Progress in the Mechanisms and Materials for CO2 Electroreduction toward C2+ Products
Yan Yang, Yun Zhang, Jin-Song Hu,*, Li-Jun Wan
1 Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Nanostructure and Nanotechnology, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P.R.China.
2 University of Chinese Academy of Sciences, Beijing 100049, P.R.China.
Abstract: Over the past decades, advances in science and technology have greatly benefitted the society.However, the exploitation of fossil fuels and excessive emissions of polluting gases have disturbed the balance of the normal carbon cycle, causing serious environmental issues and energy crises.Global warming caused by heavy CO2 emissions is driving new attempts to mitigate the increase in the concentration of atmospheric CO2.Significant efforts have been devoted for CO2 conversion.To date, the electroreduction of CO2, which is highly efficient and offers a promising strategy for both storing energy and managing the global carbon balance, has attracted great attention.In addition, the electrosynthesis of value-added C2+ products from CO2 addresses the need for the long-term storage of renewable energy.Therefore, developing catalysts that function under ambient conditions to produce C2 selectively over C1 products will increase the utility of renewable feedstocks in industrial chemistry applications.Recently, great progress has been made in the development of materials for electrocatalytic CO2 reduction (ECR) toward C2+ products; however, some issues (e.g., low selectivity, low current efficiency, and poor durability)remain to be addressed.In addition, the elementary reaction mechanism of each C2+ product remains unclear, contributing to the blindness of catalyst design.In this regard, the development of proposed mechanisms of ECR toward C2+ products is summarized herein.The key to generating C2+ products is improving the chances of C―C coupling.Test conditions significantly influence the reaction path of the catalyst.Thus, three different paths that that are most likely to occur during ECR to C2+ products are proposed, including the CO, CO-COH, and CO-CO paths.In addition, typical material regulatory strategies and technical designs for ECR toward C2+ products (e.g.crystal facet modulation, defect engineering, size effect,confinement effects, electrolyzer design, and electrolyte pH) are introduced, focusing on their effects on the selectivity,current efficiency, and durability.The four strategies for catalyst design (crystal facet modulation, defect engineering, size effect, and confinement effect) primarily affect the selectivity of the ECR via adjustment of the adsorption of reaction intermediates.The last two strategies for technique design (electrolyzer design and electrolyte pH) contributing greatly toward improving the current efficiency than selectivity.Finally, the challenges and perspectives for ECR toward C2+products and their future prospects are discussed herein.Therefore, breakthroughs in the promising field of ECR toward the generation of C2+ products are possible when these catalyst design strategies and mechanisms are applied and novel designs are developed.
Key Words: CO2 electroreduction;High value-added product;Selectivity;Faradaic efficiency;Durability
1 Introduction
Nowadays, energy issues and environmental issues are attracting widespread attention, which are the two major problems that restrict and influence the future development of mankind1-4.The main core of these two issues is how to efficiently use and manage carbon resources in the carbon recycle.As schematically illustrated in Fig.1a, dioxide (CO2) in the atmosphere released from the combustion of traditional fossil and chemical fuels is captured, and then with the addition of water it is converted into high-value chemicals and fuels through electrochemical electrolyzer.The achieved products are well stored, transported, distributed, and then consumed to give off the main industrial waste of CO2.After that, it is again captured into the reactor to close the cycle5-9.In view of this, delicately keeping the balance between carbon emission and consumption is very vital to maintain carbon neutral circulation.However, due to excessive exploitation of fossil fuels including coal,petroleum, and natural gas, CO2emissions have been soaring year by year, causing a serious damage to the natural carbon cycle.Therefore, it is extremely urgent to search for low-cost,clean, and high-efficiency approaches to eliminate excess CO2,getting a sustainable carbon neutral cycle10,11.
Among developed methods (e.g., biological, thermochemical,photochemical)12-14, electrocatalytic CO2reduction (ECR) has unique advantages over other competitive technologies,including the large-scale electrolytic cell designs, the high reaction efficiency and low energy-consumption to produce varieties of high-value products.Interestingly, it can be readily powered by carbon-free green energy sources such as wind solar,and nuclear15-17.Energy stored by the product is further realized through chemical bonds to form a green and sustainable closed carbon cycle, which attracts considerable attention.Based on above features, there has been a rapid increase in the number of publications concerning ECR in the last decades (Fig.1b).
Since the carbon has different valence states and complex C―C coupling in the reaction process, different transformation paths can occur simultaneously.Hence, the products are diversified in ECR.The most common products are C1products,mostly being the carbon monoxide (CO) and formic acid(HCOOH), both of which are not readily used fuels for applications.For example, CO needs to be downstream processed with H2by Fischer-Tropsch chemistry to synthesize the basic organic chemical products or intermediates18.In particular, compared with common C1products, C2+products enclosing the features of high energy density and added-value,like C2H4 and C2H5OH, can be well compatible with existing infrastructures and show great capabilities to substitute for fossil fuels.Moreover, hydrocarbons with unsaturated bonds are preferred to be feedstocks in polymer synthesis.For instance,ethylene (C2H4) owing a carbon double bond can participate in chain growth reactions; ethanol (C2H5OH) can act as a raw material for organic synthesis like ether, acetaldehyde and acetic acid.After several years of development, the huge progress has been achieved for ECR toward C2+products but there still present some scientific challenges as follows, which need to be addressed primarily in further works.
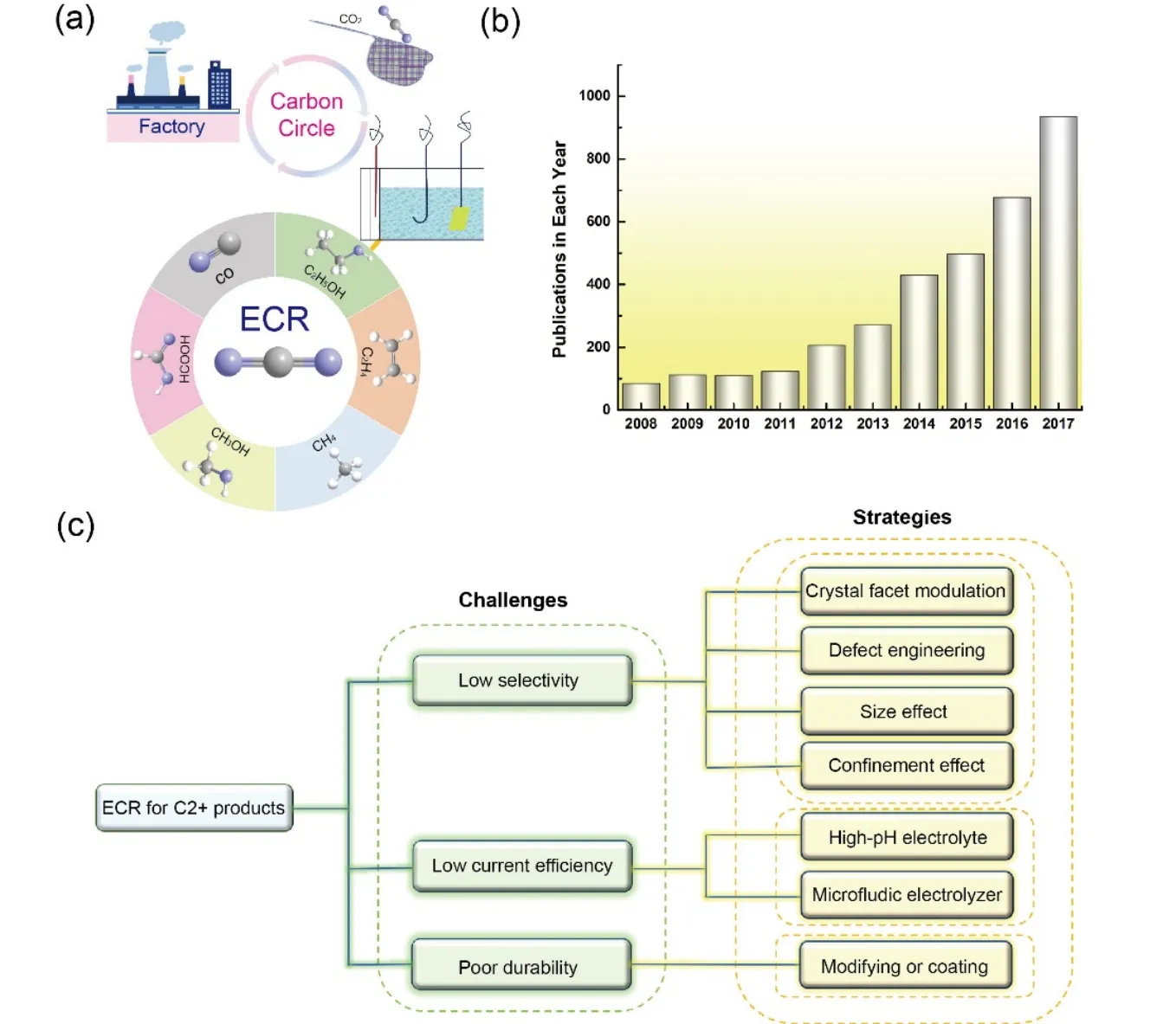
Fig.1 (a) Schematic illustration of carbon cycle.(b) Number of publications concerning electrochemical reduction of CO2 in the last decades.(c) Scientific challenges and proposed strategies on ECR toward C2+ products.
(1)Low selectivity.During ECR process, there is a general competitive side reaction, hydrogen evolution reaction, one part of electrocatalytic water splitting.Additionally, ECR is a multiple-electron transfer and multiple-pathway process.Therefore, it often gives rise to a mixed C1/C2+products due to the close thermodynamic redox potentials for different reaction pathways, thus showing a poor selectivity for a target product,especially the C2+product19-21.
(2)Low current efficiency.Traditionally performance tests for ECR are usually carried out in H-cell configuration and neutral or low pH electrolyte.The mass transfer and diffusion process of CO2, as the first step of ECR, have an essential impact on current density.Owing to a low solubility of CO2in liquid electrolyte, the achieved current density in many laboratoryscale cases is limited to be on the order of merely 1-10 mA·cm-2.However, commercially relevant current density of 100-1000 mA·cm-2is highly preferable22-24.
(3)Poor durability.From the perspective of practical implementations, durability is another key parameter beyond activity and selectivity for real-world catalysts.During ECR process, catalysts are easily deactivated under large current densities.This may mainly ascribe to the closely adsorption of active intermediates on active sites or the morphological changes driven by the negative potential, which eventually induces the catalyst degradation under sufficiently negative potential25-28.
At this juncture, the key requirement for ECR to be commercially viable is to develop proficient catalysts that can achieve excellent activity, high selectivity, and good stability toward valuable products.Therefore, the aim of developing efficient and useful ECR technologies for C2+production should focus on advancing the selectivity, current efficiency, and durability as well as researching the mechanisms involved with elementary reactions.In this review, we first present advancements of mechanism of ECR toward C2+products to establish an optimized material system.Then strategies on material designs as well as technical methods directing at tackling scientific challenges are given, as shown in Fig.1c.In the end, we make a summary and put forward a prospect of the future catalyst design based on previous mechanism and layout of catalysts.
2 Proposed mechanisms for ECR toward C2+products
The common route for catalyst discovery cannot do without long feedback loops of kinetics simulation, coupled with experimental and characterization data.However, a large number of studies show that significant breakthrough is often the result of deeper understanding of reaction mechanisms, and the maturity of mechanism makes the catalyst design less detour.Therefore, it is of great importance to propel deep research on mechanisms for ECR toward C2+products.Among developed catalysts of ECR, Cu-based materials have been intensively studied as model catalysts to probe the mechanistic pathway for ECR toward C2+products due to its high intrinsic activity to produce appreciable C2+products29.With respect to mechanistic investigations of CO2reduction on Cu, the main object of study is CO, which is the essential reaction intermediate in the initial stage of ECR.Experiments revealed that the product distribution of CO reduction was as same as that of CO2reduction30,31.Therefore, most DFT calculations about mechanisms for ECR toward C2+products focused on CO.As we all know, the process that electron transfers to adsorbate is thought to have low kinetic barrier32.The key to achieving C―C coupling is to overcome the barrier of C―C bond formation, which limits the rate and selectivity of C2+products.
Petersonet al.33firstly pointed out that the role of high applied potential in C―C coupling.It was found that C―C coupling step about possible reaction paths from C1to C2is feasible at room temperature through the nudged elastic band(NEB) calculations.However, direct dimerization of adsorbed*CO needs a high barrier and it is unfavorable from kinetic perspective.Moreover, it can be clearly seen that the barrier of dimerization of adsorbed *CO is still very high under an electric field.Fig.2a shows a free energy diagram of different reaction approaches to C―C coupling.These kinetic barriers of reaction intermediates suggest that *CO dimerization is kinetically unfavorable.However, making a single proton-electron pair transfer to *CO intermediates to form *CHO could promote the occurrence probability of coupling to other *CO derived intermediates.Namely, it is more stable for protonation of *CO to form *CHO.These results suggested C―C coupling would be more biased to occur at high applied potential.

Fig.2 (a) Free energy diagrams for C―C coupling.Reproduced with permission33.Copyright 2013, Wiley-VCH Verlag GmbH & Co.KGaA,Weinheim.(b) Lowest energy pathways for the electroreduction of CO.Reproduced with the permission34.Copyright 2013, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.(c) Proposed reaction pathways to produce CH4, CH3OH, and C2H4 for ECR on Cu(111).Reproduced with the permission35.Copyright 2013, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.(d) Pathways of CO reduction in different pH.Reproduced with permission38.Copyright 2016, American Chemical Society.
Later, Koper and his co-workers34proposed that the ratedetermining step on Cu(100) is coupling of two CO molecules accompanied by electron transfer to form *C2O2viathe Computational Hydrogen Electrode model.Fig.2b displays the lowest energy pathways and a schematic diagram for the electroreduction of CO to EtOH or C2H4.The ECR toward EtOH at 0 and -0.40 V firstly undergone the reduction of CO, then the constant protonation of intermediates through an interaction between proton and electron, and in the end escaped from surface.During this process, it was discovered that dehydration of OH groups became more reactive in reducing environments than O atoms in carbonyl groups.Meanwhile, C2H4 shared the same pathway as that of EtOH up to the fifth electrochemical steps but EtOH is inclined toward C2H4formation by approximately 0.2 eV.However, there exists some limitations that the model is purely based on thermodynamic level, ignoring the influence of reaction barriers and solvation.
Based on previous experimental studies as well as DFT works by Peterson and co-workers, Nieet al.35used DFT model to study reaction kinetics of elementary steps of ECR in 2013,indicating that the reduction of CO was the key selectivitydetermining step for ECR on Cu(111) and COH played a critical role in forming methane/ethylene.C2H4 was produced through nonelectrochemical CH2 dimerization.Electron-ion transfer reactions were taken into account for the first time.Their proposed reaction pathways were shown in Fig.2c.Though it was contrary to early experimental belief on the formation pathway of methane and ethylene36,37, the role of water was considered.Moreover, it was also underlined that the liquid phase environment and the applied potential were essential for the increase of COH intermediate content and the formation of methane/ethylene.Recently, Xiaoet al.38predicted the atomistic mechanisms for pathways of products during CO reduction at different pH conditions, as detailed illustrated in Fig.2d.At acidic pH, COH or CHO was formed through hydrogenation of absorbed CO, subsequently COH reached to C1 products through either removing the OH group or the CHOH path, while CHO got to C1products through the hydrogenation of O atoms in carbonyl groups.Compared with C1products, the formation of multi-carbon species was preferred at neutral pH and high pH conditions.Specifically, C―C coupling was realized by a novel CO-COH pathway at neutral pH, instead, adsorbed CO dimerization dominates favored developing C2 (C3) products at high pH.Furthermore, it was also confirmed to have an excellent agreement with experimental results.That is, tailoring pH and applied potential properly could boost selectivity of multicarbon products.According to the above discussions, a clear mechanism on the pathway of ECR toward C2+ products was summarized as shown in Fig.3.
3 Material design strategies for ECR toward C2+ products
With the rapid development of nanotechnologies and theoretical calculations, the material design strategies for ECR toward C2+ products gradually diversify, accompanied by the emergence of a large number of efficient catalysts.Reported ECR toward C2+performance on different materials are summarized in Table 1.It is a fact that copper is the appealing metal, which is capable of further reducing CO to hydrocarbons and alcohols in quantitatively reproducible amounts20,39.Hence,Cu-based catalysts, including nano-structure copper40,41, copper alloy42-44, copper oxide45,46, copper sulfide and so on, are the dominating materials for selective reduction of CO2 to multicarbon hydrocarbons47.In addition, it has been also reported metal-free nitrogen-doped carbon materials can achieve high selectivity for ethanol48.It is known that the performance of catalysts can be tunedviavarious material design strategies through altering size, structure, composition, surface state, and so on.Based on this, we summarize some typical material design strategies in this section with emphasis on discussing their impacts on the structure of catalysts, then their electrochemical properties.
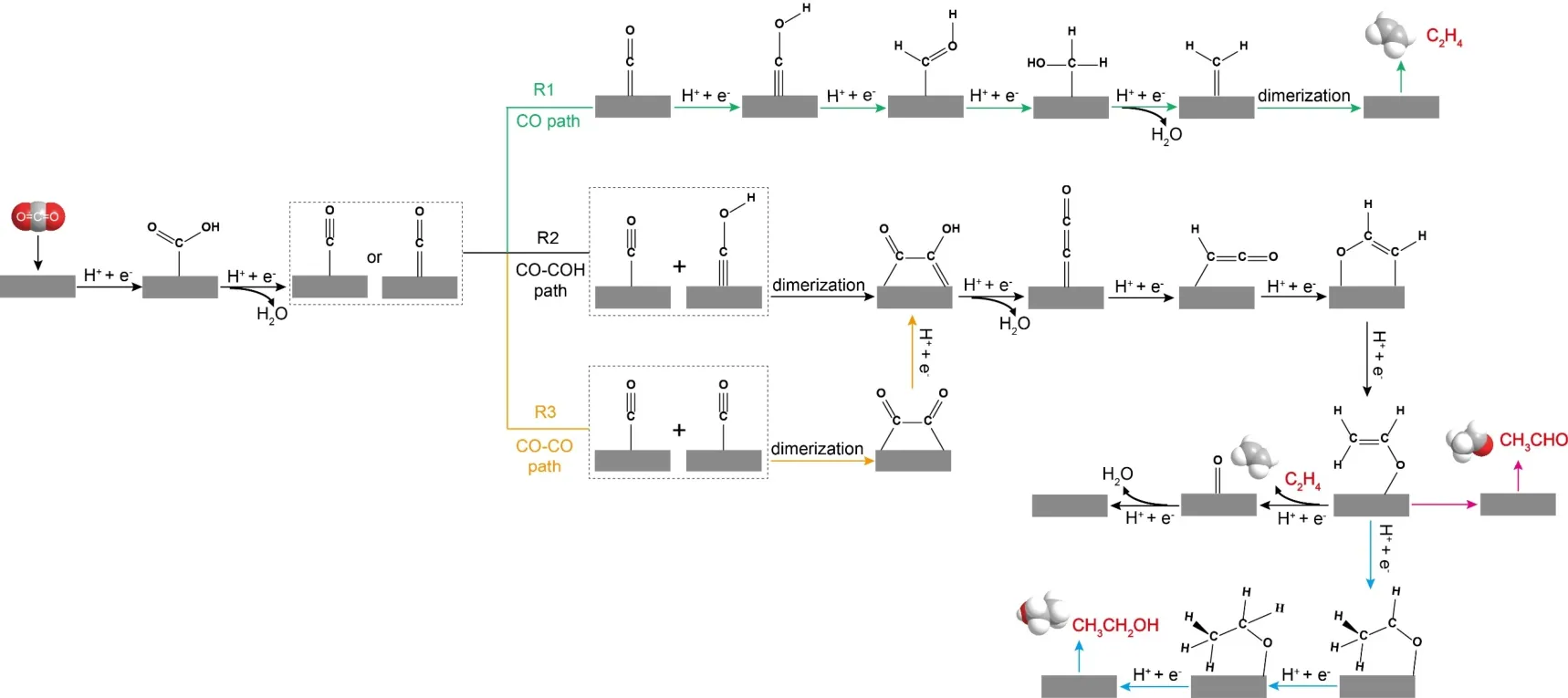
Fig.3 Three different paths for ECR toward C2+ products.
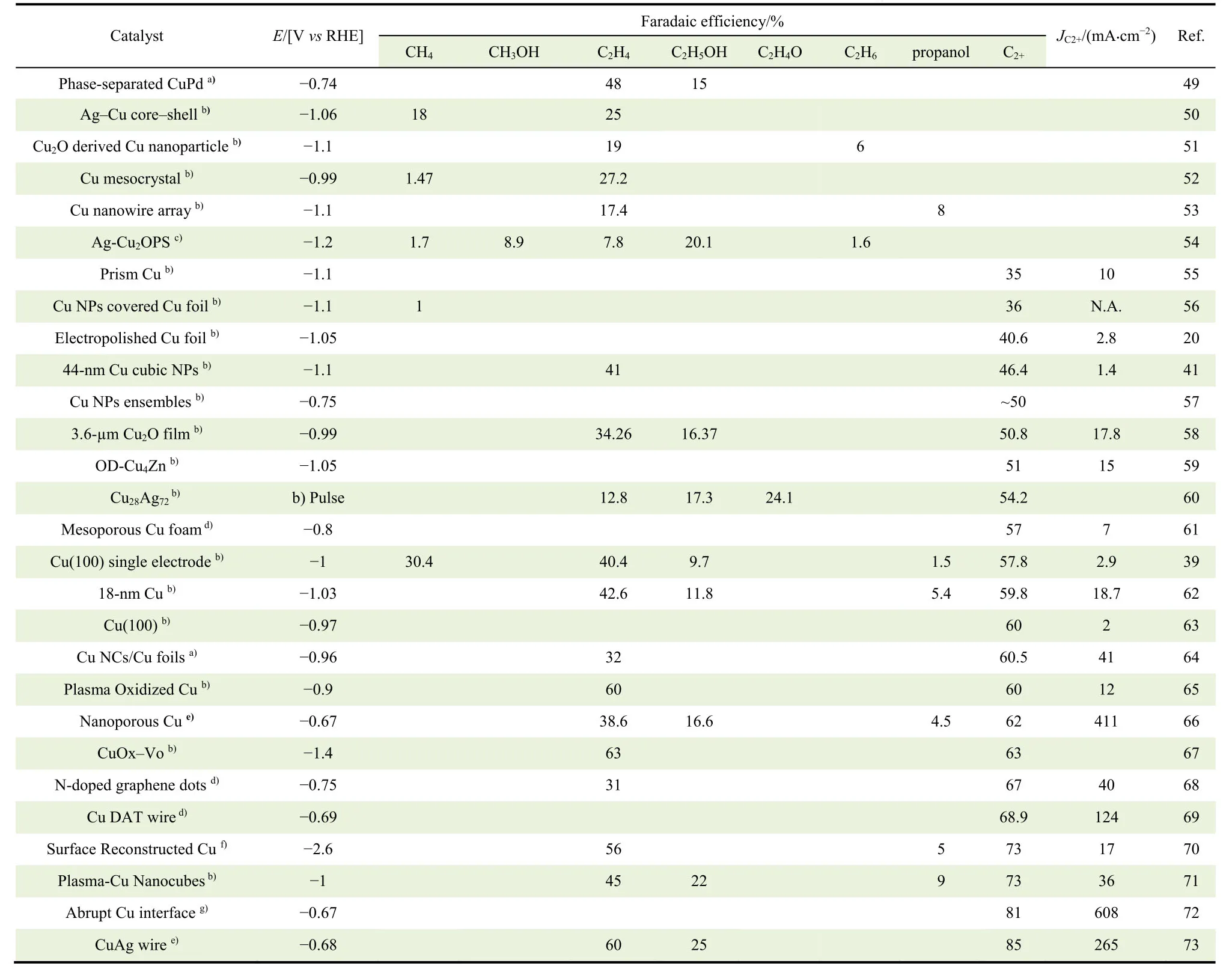
Table 1 Summary of ECR toward C2+ performance on different catalysts.
3.1 Crystal facet modulation
Generally speaking, the exposed active surface plays a very important role in the electrocatalytic reaction74,75.Particularly,the introduction of low-coordinated plane of open-structure surface, which has high density of atomic steps and kinks,normally improves reactivity of some electrocatalytic reactions76,77.Therefore, atomic-level-engineering of surface structure is an efficient way to precisely manipulate the exposure of active sites and subsequently enhance corresponding electrocatalytic activity.In the past decades, much effort has been made to study the intrinsic relationship between surface structures and electrocatalytic properties, such as electrooxidation of small organic molecules and CO,electroreduction of O2and CO278.Meanwhile, it is also found that the product selectivity highly depends on surface atomic arrangements and step atoms, especially the kink atoms77,78.
Metal single-crystal planes can provide a variety of surface structures with well-defined atomic arrangements, depending on their orientations.Therefore, they are usually employed for researching the sensitive reactions on heterogeneous surfaces.For example, a series of different copper single crystal electrodes usually are used.Hori and his co-workers found the product selectivity of ECR varied greatly with the crystal orientation39.Cu(100) benefited the C2H4 production whereas Cu(111) favored the CH4formation.After this study, a larger number of studies on C2+products formation focused on Cu(100).Recently, Jianget al.64developed an efficient Cu nanocube catalyst with significantly improved C2+ selectivity through a combined theoretical and experimental approach (Fig.4a-e).Theoretically,the energetics of the initial C―C coupling steps on different Cu facets in CO2reduction was calculated and the result showed that the presence of Cu(100) and stepped (211) facets could favor C2+product formation over Cu(111).Experimentally, it is demonstrated that the facet exposure on Cu foil could be adjustedviametal ion battery cycling method.As a result, 100-cycled Cu nanocube catalyst presented the highest selectivity of C2+products in terms of a Faradaic efficiency of over 60%,which has been attributed to the abundant exposed (100) facets.
3.2 Defect engineering
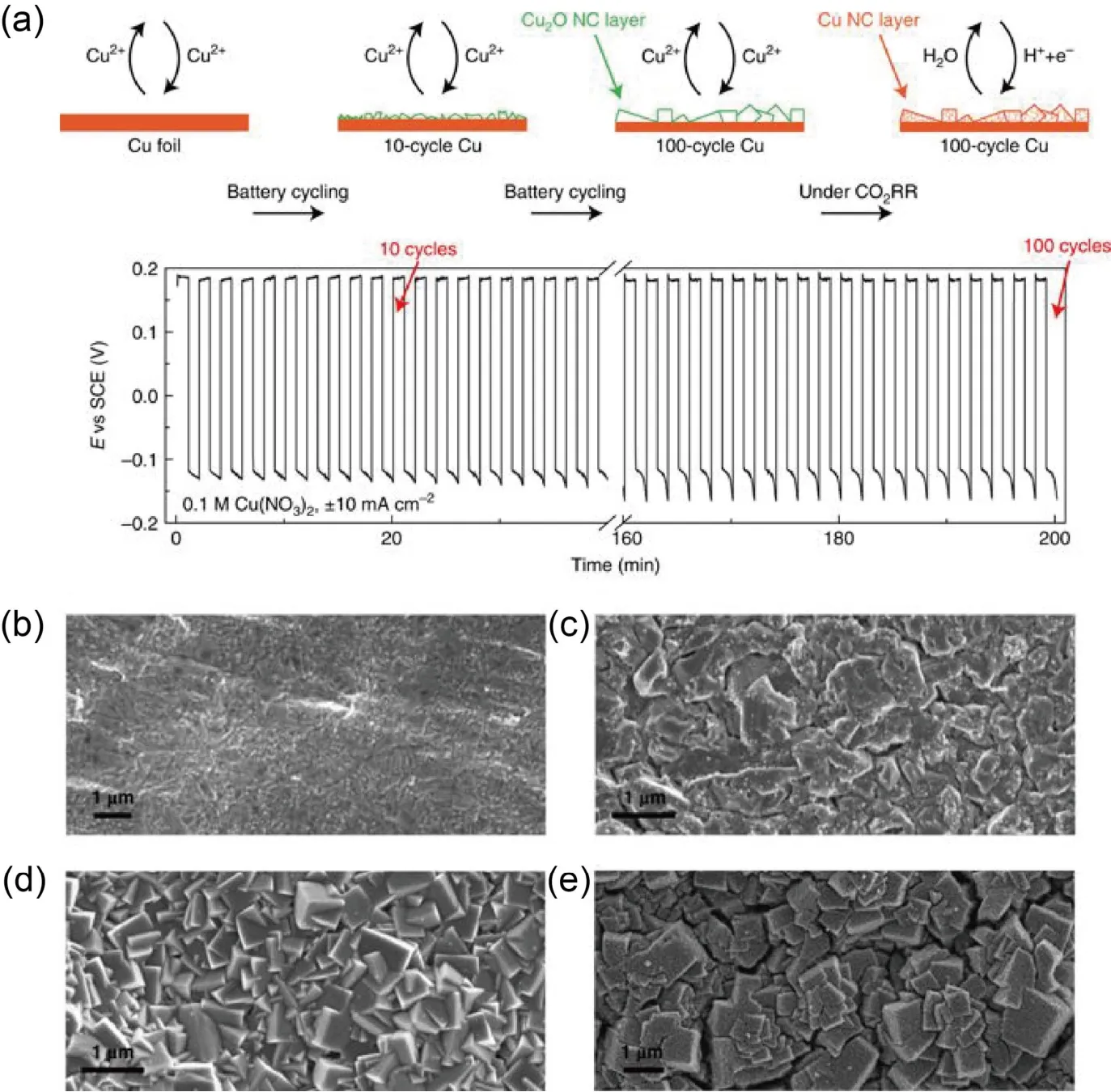
Fig.4 (a) Schematic of the Cu2+ ion cycling method for Cu2O and Cu nanocube on Cu foils.(b-e) SEM images of the pristine Cu, 10-cycle Cu,100-cycle Cu, and 100-cycle Cu after prereduction under ECR condition, respectively.Reproduced with the permission64.Copyright 2018, Springer Nature.
Defect engineering is a powerful approach to modify surface and enhance surface reactivity, which has been widely studied in electrocatalysts, such as heteroatom-doped materials, singleatom catalysts, and metal oxides79.Defects can vary the coordination environment of surface and subsurface atoms, and bring the charge imbalance near the surface to enhance the electron transfer, further effectively tuning the adsorption of surface species for catalysis80-84.For instance, the step/edge sites of nanoparticles or grain boundary between mental and carbon support can establish unsaturated environments to promote reactivity85.The defects, which are considered as any inhomogeneous compositions in a material, include dopants,atom/ion vacancies, grain boundaries, and so on79.Specific effective methods have been applied to introduce these defects,such as doping86-88, etching89, recombination90-92and pretreatment93.
During ECR process, atomic defects (e.g., oxygen vacancies or sulfide vacancies), are often introduced to improve reactivity.For example, a class of core-shell vacancy engineering catalyst(Cu2S-Cu-V) was reported by Sargent’s Group47through introducing sulfur into the Cu structure to form a modified Cu2S core with Cu surface vacancies.Even if the path toward ethylene and ethanol shares a penultimate reaction intermediate *C2H3O,DFT calculations and experimental results suggested that the Cu2S-Cu-V structure could steer products beyond alkenes and toward alcohols at certain potential, achieving a C2+alcohol production rate of (126±5) mA·cm-2with a high selectivity in terms of Faradaic efficiency of (32±1)%, as shown in Fig.5a.The finding indicates the introduction of metal atom vacancy turns out to be an alternative approach to target the suppression of unwanted C2products, rather than just C1products.
In addition, Leeet al.45developed the Cu electrocatalyst with mixed copper states through adjusting the applied potential and period of time by a simple electrochemical method.It exhibited high Faradaic efficiency of 38.1% for ethylene production at-1.08 V and excellent stability over 40 h.This good performance was attributed to mixed oxidized copper species along with high oxygen content in catalyst.Moreover, Zheng’s group67recently developed a kind of partially reduced copper oxide nanodendrites catalyst with rich surface oxygen vacancies(CuOx-Vo), probing that oxygen vacancies could serve as Lewis base sites to enhance CO2adsorption and have strong binding affinities to intermediates of *CO and *COH, but weak affinity to *CH2, contributing to the formation of C2H4with a high Faradaic efficiency (63%).In fact, not only the molecular oxygen or more reactive oxygen plasma species, but also chlorine, bromine or sulfur could oxidize Cu giving rise for the formation of cuprous/cupric precursor.Based on the similar concept, Sargent’s Group recently developed a facile surface reconstruction approach to construct a Cu(0)/Cu(I) interface,reaching Faradaic efficiency of 73% for C2+products70.Schematic illustration of the surface reconstruction process is shown in Fig.5b.
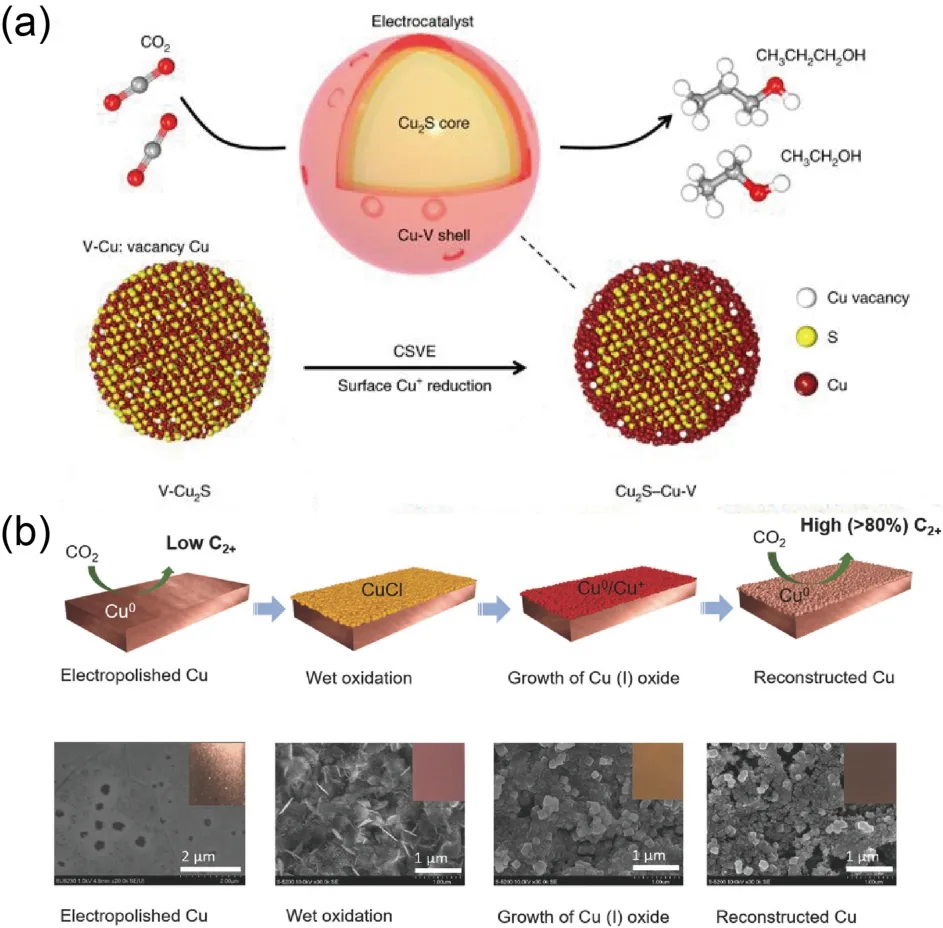
Fig.5 (a) Schematic illustration of Cu2S-Cu-V catalyst design.Reproduced with the permission47.Copyright 2018, Springer Nature.(b) Schematic illustration of the surface reconstruction process and the corresponding SEM images.Reproduced with the permission70.Copyright 2018, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.
3.3 Size effect
Another well-known morphology-based strategy is to vary the size of the catalytically active species (active site number and intrinsic activity), which tunes the surface absorption of reaction intermediates and resulting catalytic reactivity.As the size of nanoparticles decreases, the specific surface area get larger, and while dropping into below around 2 nm, quantum effects become noticeable, which is referred to as the “catalytic finitesize effect”94-96.It has been reported that even if mental nanoparticles of finite-size have higher mass activities, it is preferred for H2evolution and harmful for ECR97.Size effects had been widely reported for excellent ECR performances97-100.
For example, the effect of size of Cu nanocrystal on their CO2 electroreduction activity and product selectivity was studied in recently by Loiudice and co-workers41.They researched the ECR activity of three differently sized Cu cubes (24, 44, and 63 nm), revealing the Cu cube in a size of 44 nm exhibited the best performance for ECR in terms of Faradaic efficiency of 41% for ethylene at -1.1 V, as shown in Fig.6.They claimed that that such a unique performance derived from an optimal balance between plane- and edge- sites of nanostructured Cu nanocrystal,and predicted that a high density of edge atoms might have a large potential to further improve the selectivity toward C―C coupling for a high C2+product yield.
3.4 Confinement effect
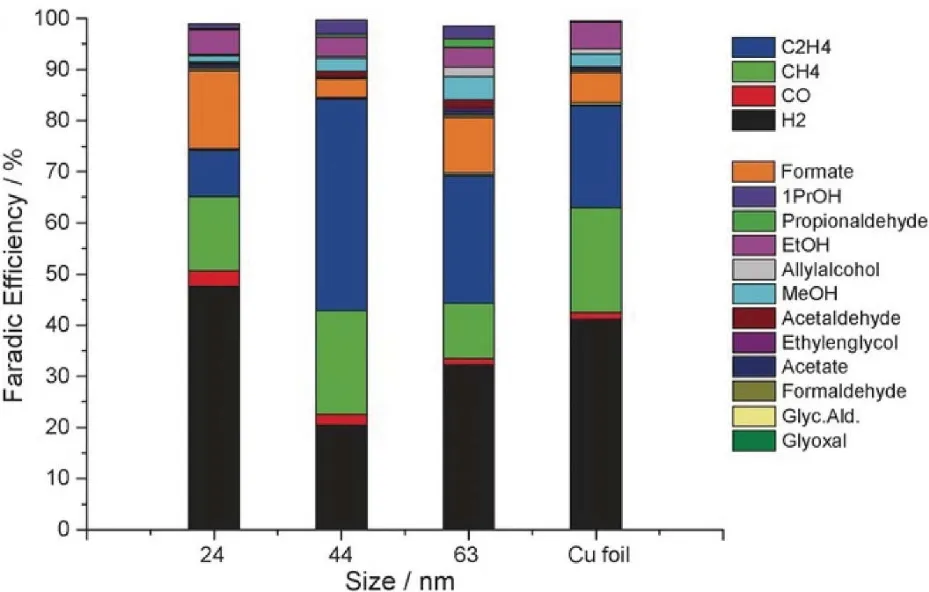
Fig.6 The Faradaic efficiencies of each product of Cu nanocubes in different size as well as the control Cu foil.Reproduced with the permission41.Copyright 2016, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.
Confinement effect is a new concept in heterogeneous catalysis.During reaction process, the micro-environment of catalytic system could restrict the physical and chemical state,thus effectively regulating the catalytic performance.For ECR,confinement effect could largely affect the product selectivity.It has been reported that decreasing the interparticle spacing of a constant nanoparticle size increased the selectivity of CH4and C2H4for ECR, which should be attributed to the increase of the lifetime of CO intermediate and the opportunity of being further reduced101.Recently, Nam’s Group studied the morphological effect of Cu mesopore electrode on the selective production of C2products (Fig.7)102.Interestingly, as the pore width decreased, the production of C1product was reduced whereas that of C2H4product was enhanced.By analyzing specific activities and performing electro-hydrodynamics study, it was found that the reaction intermediates were physically confined in the mesopores, which affected both local pH and flow field,thus, accelerating the C―C coupling reaction and prolonging retention times.These results demonstrated that the growth of C1intermediate concentration within local domain was beneficial to the formation of C2+ products.Recently, Sargent’s Group further applied the confinement effect to boost C3 production by extending the retention of C2species103.It was found that the selectivity of catalyst from C2to C3products was dependent on nanocavity morphology.
4 Other strategies for ECR toward C2+products
4.1 Electrolyzer design

Fig.7 Schematic illustration of Cu mesopore electrodes and the SEM images of samples in different mesopore with 30 nm width/40 nm depth (30/40 nm), (30/70 nm), (300/40 nm), from left to right respectively.Scale bar of 60 nm for the first and second images, and scale bar of 300 nm for the third image.Reproduced with the permission102.Copyright 2016, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.
Under ambient temperature, the continuous-flow CO2electrolyzers normally have two classifications in terms of different reaction environments, namely, liquid-phase reaction electrolyzer (type 1) and gas-phase reaction electrolyzer(type 2)104.for type 1 electrolyzer, it was operated with a liquid CO2 supplement for the cathode where CO2 was either dissolved or in ionic form.However, the cathode of this electrolyzer was limited by CO2supplement, which leads to a restriction in current density, making it difficult to be applied in practical field.For type 2 electrolyzer, it was performed with a dry concentrated CO2 supplement for the cathode incorporated with a gasdiffusion layer.Compared with the type 1 electrolyzer, the type 2 electrolyzer normally made the catalysts own a better catalytic performance for ECR toward C2+products.Firstly, in type 2 electrolyzer, ECR occurred at the three-phase-interface where CO2gas, electrolyte, and catalyst layer were directly contact;Subsequently, immediate CO2supplement enabled a fast transfer for both proton and electron, maximizing the current density and durability.Moreover, a good deal of studies based on the type 2 electrolyzer discovered that the catalytic performance was improved by many orders of magnitude.Recently, Feng Jiao’s Group66used a microfluidic electrolysis cell to explore the nanoporous Cu catalyst with a pore size of 100-200 nm.As a result, it exhibited a current density of 653 mA·cm-2with a C2+product selectivity of about 62% at an applied potential of -0.67 V.Additionally, in an H-cell configuration the reconstructed Cu catalyst reported by Sargent displayed a Faradaic efficiency of 73% for C2+products with an overall current density of 17 mA·cm-2.However, in a flow-cell configuration the Faradaic efficiency and overall current density were increased to 84% and 400 mA·cm-2, respectively.From above results, it can be clearly seen that the type 2 electrolyzer displays a large potential for commercialization and global deployment.
4.2 High-pH electrolyte
Normally, small changes in local proton concentration could induce a dramatic effect on the interfacial pH, which changes the surface coverage of OH and subsequently brings alteration of product selectivity for ECR30,36.It is proposed that the presence of OH adsorbed will reduce the adsorption of H+, leading to the decrease of H2and CH4106.During ECR process, the pH near the electrode surface is higher than that in bulk electrolyte due to the constant consumption of proton.A mathematical study by Guptaet al.105pointed out that during ECR the local pH was up to 6 units higher than in the bulk pH.The earliest study about the pH’s effect on the product selectivity was made by Horiet al30.They discovered that the formation of CH4 depended on proton concentration, while that of ethylene was not affected by the pH.Later, similarly, Koper and his co-workers investigated the influence of pH on the reduction of CO and CO2on Cu(100) and Cu(111)107, finding that the onset potential for ethylene, in particular on Cu(100), did not depend on the pH on the NHE scale, instead the formation of CH4 was dependent on the pH,which is agreeable with the results of Horiet al30.However, in previous studies, the regulation of the pH was only performed in phosphoric acid buffer system and unable to directly observe the effect on molecular influence of hydroxide.Recently, Sargent’s Group studied the direct molecular effects of hydroxide on ECR72, discovering that the onset potential for ECR toward ethylene shifted markedly to more positive potentials with increasement of KOH concentrations.This indicated that hydroxide ions adsorbed or adjacent to metal surface may play an important role in modulating the active catalytic sites.
5 Summary and perspectives
In this review, we have briefly summarized the recent research advances on mechanisms and materials design strategies for ECR toward C2+products.However, to achieve the target of utilizing renewable energy to close carbon circle through CO2conversion, serval key challenges still exist and must be overcome as follows (Fig.8):
(1) In terms of selectivity, although the yield of C2+products is higher than previously reported, the selectivity of single product among C2+products is still poor, which is failing to meet practical needs and brings a new challenge for the mechanism of single product production.In situtechnologies may make it possible to help to identify the configuration of key intermediates and find out preferred reaction pathways.Therefore, a concerted and interdisciplinary effort need to be made to apply advanced characterization techniques to investigate CO2RR under real operating condition.
Fig.8 Schematic perspective for future ECR toward C2+ products.
(2) With respect to the current efficiency, it is an efficient solution to increase the number of the intrinsic active sitesviadefects, doping and so on.However, so as to close the gap between laboratory discovery and industrial needs, adverse effects of mass transfer factors, that is, the limitation of solubility of CO2in electrolyte is most responsible to low current efficiency, which has high requirements to design the structure of gas diffusion electrode and optimize the property of threephase contact interface.Besides, due to a several-fold higher CO2solubility than water, some organic solvents and ionic liquids can be served as an alternative reaction medium.
(3) In regard to the durability, it remains a big challenge so far.A large number of experiments discovered the stability of catalysts will drop a lot compared to the initial activity as time extended, the internal cause is still ambiguous65.For a high stability, it will be better to modulate the chemical and physical properties of active materials through various methods (such as,doping, defecting, and confining), meanwhile, which may improve the catalytic activity of materials.In addition to improve the stability of catalyst body, the durability of interaction between electrode surface and catalyst is also very key for an excellent catalytic performance.Coating or compositing with some materials may be an efficient way to improve the electrode stability.
From the perspective of practical application, further research is largely required to develop efficient strategies to increase CO2concentration at catalytic interface, product selectivity at high current density, and durability of electrode.With continuous efforts in exploring multifunctional material as well as electrolyzers, the realization and optimization of ECR toward C2+products may provide an insight into the field of energy storage and utilization.

