin bonds is given.Atomic scheme are violet blue Co, black/green C, red O, cyan N, and white H.
It should be mentioned that the pure Co and Zn compounds,Co100 and Co0, underwent structural phase transitions around 120 K, from the above high temperature (HT) phases to LT incommensurate phases17, in which the wave vectors ofq~ (1/7) ×c* were observed (Fig.S2 (SI), for Co100 only).We have observed, in fact, that all members of [CH3NH3][M(HCOO)3](M = divalent Mn to Zn and Mg) exhibited incommensurate phase transitions10a.Such transitions were ignored or not detected before4a,5a,6a, probably due to the occurrence at quite low temperatures and the difficulty to be observed.The detailed incommensurate structures merit further investigation.Incommensurate phase transitions are still rarely observed or reported for AMFF compounds even metal-organic framework materials18.
3.3 Magnetic properties
The magnetic properties of Co10 to Co100 were investigated by dc (direct current) and ac (alternating current) magnetic measurements using the polycrystalline samples, and the basic magnetic data are given in Table 3.As the octahedral Co2+ion is strongly magnetically anisotropic19,20, showing an effectiveS=1/2 at LT from aS= 3/2 at HT due to the depopulation of the higher energy Kramers doublets (±3/2 and ±5/2), the detailed analyses on the magnetic data of the materials are difficult.At present time we focus on the dilution effect and the features differences from the isotropic Mn-Zn system, and our discussion is qualitatively.
The pure Co compound, Co100, was reported quite earlier4a,21.However the magnetism was reported by Zapfet al.in 20166a.The material is a weak ferromagnet or spin-canted antiferromagnet with the reported Néel temperatureTN of 15.9 K, and shows strong magnetic anisotropy based on the magnetic measurements against single crystal.Our results are comparable to the previous ones but show some other features (Table 3, Figs.2 and 3, and Fig.S3 (SI)).Under the field of 100 Oe (below our magnetic data at 100 Oe are presented, if not otherwise specified), theχTvalue of 2.99 cm3·K·mol-1at room temperature and the Curie constant,C= 3.51 cm3·K·mol-1, are almost the same as the previously reported values (under 1 kOe field), and typical for octahedral Co2+ion22.However the Weiss temperature,Θ= -51.7 K (derived from the Curie-Weiss fittingfor the HT susceptibility data above 65 K, Fig.S3a (SI)), is somewhat different from the reported -43.5 K (derived from magnetic data under 1 kOe field and above 20 K).TheTNof 14.2 K, defined by the peak positions in the derivatives (-dFC/dT,Fig.S3b (SI)) of field-cooled (FC) and/or zero-field-cooled(ZFC) magnetization traces under 10 Oe (Fig.2a, inset), as typical for weak ferromagnets3,4,5a, is also different from the reported 15.9 K defined at the arising point of the FC/ZFC plots on cooling6a.By using the fine temperature steps of 0.1 K in measurements, the present derivative FC or ZFC (-dZFC/dTnot given in Fig.S3b (SI)) traces in fact showed shoulders on both sides of the peaks, indicating the possible spin-reorientations aroundTN.The doublet or triplets peaks appeared in ac susceptibilities of both in-phase (χ′) and out-of-phase (χ′′) (Fig.S3c (SI)) confirmed such observations.These were not observed in the previous work6aprobably because of the larger temperature steps of 0.5 K used in the measurements.The incommensurate LT structure of Co100 has multiple sub-lattices of slightly different molecular geometries17.These might lead to the possible spin-reorientations.The frequency-independent peak temperatures of ac susceptibilities are close toTNof 14.2 K(Table 3).Under the high field of 10 kOe, the maximum ofχTtrace is suppressed (Fig.S3d (SI)), characterizing the weak magnetism4,5a.The isothermal magnetization trace at 2 K (Fig.2b) also revealed the occurrence of weak magnetism, and the coercive field (HC) 4.98 kOe, remnant magnetizations (MR)0.031Nβ(N= Avogadro number,β= Bohr magneton, orNβ=per mole Bohr magneton), and magnetization of 0.36Nβat the highest applied field of 50 kOe (Table 3), are all typical for Co-AMFF perovskites4,5a,6.It should be mentioned that because of the magnetic anisotropy of Co2+ion, Co100 shows significantly larger spin-canting thus larger magnetizations than the isotropic Heisenberg weak magnet of [CH3NH3][Mn(HCOO)3]4a,9b, as observed in other AMFF perovskite series.The antiferromagnetic coupling (~ -4 K) between Co2+ions is also ten times stronger than that (~ -0.4 K) between Mn2+ions3-6.
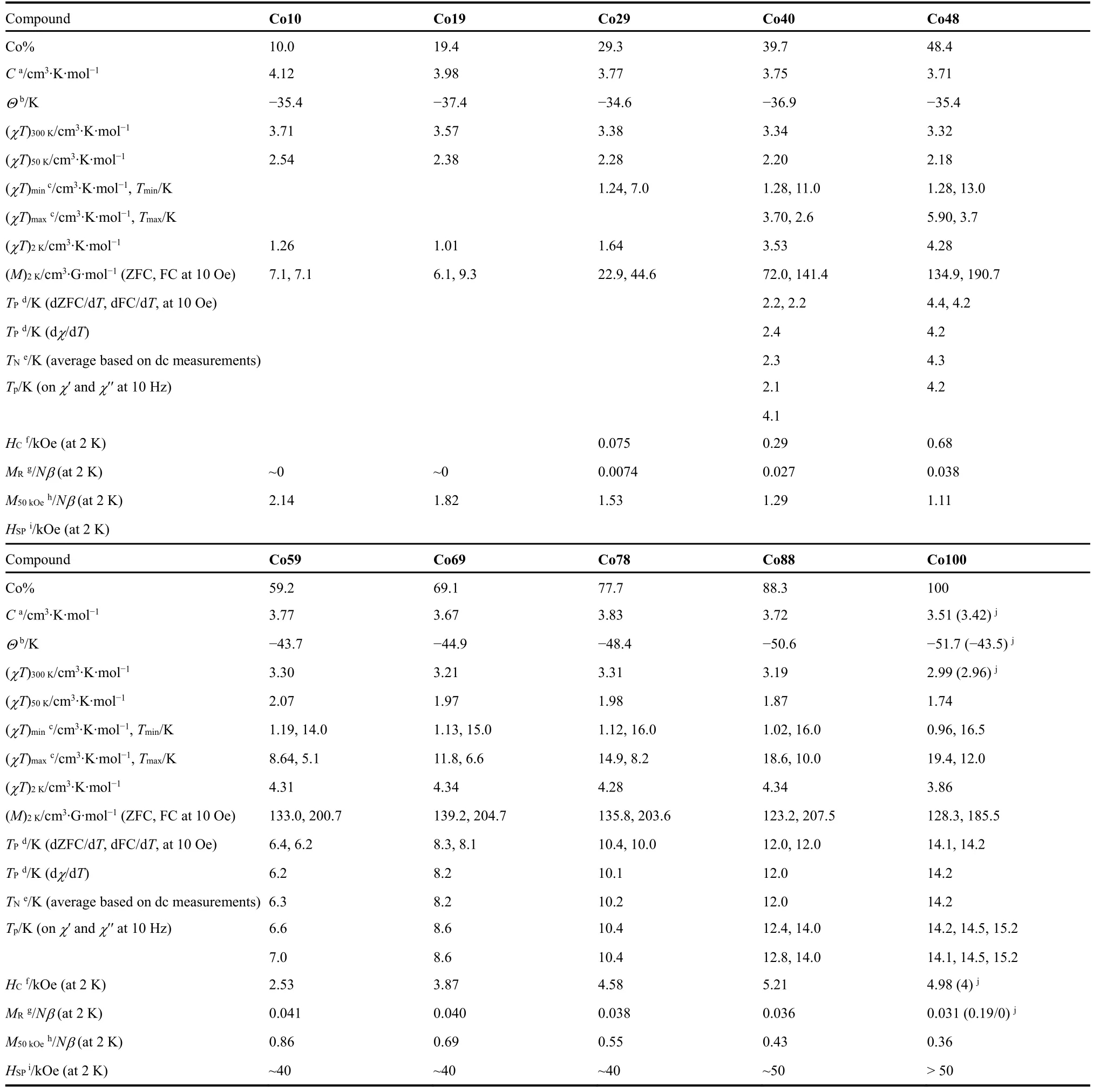
Table 3 Summary of magnetic properties of Co10 to Co100.Magnetization and susceptibility data are represented for per mole Co and under 100 Oe if not otherwise specified.In last column the data in parentheses were taken from Ref.6a (under different fields, temperatures and orientations of single crystal, see the reference6a).
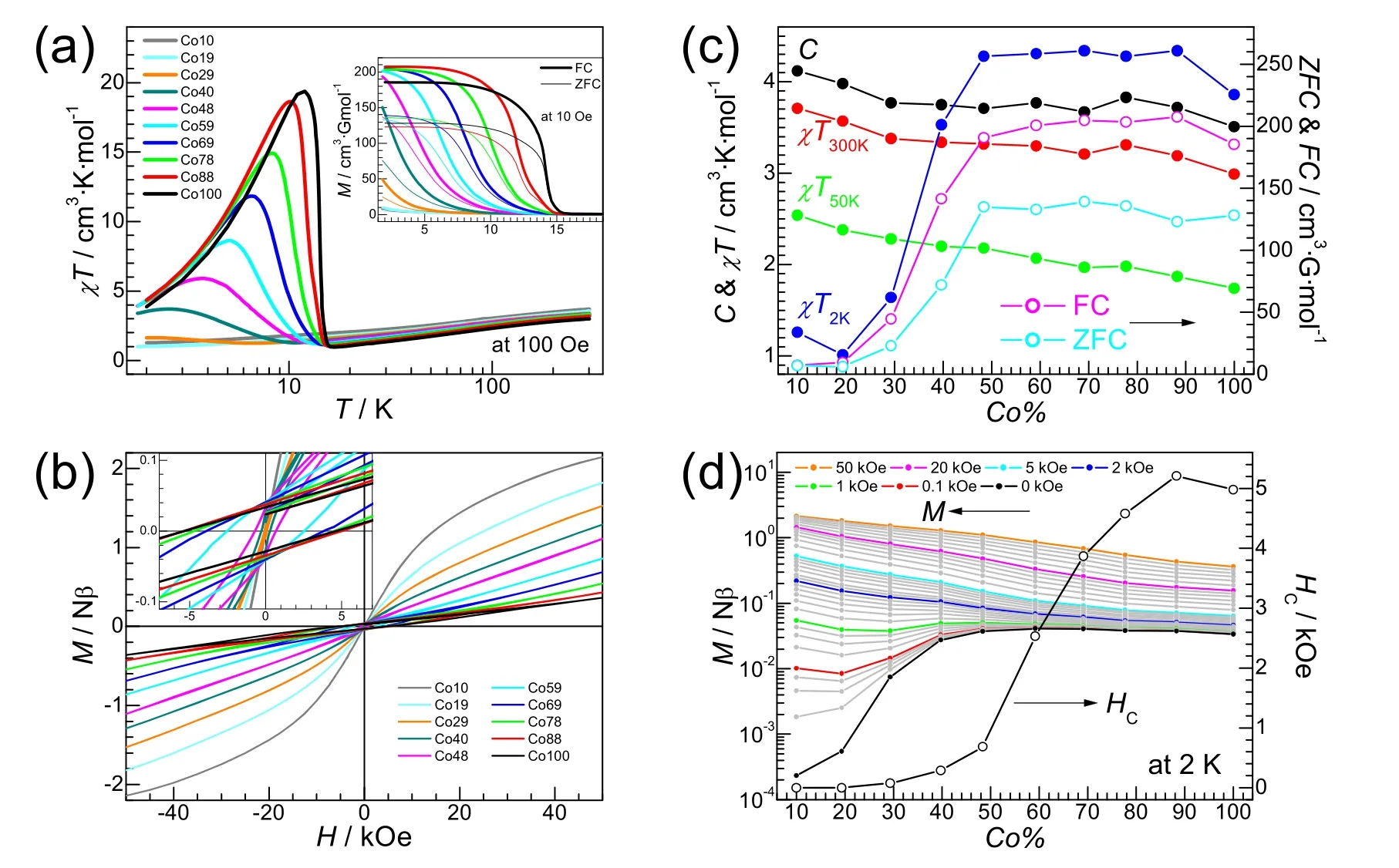
Fig.2 Magnetism for Co100 to Co10.(a) Plots of χT vs T (under 100 Oe field and T axis in logarithmic scale) and inset ZFC/FC plots (under 10 Oe).(b) The isothermal magnetization plots at 2 K and inset the plots in low field region.(c) The Co% dependence of the Curie constants, χT values at 300 K, 50 K and 2 K, and of the ZFC/FC magnetizations (under 10 Oe) at 2 K.(d) The Co% dependence of magnetizations (in logarithmic scale) under different fields, with plots at some fields highlighted, and the Co% dependence of HC.
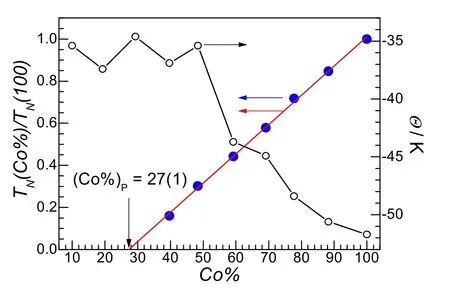
Fig.3 The Co% dependence of Weiss temperatures (Θ) and the normalized Néel temperatures (TN(Co%)/TN(100)) for Co100 to Co40.The red line is linearly fitted one, see text.
The present dilute solid solution compounds mainly show antiferromagnetic (AF) character (Table 3 and Figs.2 and 3, and Fig.S3 (SI)).In HT region they behaved similarly.With the decrease of Co%, theCvalues and theχTvalues at 300 K and 50 K increased somewhat (Fig.2a, c).TheΘvalues changed first nearly linearly from -51.7 K of Co100 to -35.4 K of Co48(Table 3 and Fig.3), then oscillated in a range of -37.4 to -34.6 K for lower Co%.These observations could be resulted from the fact that the dilution by non-magnetic Zn2+gradually reduced the antiferromagnetic couplings in the dilute compounds, and the contribution toΘowing to the spin-orbit coupling of octahedral Co2+ion19,20, could be about -35 K in the present cases.In LT region (Fig.2a), from Co100 to Co40, theχTmaxima decreased significantly and shifted to lower temperatures, and the peaks became more flatted.For lower Co% noχTmaxima were observed, though a rise was observed for Co29.At 2 K, theχTvalues (in cm3·K·mol-1) slightly increased from 3.86 of Co100 to 4.34 of Co88, kept constantly around 4.3 to Co48, then quickly dropped to ~1.0 from Co40 to Co10, with the fast descending in the range of 40 to 30% of Co% (Fig.2c).Therefore, the magnetizations were suppressed on the decrease of Co% at LT.The magnetization suppression by dilution for the present Co-Zn system is completely different from the magnetization enhancement observed in the reported Mn-Zn system9b.In the isotropic Mn-Zn system, the enhancement was caused by local fluctuations in the total magnetic moment which come from the destroyed balance of the antiferromagnetic sublattices in the vicinity of the non-magnetic Zn2+sites11a,23, or the percolated-induced ferrimagnetism24.In the present anisotropic Co-Zn system, however, such enhancement could be suppressed by the anisotropy of Co2+because the moment was forced to be parallel to the easy (or Ising) axis11a,23.This mechanism was documented in several inorganic, anisotropic diluted systems23.Owing to the larger spin-canting between coupled Co ions3-6,we could suggest that the broken couplings by non-magnetic Zn2+also significantly reduced the magnetization originated from the spin-canting or weak ferromagnetism.In Mn-Zn system, however, magnetization from spin-canting is small because of much smaller spin-canting, so that the magnetization loss by broken spin-canting is also small or could be neglected.The ZFC/FC traces under 10 Oe displayed similar suppressed magnetizations, and the ZFC/FC magnetizations at 2 K exhibited the same dependence upon Co% as thatχTvalues at 2 K.The bifurcation behaviors in ZFC/FC traces (Fig.2a, inset) were observed for Co88 to Co19, indicating that the magnetization irreversibility or the onset of spontaneous magnetization of the spin-canted AF characteristics, and the bifurcation temperatures lowered for lower Co%.However, the peaks on the derivatives(e.g., -dFC/dT, Fig.S3b) could be observed only from Co88 to Co40.For each dilution member from Co88 to Co40, the peak temperatures on the -dZFC/dTand -dFC/dTplots at 10 Oe, as well as the dχ/dTat 100 Oe, could be fairly located and they are very similar (Table 3), though the peaks became broader upon lowering Co%.The averaged peak temperatures by these dc measurements defined theTN’s for the individual dilution members from Co88 to Co40, as given in Table 3.TheseTN(Co%) data, normalized byTN(100) of 14.2 K, i.e., the reducedTN(Co%)/TN(100), were plotted against Co% in Fig.3,showing linearity upon Co%.The linearity was fitted, then the percolation limit was obtained to be (Co%)P= 27(1)% (orxP=0.27(1)) by extrapolation.This value is slightly lower than the theoretical 31% for simple cubic lattice of site percolation according to percolation theory11, and this could be due to the anisotropy or Ising-like character of the present Co-Zn dilute magnetic system, which causes the higher values ofTN(Co%)/TN(100) as well as lower slope of the line, than Heisenberg dilute magnetic systems such as the Mn-Zn one11,25.The relevant peaks appeared inχ′ andχ′′ traces for Co88 to Co40 (Fig.S3c) confirmed the occurrence of long range magnetic ordering within the materials, and the temperatures for peak positions are close to theTN’s determined from dc measurements.
The isothermal magnetizations at 2 K for various Co% are shown in Fig.2b, d.Magnetic hystereses were clearly observed for Co88 to Co40, indicating the long range magnetic orderings.On lowering of Co%, the loop widths decreased, and theHC values continuously decreased from 5.21 kOe of Co88 to 0.29 kOe of Co40.TheHC value of Co88 in fact is slightly higher than Co100.The next member Co29 also showed a smallHCof 0.07 kOe.TheMRvalues first slightly arose from 0.031Nβ of Co100 toca.0.040Nβfor Co88 to Co48, then dropped to 0.029Nβof Co40, 0.0074Nβfor Co29, and further to nearly zero for Co19 and Co10 (Fig.2d), indicating again the suppressed magnetizations in LT and low field region, as observed in the temperature-dependent measurements aforementioned.In high field region (Fig.2b), for samples of Co% higher than 40% the magnetizations were nearly linear with field, typical for the antiferromagnetic ordering occurred in the samples.The magnetization plots gradually became convex as Co% decreased from 40%.This gradual convexness came from the contribution in magnetization from the isolated, non-coupled finite clusters and single Co2+ions and their increasing numbers when lowering Co%26.As can be seen from Fig.2d, with the increase of field, the suppression of magnetizations was gradually released, showing the turning region around Co% in 30%-40%and field ~2 kOe, and this also revealed the percolation limit of Co% ~ 30%.Above 2 kOe, the magnetizations increased with the decreasing Co%.For members of low Co%, the high field forced the magnetizations of uncoupled, isolated finite clusters and single Co2+ions to turn to the direction of the applied field,resulting in the increasing magnetizations.For Co10 of the lowest Co% in the series, the magnetization at 2 K and 50 kOe is 2.14Nβ, being close to the expected 2.2NβassumingS= 1/2 andg= 4.3 for octahedral Co2+ion in LT19,20.
4 Conclusions
The members of a diluted magnetic hybrid perovskite series of [CH3NH3][CoxZn1-x(HCOO)3] forx= 0-1 (or the molar percent Co% = 0-100) have been successfully prepared and characterized.Single-crystal and powder X-ray diffraction analyses revealed that in the full range the solid solution compounds are iso-structural, and the perovskite structures consist of simple cubic anionic metal-formate framework and the CH3NH3+cations locating in the cubic framework cavities.The cell parameters and respective molecular geometries within the series show very small changes less than 0.4%.The strong magnetic anisotropy of Co2+ion leads to an anisotropic or Isinglike diluted magnetic system.In low temperature and low field region, the system shows a significantly suppression of magnetization upon the lowering Co% orx, in opposite to the previously reported isotropic, Heisenberg diluted magnet series of [CH3NH3][MnxZn1-x(HCOO)3] under the same perovskite structure.The series shows systematical evolution from long range ordering of spin-canted antiferromagnetism to paramagnetism, and the percolation limit was estimated(Co%)P = 27(1)%, slightly lower than 31% by percolation theory for the simple cubic lattice.Besides, the incommensurate phase transitions have been primarily detected belowca.120 K for the pure Co and Zn members, which might add some effects on the magnetism.Further researches could include the studies on the low temperature incommensurate structures and investigations on other diluted and mixed magnetic metal perovskite systems, and these researches are in progress.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.

