Progress and Prospects of Non-Metal Doped Graphitic Carbon Nitride for Improved Photocatalytic Performances
Yiqing Wang, Shaohua Shen
International Research Center for Renewable Energy, State Key Laboratory of Multiphase Flow in Power Engineering,Xi’an Jiaotong University, Xi’an 710049, P. R. China.
Abstract: Since Fujishima and Honda demonstrated the photoelectrochemical water splitting on TiO2 photoanode and Pt counter electrode, photocatalysis has been considered as one of the most promising technologies for solving both the problems of environmental pollution and energy shortage. This process can effectively use solar energy, the most abundant energy resource on the earth, to drive various catalytic reactions, such as water splitting, CO2 reduction, organic pollutant degradation, and organic synthesis, for energy generation and environmental purification. Except for the various metal-based semiconductors, such as metal oxides, metal sulfides, and metal oxynitrides, developed for photocatalysis, graphitic carbon nitride (g-C3N4) has attracted significant attention in the recent years because of its earth abundancy, non-toxicity,good stability, and relatively narrow band gap (2.7 eV) for visible light response.However, g-C3N4 suffers from insufficient absorption of visible light in the solar spectrum and rapid recombination of photogenerated electrons and holes, thus resulting in low photocatalytic activity. Until now, various strategies have been developed to enhance the photocatalytic activity of g-C3N4, including element doping, nanostructure and heterostructure design, and co-catalyst decoration. Among these methods, element doping has been found to be very effective for adjusting the unique electronic and molecular structures of g-C3N4, which could significantly expand the range of photoresponse under visible light and improve the charge separation. Especially, non-metal doping has been well investigated frequently to improve the photocatalytic activity of g-C3N4. The non-metal dopants commonly used for the doping of g-C3N4 include oxygen (O), phosphorus (P), sulfur (S), boron (B), and halogen (F, Cl, Br, I) and also carbon (C) and nitrogen (N) (for selfdoping), as they are easily accessible and can be introduced into the g-C3N4 framework through different physical and chemical synthetic methods. In this review article, the structural and optical properties of g-C3N4 is introduced first, followed by a brief introduction to the modification of g-C3N4 as photocatalysts. Then, the progress in the non-metal doped g-C3N4 with improved photocatalytic activity is reviewed in detail, with the photocatalytic mechanisms presented for easy understanding of the fundamentals of photocatalysis and for guiding in the design of novel g-C3N4 photocatalysts. Finally,the prospects of the modification of g-C3N4 for further advances in photocatalysis is presented.
Key Words: Graphitic carbon nitride (g-C3N4); Non-metal doping; Photocatalysis; Band structure; Electronic structure
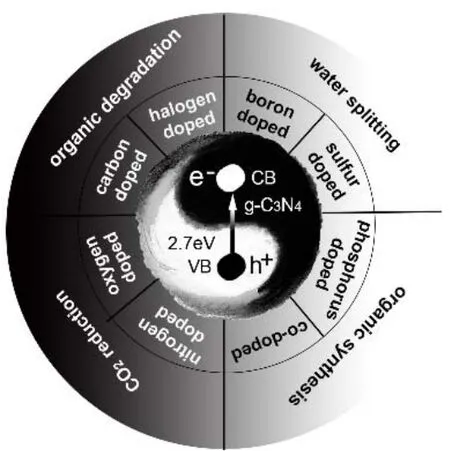
1 Introduction
The growing global energy shortage and environmental pollution have been seriously threatening the sustainable development of human society. Utilizing the clean and inexhaustible solar energy is undoubtedly the most ideal way to solve the above-mentioned problems. Since the seminal demonstration on photoelectrochemical water splitting using TiO2as semiconducting photoanode by Fujishima and Honda1,semiconductor-based photocatalysis has emerged as an alternative and promising technology for solar energy conversion and utilization by driving different catalytic reactions in a variety of applications, such as organic degradation2-6,water splitting7-10, CO2and N2reduction11-15, and organic synthesis16-18. Until now, various types of semiconductors have been developed and widely studied for photocatalysis, mainly including metal oxides (such as TiO21,19-21, ZnO22-24, Fe2O325,etc.), metal sulfides (such as CdS26-34, ZnS22,26,28,30,35,etc.) and metal (oxy)nitrides (such as Ta3N536,37, TaON36,38-40,etc.).However, these developed semiconductor photocatalysts have their own intrinsic deficiencies, limiting their applications in applicable photocatalytic reactions. For example, the most studied TiO2photocatalyst, with a large band gap of ~3.2 eV can be only activated by the 4% ultraviolet light in the solar spectrum. Although CdS has a narrow band gap of ~2.4 eV for efficient absorption and utilization of the visible light (λ< 600 nm) in solar spectrum, its poor photostability and high toxicity of Cd element can hardly satisfy the highly stable and environmentally friendly solar energy conversion. Therefore, it is highly desired to find a photocatalyst with suitable band gap and good stability for efficient and stable photocatalytic reactions; and meanwhile this photocatalyst should be also noble and toxic element free and easily synthesized for large scale and low-cost applications. Fortunately, in 2009, Wanget al.reported for the first time that a new non-metallic semiconductor polymer,graphitic carbon nitride (g-C3N4), can photolyze water to produce hydrogen under visible light41. Since then, g-C3N4has become one of the most popular photocatalysts for different photocatalytic reactions, due to its relatively narrow band gap,high chemical stability and earth abundancy42,43.
2 Brief introduction to g-C3N4 photocatalyst

Fig. 1 Diagrams of g-C3N4 single layer composed of (a) triazine structural unit, (b) heptazine structural unit (1 Å = 0.1 nm).
As a metal-free polymer semiconductor, polymeric carbon nitride composed of two earth-abundant non-metal elements (C and N), has five different phases, including α-C3N4, β-C3N4,cubic C3N4, pseudo cubic C3N4, and g-C3N4. In comparison to other four phases, g-C3N4is the most thermodynamically stable,with the basic single-layered structure composed of triazine structural unit (Fig. 1a) and heptazine structural unit (Fig. 1b).The heptazine ring-based g-C3N4structure is more stable than the triazine ring-based g-C3N4structure, as predicted by density functional theory (DFT) calculations44,45. As a direct band gap organic semiconductor, g-C3N4exhibits optical absorption onset at about 420 nm, with band gap determined to be 2.7 eV, which makes g-C3N4itself yellowish41. As revealed by DFT calculations on the band structure of g-C3N4, the lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) are composed of carbon atomicPzorbital and N atomicPzorbital, respectively, with potentials determined to be about -1.3 eV and 1.4 eV, perfectly straddling over the water redox potentials46. Thus, it could be well expected that the band structure of g-C3N4satisfies the thermodynamic requirements for photocatalytic water splitting for hydrogen and oxygen evolution under visible light. Except for photocatalytic water splitting, g-C3N4has been well evidenced effective to drive other photocatalytic reactions, such as photodegradation of organic pollutants2-6, photocatalytic CO2reduction11-15,etc., which will be also discussed in this review article.
Until now, different methods have been developed for the synthesis of g-C3N4including hydrothermal and solvothermal method47,48, physical and chemical deposition49-52, thermal polymerization method53-57, and electrochemical synthesis58,etc.Among these reported synthetic methods, thermal polymerization has attracted the most intensive attention, due to its well-recognized advantages of simple and safe operation as well as easy control of reaction conditions for up-scaling synthesis. Through the thermal polymerization process, g-C3N4could be obtained from various carbon and nitrogen-rich compounds as precursors, such as cyanuric acid48,melamine59,60, melamine chloride61-63, dicyandiamide54,64,urea57,65, and other triazine compounds66,etc.Taking melamine as the example, the detailed process for the synthesis of g-C3N4is schemed in Fig. 2. In the thermal polymerization process, the melamine precursor is polycondensed to be a small molecule intermediate “melem”, which is further polymerized into lamellar structured g-C3N467,68.

Fig.2 Schemed synthetic process of g-C3N4 from melamine adapted from Angew. Chem. Int. Ed. Engl., Wiley publisher 68.
In comparison to other modification approaches, elemental doping could be more versatile with metal or non-metal elements as dopants to engineer both the molecular and electronic structures for the improved optical absorption ability and the promoted charge transfer processes, thereby resulting in the great enhancement in photocatalytic activities. Metal dopants(e.g., Fe, Cu, W, Zn, Mo, Zr,etc.8,89,91-96) imparts unique photocatalytic properties to g-C3N4by improving the visible light absorption and/or accelerating charge migration and prolonging carrier lifetime. For example, the introduced Mo dopants could effectively reduce the recombination rate of photogenerated charges and expand visible light response, and then the obtained Mo-doped g-C3N4exhibited much higher activity than the undoped one for the photoreduction of CO2,with the highest CO and CH4production rates reaching 887 μmol·g-1·h-1and 123 μmol·g-1·h-1, respectively96. Given the easy accessibility of non-metal elements and their easy introduction into the g-C3N4framework, non-metal element doping has been well and frequently investigated to improve the photocatalytic activity of g-C3N4. In this article, the recent advances in the non-metal doped g-C3N4with tunable molecular and electronic structures for effective optical absorption and efficient charge transfer will be reviewed, with oxygen (O)97-99,phosphorus (P)69,100-102, sulfur (S)103-106, boron (B)107-110,halogen (F, Cl, Br, I)111-113and other non-metallic elements (like C and N self-doping)114-119as dopants, for the improved photocatalysis.
3 Non-metal doping of g-C3N4
The non-metal doping of g-C3N4could be mainly achieved by copolymerization120-122, ball milling123, hydrothermal and solvothermal methods124,125, and chemical oxidation126,127,etc.The non-metal doping can promote the delocalization ofπconjugated electrons in g-C3N4, thus improving the conductivity,mobility and separation of photoelectrons. It can also reduce the band gap of g-C3N4and enhance its optical absorption ability,especially in visible light, for the improved photocatalytic performances. The different carbon or nitrogen sites substituted by various types of non-metal dopants are shown in Fig. 3. In the following sections, various non-metal elements used as the dopants to modify the molecular and electronic structures of g-C3N4will be introduced, for the thermodynamically and kinetically improved photocatalysis.
3.1 O-doped g-C3N4
As an element widely existed in nature, O has been well investigated for the doping of g-C3N4viadifferent oxidation methods, such as hydrothermal treatment of g-C3N4by hydrogen peroxide (H2O2)128, pretreatment of synthetic precursors with H2O299, oxidizing g-C3N4using sulfuric acid and nitric acid127,and thermal oxidation of g-C3N4in air98.

Fig. 3 Doping sites of different non-metal elements in g-C3N4.
O-doped g-C3N4was synthesized for the first time by Liet al.viaa simple H2O2hydrothermal treatment process128, which showed the optical absorption edge extended to 498 nm for the enhanced visible light activity. It was revealed that O doping formed an N=C―O structure in the crystal lattice of g-C3N4,indicating that the O atom can be directly bonded tosp2hybridized carbon atoms, with the conduction band (CB)minimum reduced by 0.21 eV and the valence band (VB)maximum unchanged. The H2release rate on the O-doped g-C3N4was 121 μmol·h-1, which was 12 times that of g-C3N4. Sunet al.used the melamine oxidatively modified via a H2O-H2O2assisted hydrothermal reforming process as the precursor to synthesize O-doped g-C3N4nanosheets (Fig. 4a)99. Compared with the conventional g-C3N4, the O-doped g-C3N4nanosheets showed greatly improved photocatalytic activity under visible light, with hydrogen evolution rate increased by 10.7 times and an AQY achieving 13.04% at 420 nm.
By chemical oxidation in mixed acids (H2SO4and HNO3) at room temperature, Sheet al.obtained a 2D porous ultrathin Odoped g-C3N4nanosheet127, which exhibited quite high photocatalytic activity, with the average H2evolution rate increased by ~5.2 times relative to g-C3N4and reaching ~189.3 μmol·h-1. The enhanced photocatalytic activity was due to more adsorption sites and more active sites, resulting in less recombination and more efficient separation of photogenerated electron and hole pairs, enhanced redox capability and improved electron transport capability.
Except for photocatalytic hydrogen production, O-doped g-C3N4has also been reported of great potential in photocatalytic reduction of CO2. Yuet al. prepared fractionated porous Odoped g-C3N4nanotubes (OCN-tubes) by continuous thermal oxidation stripping and crimp-condensation of bulk g-C3N4(Fig.4b)98. The graded OCN-tubes showed excellent photocatalytic CO2reduction performance under visible light, with methanol release rate reaching 0.88 μmol·g-1·h-1, which was 5 times higher than the bulk g-C3N4(0.17 μmol·g-1·h-1). The O-doping enhanced photocatalytic activity of OCN-tubes was synergistically attributed to the narrower band gap, the greater CO2affinity and absorption capacity, and the more efficient separation of photogenerated charge carriers.
3.2 S-doped g-C3N4
As firstly demonstrated by Liuet al., the doping of S into g-C3N4could be achieved by thermal treatment under H2S atmosphere129, and thermal polymerization of S-containing precursors such as thiourea and trithiocyanuric acid130. The S atom preferentially replaces the nitrogen atom at the edge of hexazine ring nitrogen units in g-C3N4, which will enhance the redox ability in the photocatalytic reactions. S doping could also modify the electronic structure of g-C3N4to increase light absorption ability, charge carrier mobility, and then the photocatalytic reactivity131-133.
I vividly1 recall entering their driveway and being overwhelmed by the size of their home, the beauty of the furnishings, the manicured grounds and the pecan orchard2
Liuet al. for the first-time prepared S-doped g-C3N4by treating pure g-C3N4powder at 450 °C under the H2S atmosphere (Fig.5a)129. The homogeneous substitution of S atoms at nitrogen sites created a unique electronic structure, with VB width increased and CB minimum elevated and absorbance slightly reduced. As a result, the obtained S-doped g-C3N4showed excellent photocatalytic activity with hydrogen evolution photoreactivity increased by 7.2 and 8.0 times underλ> 300 and 420 nm, respectively, in comparison to the pristine g-C3N4.
Instead of the poisonous and foul-odor H2S gas as S precursor,Wanget al. successfully synthesized S-doped g-C3N4by simply calcinating thiourea at 520 °C134. It was found that S doping extended the visible light absorption region and reduced the band gap from 2.7 eV for the undoped g-C3N4to 2.63 eV for the obtained S-doped g-C3N4, along with impurity levels created for the easy photoexcitation of electrons from the impurity states to the CB or from the VB to the impurity states, which gave rise to the great increase in photocatalytic yield for CO2reduction to CH3OH. Fenget al.prepared a nanoporous S-doped g-C3N4microrod (CN-MT) by directly thermally condensing a melamine-trithiocyanate supramolecular under N2atmosphere130,providing a photocatalytic activity for H2production under visible light irradiation about 9.3 times higher than that of g-C3N4prepared using melamine as a precursor. In addition, the material also exhibited satisfactory stability, without loss in catalytic activity after catalyzing H2production for 60 h. Yanet al.synthesized S-doped g-C3N4by copolymerization, which displayed much higher photocatalytic activity for CO2reduction than g-C3N4, with CH3OH production rate reaching 1.12 μmol·g-1·h-1135. DFT calculations revealed that S doping narrowed the band gap as well as reduced the Gibbs free energy of the rate determining step from 1.43 eV to 1.15 eV (Fig.5b)135. Moreover, it was theoretically demonstrated that CO was easily adsorbed on S-doped g-C3N4, while CH3OH was easily desorbed from S-doped g-C3N4compared to g-C3N4(Fig. 5c,d),which well explained why CH3OH was the main product.

Fig. 4 (a) Schematic illustration of the precursor pre-doping strategy to synthesize O-doped g-C3N4, reproduced with permission from Inorg. Chem. Front., Royal Society of Chemistry 99. (b) Fractionated porous O-doped g-C3N4 nanotubes (OCN-tubes),reproduced with permission from Small, Wiley 98.
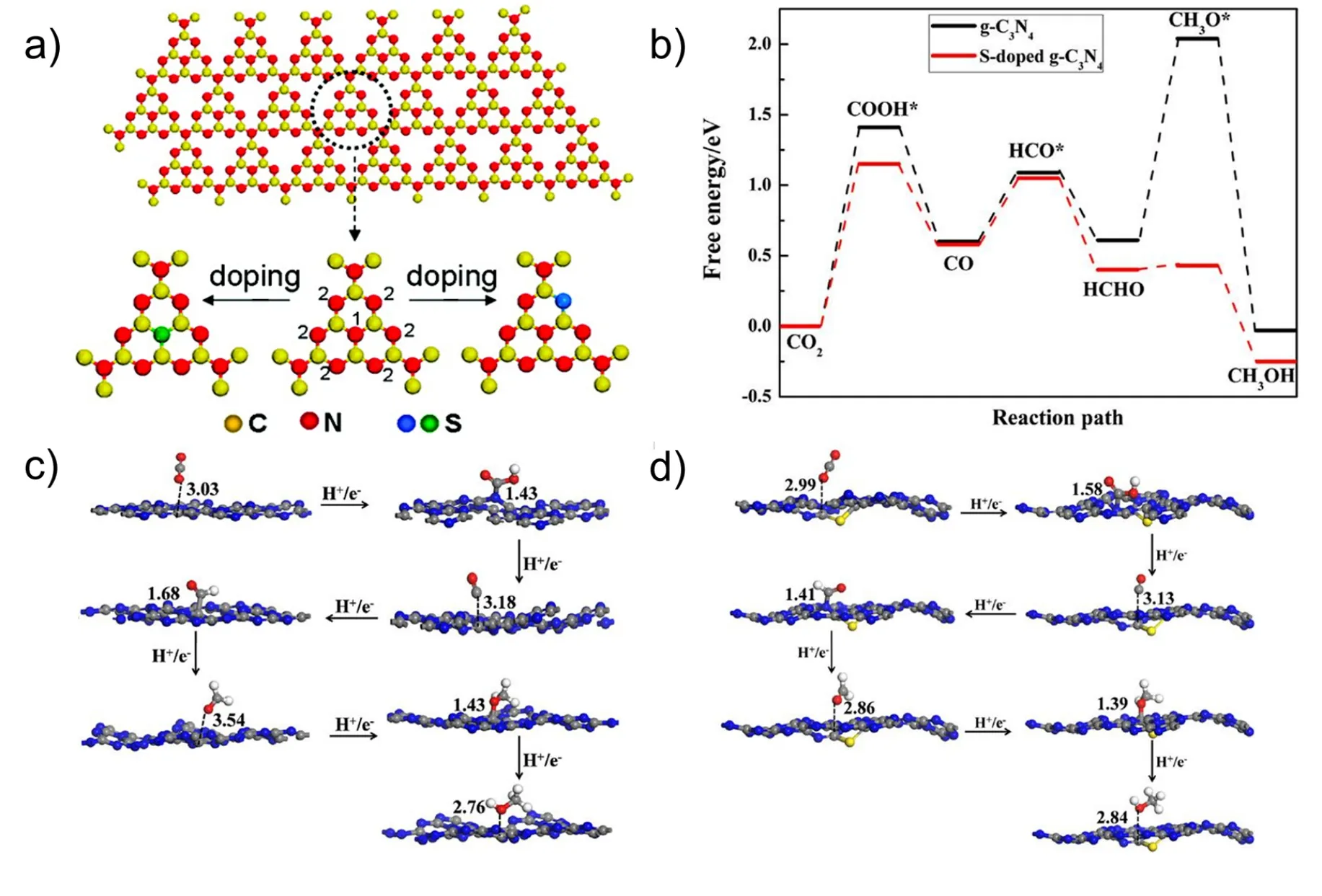
Fig. 5 (a) Schemed process for the synthesis of S-doped g-C3N4, reproduced with permission from J. Am. Chem. Soc., American Chemical Society 129.(b) Energy profile for the HER on the S-doped g-C3N4. Calculated structures corresponding to the optimal reaction path followed by the CO2 conversion on (c) g-C3N4 and (d) S-doped g-C3N4. Selected distances are shown in angstrom. Chemistry (bound) species are indicated by full bonds,whereas physiography species are indicated by dashed bonds, reproduced with permission from J. Phys. Chem. C, American Chemical Society 135.
3.3 P-doped g-C3N4
There are different methods developed and different precursors used for introducing P into g-C3N4, such as hexachlorocyclotriphosphazene69, BmimPF6120,(NH4)2HPO4121, phosphorous acid125, and NH4PF6136,etc.Zhanget al. treated dicyandiamide and sodium 1-butyl-3-hexamethyl phosphate (BmimPF6) in a phosphorus-containing ionic liquid at different temperatures by solvothermal method to obtain P-doped g-C3N4137. It was found that as the temperature increasing, P would react with the amine group of g-C3N4,resulting in the attachment of the P atom to the backbone of g-C3N4. As a result, a serious change happened to the electronic characteristics of g-C3N4, which improved the conductivity by 4 orders of magnitude and provided an improvement in photocurrent generation.
Except for hydrothermal and solvothermal methods, thermal copolymerization could be another effective method to prepare P-doped g-C3N4. Huet al. prepared P-doped g-C3N4by a simple copolymerization for the improved photocatalytic activity for RhB degradation, as P doping could reduce the band gap energy and improve the separation efficiency of photogenerated electrons and holes (Fig. 6a, b)121. Moreover, they found that the P doping sites and then the photocatalytic activities were greatly influenced by different P sources. Interstitial P doping with(NH4)2HPO4used as P source was more effective in improving the photocatalytic activity of RhB degradation compared with substitutional P doping with ionic liquid [Bmim]PF6.
The Zhang group used guanidiniumhydrochloride (GndCl)and hexachlorocyclotriphosphazene (HCCP) as the precursors of g-C3N4and P sources, respectively, to synthesize P-doped g-C3N469. The obtained P-doped g-C3N4showed excellent photocatalytic performance, with H2production rate reaching 50.6 mmol·h-1, which was 2.9 times higher than that of pure g-C3N4. With Lewis acid and Lewis base units coupled to g-C3N4with P-doping (Fig. 6c)69, it was believed that the P atoms replacing some of the carbon atoms in the frame column of g-C3N4resulted in the altered electronic properties, which inhibited the photogenerated charge carrier recombination and thereby improved the photocatalytic performance. Zhuet al.synthesized P-doped g-C3N4mesoporous nanostructures by cocondensation of melamine and (hydroxy ethylidene) phosphonic acid. They found that P can chemically bond with carbon and nitrogen in the g-C3N4framework. In addition, the lone pairs of electrons could be delocalized to P-doped g-C3N4, which enhanced the conductivity and then the electron transfer ability by acting as an active site (Fig. 6d)122.

Fig. 6 The possible doping sites of P atoms in g-C3N4, using (a) (NH4)2HPO4 and (b) ionic liquid [Bmim]PF6 as P sources, reproduced with permission from RSC Adv., Royal Society of Chemistry 121. (c) Molecular structure of P-doped g-C3N4, reproduced with permission from J. Mater.Chem. A, Royal Society of Chemistry 69. (d) Schematic diagram of P-doped g-C3N4 for photocatalytic reduction of carbon dioxide, reproduced with permission from ACS Appl. Mater. Appl. Interfaces, American Chemical Society 122.
3.4 Halogen doped g-C3N4
Halogen elements, such as F, Br and I, have also been incorporated into g-C3N4for the improved photocatalytic activity. Yonget al.demonstrated that F could enter the g-C3N4framework by hydrothermally treated in ammonium fluoride solution138. Due to the difference in electronegativities between nitrogen and carbon, the F atom was easy to bind to carbon instead of nitrogen, resulting in partial conversion of C-sp2to Csp3. DFT calculations indicated that VB and CB moved to higher energy values, and thus the F-doped g-C3N4exhibited a photocatalytic hydrogen evolution activity about 2.7 times higher than unmodified g-C3N4.
Viaa copolymerization synthesis strategy139, Zhanget al.successfully obtained I-modified g-C3N4, which exhibited photocatalytic activity for hydrogen evolution increased by three times as compared to g-C3N4, due to the well-tuned electronic and optical properties. Later, Hanet al.developed a post-doping method to prepare the iodized g-C3N4nanosheet (IGCNS) by easily grinding g-C3N4in the presence of iodine (Fig. 7a). With the I atom content optimized to be 0.34%, the obtained IGCNSs sample showed a high H2yield of 44.5 μmol·h-1under visible light irradiation123.
Br could be doped into g-C3N4by co-condensation of urea and ammonia bromide (Fig. 7b)140. It was found that the incorporation of Br into the g-C3N4backbone modulated the framework structure, light absorption, electron conductivity, and charge-carrier separation rate. The optimized Br-doped g-C3N4sample achieved a high H2evolution rate under visible light irradiation, which was more than 2 times higher than that of the pure g-C3N4sample.
3.5 B-doped g-C3N4
B-doped g-C3N4was first reported by Yanet al. by heating the mixture of melamine and boron oxide141. With B doping, the photocatalytic degradation of RhB was improved by 3.6 times,due to the enhanced visible light absorption ability and charge separation efficiency. With the mixture of melamine and dicyandiamide and BH3NH3as precursor for copolymerization,Ohnoet al. prepared B-doped g-C3N4, which showed photocurrent response about 5 times that of pure g-C3N4. Under photoelectrochemical conditions, it was further observed C2H5OH as major product in the aqueous phase with production rate up to 150 nmol·h-1and only a small amount of CO and H2in the gas phase142.
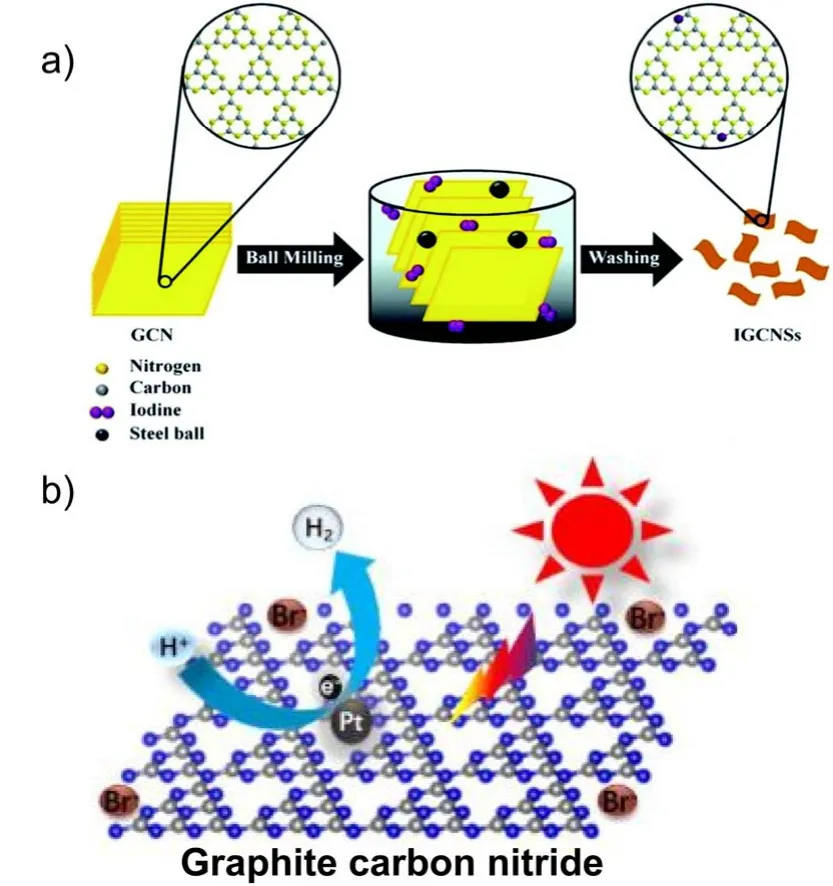
Fig. 7 (a) Synthesis methods and charge carrier transfer processes in I-doped g-C3N4, reproduced with permission from J. Mater. Chem. A,Royal Society of Chemistry 123. (b) Charge carrier transfer processes Br-doped g-C3N4, adapted from Appl. Catal. B, Elsevier 140.
Zhanget al.successfully synthesized B-doped g-C3N4nanosheets (B-CN) and their nanocomposites with nanocrystalline anatase TiO2(T/B-CN) by thermal polymerization and self-assembly approach (Fig. 8a)143. The prepared T/B-CN nanocomposite containing an appropriate amounts of B dopants and TiO2exhibited relatively high photocatalytic activity under visible light irradiation, with AQY reaching 3.08% for hydrogen evolution and 1.68% for CH4production from CO2reduction atλ= 420 nm, respectively,which was 5.1 and 7.6 times higher than g-C3N4. It was demonstrated that such great photocatalytic enhancement should be attributed to the B doping and the subsequent coupling with TiO2, resulting in the extended visible light absorption region and the promoted charge transfer ability in the formed heterojunction.
Zhuet al.thermally treated g-C3N4in the presence of NaBH4under a N2atmosphere (Fig. 8b)144. They believed that the N vacancies and B dopants introduced into g-C3N4gave rise to the extended optical absorption in the visible region as well as the promoted spatial separation of photoexcited electrons and holes.As a result, the reduced g-C3N4exhibited high photocatalytic activity for H2O2production (170 μmol·g-1·h-1) in the absence of an organic electron donor, with solar energy conversion efficiency and apparent quantum yield close to ~0.26% and~4.3%, respectively.
3.6 Self-doped g-C3N4

Fig. 8 (a) Schematic mechanism of T/B-CN for photocatalytic pollutant degradation, hydrogen production, carbon dioxide reduction.reproduced with permission from J. Phys. Chem. C, American Chemical Society 143. (b) The molecular structure of g-C3N4 reduced with NaBH4, adapted from Appl. Catal. B, Elsevier 144.
Some interesting advances have been achieved over the C and N self-doped g-C3N4in recent years. Nitrogen atoms could be successfully doped into g-C3N4(C3N4+x) through the thermal condensation of melamine pretreated with hydrazine hydrate by providing more nitrogen in precursor145. N self-doping redshifted the light absorption edge of g-C3N4and reduced the band gap from 2.72 eV to 2.65 eV. Moreover, N-doping could greatly promote charge separation and migration ability. In terms of photocatalytic hydrogen evolution, the performance was improved by 5.6 times compared to unmodified g-C3N4,reaching 44.28 μmol·g-1·h-1.
Panneret al.synthesized the porous g-C3N4nanosheets with high surface area by thermal decomposition of the urea-thiourea mixture, which was spray-granulated into microspheres using 2% (w) of polyvinyl alcohol (PVA) as a binder. It was revealed that the delocalizedπbond produced by C doping and the macroscopic mesoporous structure produced by the granulation process contributed to the high adsorption capacity, allowing tetracycline antibiotics adsorption increased by multiple times(about 75% in 60 minutes) for fully photocatalytical degradation under visible light (> 95% in 90 min)146. Z. Zhaoet al.developed a C self-doped g-C3N4photocatalyst using porous carbon foam as a soft template for photocatalytic NO purification in air147. They found that C-doped g-C3N4possessed an increased surface area (65 m2·g-1), extended optical absorption up to the near-infrared range (800 nm) and accelerated electron-hole separation, which together contributed to an increase in photocatalytic performance, resulting in a 3.8-time increase in the reaction rate constant. Baoet al.synthesized a porous C-doped g-C3N4nanosheet photocatalyst using an anionic polyacrylamide as an intercalator and a carbon source. It was evidenced that C doping narrowed the band gap from 2.75 eV to 2.41 eV, and two-dimensional nanostructures promoted charge separation by shortening the charge diffusion distance to the photocatalyst surface, contributing to the excellent photocatalytic activity with and RhB degraded by 95% under visible light for 30 minutes148.
3.7 Non-metal co-doped g-C3N4
As discussed in the previous sections, many studies have investigated the single non-metal doped g-C3N4photocatalysts.However, the charge balance can be hardly maintained in singleelement doped photocatalyst systems, and the dopants would introduce recombination centers of photogenerated electronhole pairs, resulting in effective visible light absorption property but poor charge transfer ability. Interestingly, co-doping of different heteroatoms could improve the utilization of visible light by engineering the band structures, as well as overcome charge transfer limitation of single-element doping through the charge compensation effect112,149,150. P and S151, S and N152,153,I and P154and B and F112co-doped g-C3N4have demonstrated encouraging photocatalytic activities in different applications including CO2conversion, pollutant degradation and H2production. However, in the co-doping process, surface decoration and framework doping usually occur simultaneously.Moreover, the mechanism of photoactivity enhancement depending on surface or internal doping is not fully understood.
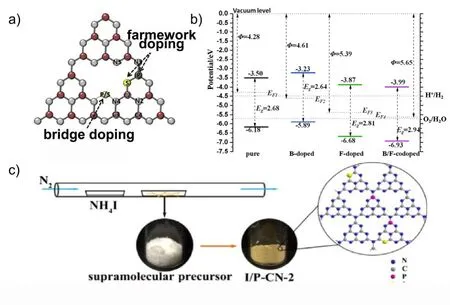
Fig. 9 (a) Molecular structure of P and S co-doped g-C3N4, adapted from Carbon, Elsevier 155. (b) Calculated band edge positions of pure, B-doped,F-doped, and B/F co-doped g-C3N4 in comparison with water redox potentials. Φ is the work function, Eg is the band gap, and EF1, EF2, EF3, and EF4 are the Fermi levels of pure, B-doped, F-doped, and B/F co-doped g-C3N4, respectively, reproduced with permission from Phys. Chem. Chem.Phys., Royal Society of Chemistry 112. (c) Schematic synthesis illustration of the fractionated porous iodine and P co-doped carbon nitride(I/P-CN), reproduced with permission from ChemCatChem, Wiley 154.
Huet al. synthesized O-functionalized S-P co-doped g-C3N4nanorodsviacopolymerization followed by hydrothermal posttreatment, for the anoxic removal of organic pollutants under visible light151. They found that S and P co-doping inhibited the crystal growth of g-C3N4, reduced the band gap energy, and improved the separation efficiency of photogenerated electrons and holes, which increased the hypoxia-catalyzed RhB degradation constant by about 6.5-fold. Moreover, O functionalization not only increased the adsorption capacity of g-C3N4, but also captured photogenerated electrons, thereby producing photogenerated holes for RhB degradation under anoxic conditions, which resulted in a further doubling of the RhB degradation constant. Furthermore, Huet al.proposed that P and S co-doping into g-C3N4can greatly enhance photocatalytic reactivity by rapidly transporting charge carriers through the N-S-N-C-N-P pathway (Fig. 9a)155, which allowed electrons to pass through the hexadazine unit for photocatalytic reaction and then increased the rate constant by about 5 times for hydrogen evolution.
Jianget al.154prepared a novel layer stack of I and P co-doped g-C3N4(I/P-CN) from a supramolecular precursor (Fig. 9c). The obtained I/P-CN showed significantly enhanced visible light photocatalytic activity for hydrogen production (93.9 μmol·h-1)compared to bulk g-C3N4. The reason for the significant improvement in photocatalytic activity should be related to the co-doping of I and P, resulting in a decrease in band gap and an increase in absorption intensity. The unique supramolecular layer-stack structure also increased the specific surface area and provided more active reactive sites for hydrogen evolution.
Cuiet al.prepared porous B and F co-doped g-C3N4using[Bmim]BF4as doping precursor, by post-calcination in air to obtain156. It was distinguished that B-doping was mainly present in the internal framework of g-C3N4, but F-doping was only present in the surface layer. The internally doped B served to enhance visible light absorption, while B and F co-doping synergistically enhanced photoelectron generation, transport and separation, which resulted in 13-fold increase in photocatalytic activity, with H2evolution rate reaching 351 μmol·h-1.Furthermore, Dinget al. investigated the electronic and optical properties of B, F single-doped and B/F co-doped g-C3N4by density functional theory (DFT) calculation (Fig. 9b)112. It was found that B and F co-doping could narrow the band gap for increased visible light utilization without localized states created in the mid-gap for charge recombination, and meanwhile the band edge positions satisfied the need of overpotential for water redox reactions.
4 Conclusions and perspective
g-C3N4is an inexpensive non-metallic photocatalyst consisting of only carbon and nitrogen with a band gap of approximately 2.7 eV. Under the excitation of sunlight, electrons are excited from the valence band to the conduction band to form electron-hole pairs, which could be used to photocatalyze pollutant degradation, water decomposition and organic synthesis. However, the photocatalytic efficiency of g-C3N4is unsatisfactory due to insufficient solar absorption, low surface area and rapid recombination of photoelectron-hole pairs.
This review summarizes the recent advances in non-metal doped g-C3N4photocatalytic systems. Non-metallic doping can narrow the band gap, extending the spectral response region, as well as reduce the recombination rate of electron-hole pairs for efficient charge separation, therefore, resulting in the improved photocatalytic performances.
Despite some encouraging results, the development of nonmetal doped g-C3N4in the field of photocatalysis is still in its infancy, and many challenges still exist. Exploring controllable and facile synthesis techniques, to build a stable and efficient g-C3N4photocatalytic system for its application in the solar energy field, is still a delicate and long-term work. Although different modification methods have been developed, it is still difficult to achieve the precise control of crystal and molecular structures of g-C3N4with dopants and defects well identified.
Except for the controllable non-metal doping, we firmly believe, novel approaches would be also highly desired for the application of g-C3N4in photocatalysis in the future. Firstly, codoping should be a viable strategy that combines the advantages of these dopants to have a synergistic impact on structural and optical properties. The use of multi-nonmetal co-doping to explore a stable and efficient co-doping system has a very broad significance for the performance improvement of g-C3N4.Secondly, the simultaneous introduction of dopants and defects could have a good effect on the photocatalytic performance of g-C3N4by further improving the separation of photogenerated electrons and holes, along with extending optical absorption region. Finally, doping and heterojunction engineering can further promote the separation of photogenerated electrons and holes and thus improve photocatalytic activity. Element doping is used to alter the molecular and electronic structures of g-C3N4,while the heterojunction structure always benefits charge carrier separation.

