Are all prostate cancer patients "fit" for salvage radiotherapy?
Carmen González-San Segundo, Alfonso Gómez-Iturriaga, Felipe Couñago
Abstract The indication for salvage radiotherapy (RT) (SRT) in patients with biochemically-recurrent prostate cancer after surgery is based on prostate-specific antigen (PSA) levels at the time of biochemical recurrence. Although there are clear criteria (pT3-pT4 disease and/or positive margins) for the use of adjuvant radiotherapy, no specific clinical or tumour-related criteria have yet been defined for SRT. In retrospective series, 5-year biochemical progression-free survival(PFS) ranges from 35%-85%, depending on the PSA level at the start of RT. Two phase 3 trials have compared SRT with and without androgen deprivation therapy (ADT), finding that combined treatment (SRT+ADT) improves both PFS and overall survival. Similar to adjuvant RT, the indication for ADT is based on tumour-related factors such as PSA levels, tumour stage, and surgical margins.The number of patients referred to radiation oncology departments for SRT continues to rise. In the present article, we define the clinical, therapeutic, and tumour-related factors that we believe should be evaluated before prescribing SRT. In addition, we propose a decision algorithm to determine whether the patient is fit for SRT. This algorithm will help to identify patients in whom radiotherapy is likely to improve survival without significantly worsening quality of life.
Key words: Prostate cancer; Salvage radiotherapy; Comorbidity; Fit; Androgen deprivation therapy
INTRODUCTION
Salvage radiotherapy (RT) (SRT) is the standard treatment for patients with biochemically-recurrent prostate cancer (PCa) following radical prostatectomy[1,2].Findings from several phase 3 clinical trials demonstrating the value of adjuvant RT in these patients[3-5], together with the growing interest among urologists in the surgical treatment of high-risk PCa, have led to an increase in the number of patients who receive RT postoperatively.
After the findings of those clinical trials confirmed the benefits and efficacy of SRT -especially for early recurrences [defined as prostate-specific antigen (PSA) < 0.5 ng/mL][6-8]- most subsequent studies have focused on the role of tumour-related variables (e.g., PSA levels, PSA kinetics, Gleason score, and surgical margins) in establishing the treatment indication. However, those studies have largely ignored the clinical characteristics that could potentially contraindicate this treatment.
A significant proportion of patients who develop biochemical recurrence (BCR)undergo SRT. However, the use of high-dose, hypofractionated RT in tissues previously subjected to surgery, together with the poor anatomical condition of these tissues (often associated with urinary incontinence), are important factors to consider when deciding whether SRT is indicated given the increased risk of radiation-induced toxicity and the potential to worsen quality of life (QoL).
In the present article, we propose a decision algorithm for SRT. This algorithm was developed after a careful analysis of the literature involving an assessment of a wide range of factors - apart from the well-known tumour characteristics associated with progression-free survival (PFS) - including comorbidities, life expectancy, expected toxicity, and dosimetric variables.
CLINICAL ASPECTS
Life expectancy
Compared to other malignant tumours, PCa has a long clinical course, which explains why survival outcomes are usually reported at a median follow-up of 10 years. In the United States, data from population registries show that 5-year survival rates in patients with PCa are greater than 90%[9]. In most clinical guidelines, life expectancy ≥10 years is an important criterion for treatment selection, especially in patients with low-grade tumours[1,2]. However, in patients with biochemically-recurrent PCa, life expectancy is not usually considered in the treatment selection process, as evidenced in phase 3 trials of postoperative adjuvant RT in which age (< 75 years) is an inclusion criterion but life expectancy is not[3-5]. However, the two randomized clinical trials(RCT) that compared SRT with or without androgen deprivation therapy (ADT)[10,11]did include life expectancy (< 10 years) as an exclusion criterion. Patients who develop BCR after prostatectomy are, on average, 3-5 years older than when the surgery was performed. For this reason, it is important to statistically determine life expectancy, especially in patients with late onset, non-aggressive BCR (based on PSA kinetics and Gleason score). Importantly, patients whose life expectancy is < 10 years at detection of BCR are unlikely to benefit from SRT, except for those with symptomatic, locally-recurrent disease with elevated PSA levels[12], in which case SRT plus ADT can be considered on an individual basis.
Comorbidities
Many studies have found that the presence of significant comorbidity is associated with worse survival in PCa patients who undergo radiotherapy[13,14]. Most clinical guidelines recommend the use of validated scales to assess comorbidity in order to facilitate decision-making[1,2]. Specific scales are available to assess comorbidity in patients with PCa[15]and these scales can be used both to predict QoL in the six month period following diagnosis and to estimate the probability of survival in the next 3.5 years. Patients with greater comorbidity, as determined by the total illness burden index for PCa (TIBI-PCa), have a 13-fold greater risk of dying from causes other than PCa in the 3.5 years after diagnosis[15]. Crawford et al[13]showed that survival outcomes in patients with significant comorbidities who underwent RT were significantly worse than in patients who did not receive oncological treatment. At 10-years of follow-up,those patients had a higher risk of PCa-specific mortality (PCSM; 62 deaths in the treatment group vs 42 in the supportive care group, P = 0.08). Moreover, patients with significant comorbidities had a greater risk of mortality of non-PCSM than patients with no or minimal comorbidity (16.1% vs 8.2%)[13].
The RCTs published to date that have evaluated SRT plus ADT have only included patients with performance status ranging from 0-2[10,11]. The TROG 03.06 trial[16]excluded patients with a life expectancy < 5 years (due to the presence of comorbidities). Based on these data, we recommend the use of comorbidity scales at the time of BCR; in addition, patients with a TIBI-PCa > 11 or a Charlson index > 3 should not be offered active treatment because the presence of these risk factors implies a high probability (> 50%) of non-PCSM mortality in the following 3 years.
Baseline urinary status
The use of validated scales such as the International Prostate Symptom Score (IPSS) or the Expanded Prostate Cancer Index to obtain an accurate assessment of urinary symptoms is crucial before deciding whether SRT is indicated. Most studies of postoperative RT have found a direct association between baseline urinary status and the risk of radiation-induced toxicity[17-19]. Patients with poor postoperative urinary function, a previous history of transurethral radical prostatectomy (TURP), or who require repeated bladder catheterization present an increased risk of developing stenosis of the bladder neck and urethra, which can cause a significant deterioration in urinary function. Although the studies that have reported toxicity outcomes associated with postoperative RT have reported similar findings with regard to the impact on urinary function[17-19], this variable was not included in the selection criteria of the prospective trials conducted to date. Neither of the two phase 3 trials that evaluated SRT with or without ADT[10,11], and none of the three phase 3 trials that assessed adjuvant RT[3-5], have reported data on urinary function, nor have they described whether RT negatively impacted urinary function. The SWOG trial only excluded patients who developed total urinary incontinence after surgery[5].
The recently published study by Pollack et al[20]on hypofractionation in patients undergoing primary RT found that late urinary toxicity was significantly higher in patients with high IPSS scores and a history of TURP. The poor urinary status prior to RT in patients who had previously undergone prostatectomy (versus surgery-naive patients) may explain why hypofractionation is not considered standard in this group of patients. In the study by Cozzarini et al[21], the 5-year rate of urinary toxicity rate ≥grade 3 was 18.1% in the hypofractionated group (2.3-2.9 Gy) versus only 6.9% in the conventional fractionation group.
Given the lack of validated data from prospective studies on the role of urinary function, we cannot recommend a definition of “unfit” based on urinary parameters,nor can we recommend the routine use of hypofractionated schemes. Patients who present poor urinary function prior to RT should be informed of the increased risk of urinary complications (stenosis, hematuria, stranguria, etc.). In addition, it is essential to analyse the risks and benefits of performing RT in patients with poor urinary function. In these patients, dosimetric parameters and clinical variables must be considered together. If the rectal and bladder constraints cannot be met (Table 1), then RT is contraindicated and the recommended treatment approach should be either observation or, in high-risk patients, hormonotherapy.
Concomitant medications
Although no specific drugs are contraindicated in patients scheduled to undergo SRT,the use of anticoagulant and antiplatelet medications increases the risk of rectal and/or urinary bleeding[17,19,22]. Takeda et al[23]found that anticoagulant use was significantly correlated (P = 0.027) with higher rates of chronic rectal toxicity ≥ grade 2. Even if the use of such medications does not contraindicate RT per se, patients should be informed about the increased risk of bleeding. By contrast, the available evidence indicates that hormonotherapy - sometimes administered concomitantly with SRT - does not increase urinary or radiation-induced rectal toxicity[17,24].
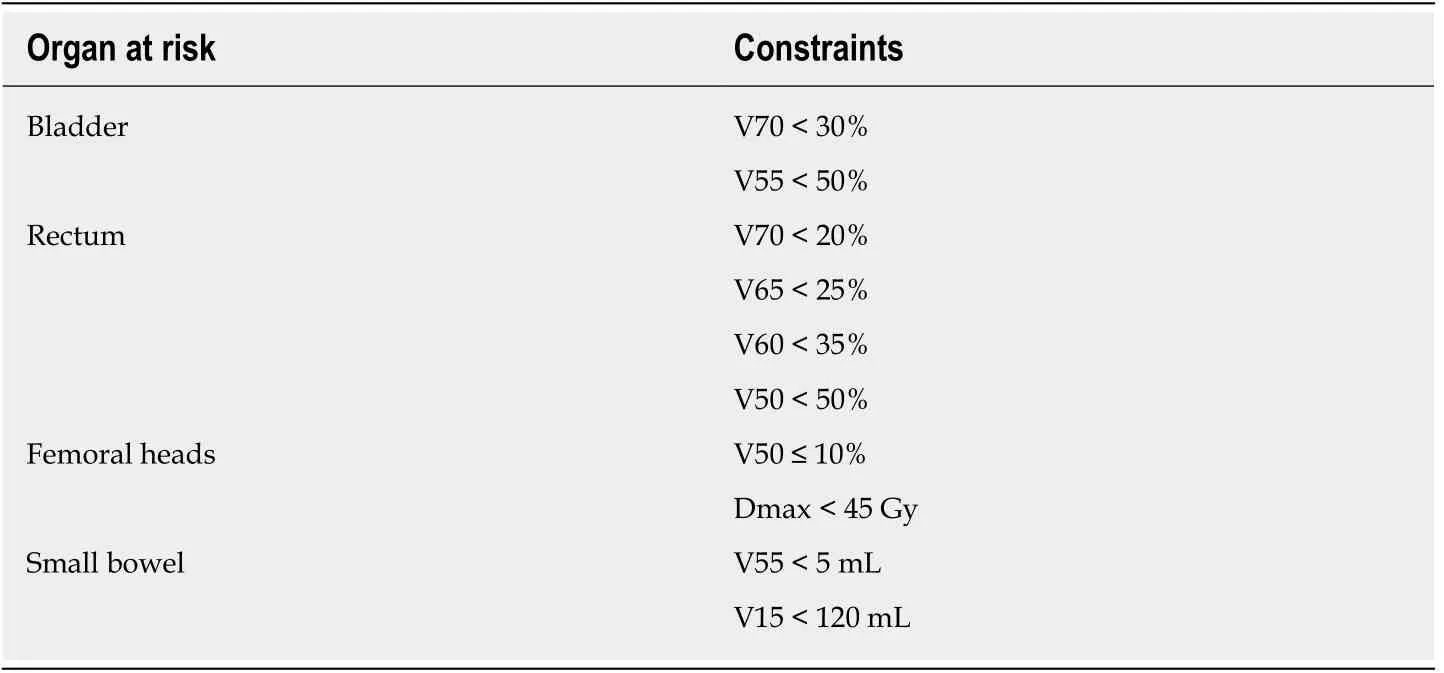
Table 1 Constraints recommended in salvage radiotherapy with conventional fractionation
However, in patients with cardiovascular risk factors, the prolonged use of hormonotherapy with SRT should be limited to patients with a poor prognosis,defined as the presence of local and/or regional recurrence, a PSA doubling time(PSADT) < 6 mo, and/or Gleason score > 7.
TUMOUR-RELATED VARIABLES
Recently, our group proposed a risk classification system - similar to the risk stratification used in patients at the initial diagnosis of PCa - to classify patients with biochemically-recurrent PCa into three risk groups[25]. That framework was designed to facilitate decision-making for the use of ADT based on several key prognostic variables (Table 2) assessed at the time of BCR. Low-risk patients, in whom ADT is not indicated, fulfil all of the conditions for good prognosis: PSA ≤ 0.5 ng/mL;PSADT> 12 mo; interval from surgery to recurrence > 18 mo; Gleason score 6 or 7 (3 +4); free margins; and stage pT2pN0. This subgroup of low-risk patients has the best survival outcomes (PFS) after SRT, which is expected given that they have the least aggressive disease. However, the benefits of RT in this subgroup must be carefully weighed against the risk of radiation-induced toxicity. Two other variables - age and(especially) comorbidities - play a key role in deciding whether to prescribe active treatment or not. We believe that low-risk patients, patients over age 75, and/or those with comorbidities that reduce their life expectancy to < 5-10 years (based on validated scales) should be considered “unfit” for SRT because the treatment is likely to worsen QoL without providing a clear survival benefit.
PSA at diagnosis of BCR
As early as 2002, Choo et al[26]described the lack of efficacy of SRT - with 5-year biochemical control rates < 35% - in patients with PSA levels > 2 ng/mL or with local macroscopic recurrence. In the meta-analysis by King and colleagues[27], the PSA level prior to SRT was directly related with the probability of disease response and control:for each 0.1 ng/mL increase in the PSA level at the time of BCR, the biochemical relapse-free survival (BRFS) rate decreased by 2.6%. Numerous authors consider PSA≤ 0.5 ng/mL as the optimal level at which to initiate “early" SRT[6-9]. In their study,Fossati et al[7]found that biochemical control in patients who underwent SRT with PSA levels ≤ 0.5 ng/mL was comparable to that obtained in patients who received adjuvant RT; however, patients with persistently elevated postoperative PSA levels were excluded from the comparison.
The available evidence indicates that the lower the PSA level at the time of BCR, the better the outcomes of SRT. To date, however, no PSA cut-off levels have been established to contraindicate SRT. Choline positron emission tomography/computed tomography (PET/CT) should be performed in patients with PSA values > 1 ng/mL or a PSADT < 6 mo[28]. According to current European Association of Urology Guidelines, prostate-specific membrane antigen (PSMA) PET/CT should be performed prior to SRT in patients with PSA > 0.2 ng/mL at the time of BCR[29]. It is important to keep in mind that administering SRT in patients with PSA levels > 1 ng/mL without first localizing the lesion via imaging tests increases the risk that the affected area (particularly lymph node regions) will not be adequately irradiated.
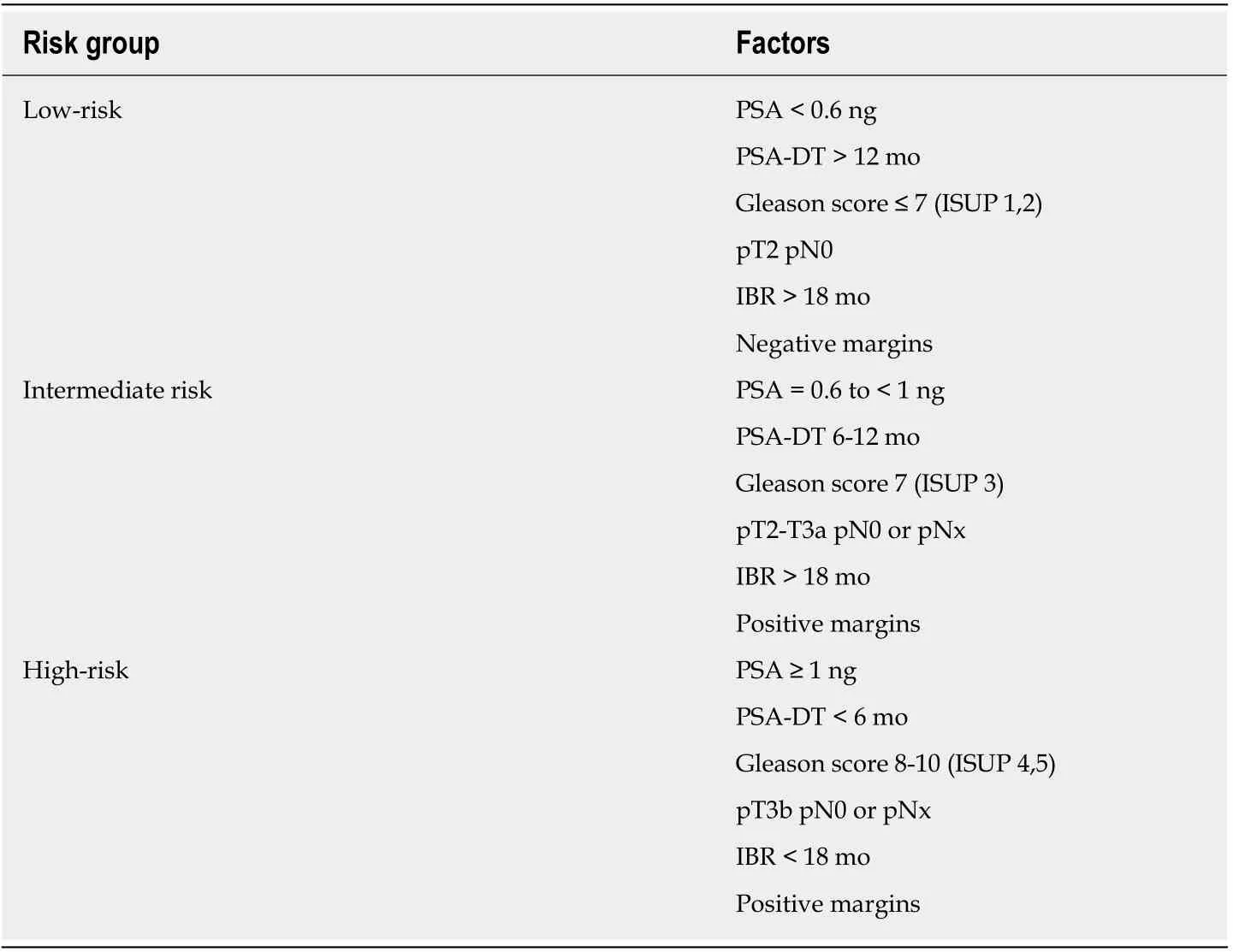
Table 2 Risk groups for salvage radiotherapy
We recommend performing SRT in patients with PSA values < 0.5 ng/mL provided that the patient has a life expectancy > 10 years and no medical contraindications.Choline or PSMA PET/CT (based on availability) should be performed when PSA values exceed 0.2 ng/mL and/or in cases with PSADT < 6 mo. If there is a visible locoregional recurrence without evidence of distant metastasis, then the radiation target volume can be adjusted to the findings of the imaging tests; in these cases,concomitant ADT is indicated, even in patients with PSA values > 2 ng/mL. Local SRT is not indicated in cases with extrapelvic involvement; instead, systemic therapy should be prescribed after a multidisciplinary tumour board has reviewed and approved the treatment. Finally, in patients with normal imaging tests and PSA values ranging from 0.5-2 ng/mL, the recommendations of the phase 3 GETUG and RTOG trials should be followed[10,11].
PSA doubling time
Many authors consider the PSADT to be the most important prognostic factor at the time of BCR, even though this variable was not an inclusion criterion in any of the RCTs published to date, nor was it used for risk stratification[3-5,10,11]. However, most clinical guidelines recommend the application of systemic therapy in patients with a PSADT < 6-10 mo at BCR[1]. The PSADT plays no role in determining whether SRT is contraindicated or not, nor should it be used to determine radiation volumes.However, when the PSADT is < 6 mo, ADT should be prescribed, in addition to SRT.
Disease-free interval
The GETUG study evaluated the influence of the time interval between radical prostatectomy and BCR on treatment outcomes in patients undergoing SRT plus androgen suppression therapy (goserelin)[10]. Patients were grouped into early (< 30 mo) or late BCR. However, no significant differences in biochemical control were observed. By contrast, other authors have found that biochemical control rates are worse in patients with a disease-free interval (DFI) < 18 mo and in patients with persistently-elevated PSA levels after prostatectomy[30], which suggest the presence of high-risk tumours and/or involved surgical margins. Nevertheless, the DFI does not condition the use of SRT, although ADT should be started in patients with a DFI < 18 mo, especially in cases with a short PSADT (< 6 mo). In patients with late onset BCR(> 10 years), the indication for SRT should be evaluated in the context of the patient’s age and comorbidities.
Risk group: Gleason score
In patients who develop BCR after primary external beam RT, eligibility for salvage should include the patient’s risk group classification at the initial diagnosis of PCa.Local salvage treatment is not advised in high-risk patients and/or those with Gleason 8-10[31]. The phase 3 trials that evaluated adjuvant RT did not include the Gleason score as an inclusion criterion[3-5]. However, the RCTs that have evaluated SRT with and without ADT found no significant between-group differences in survival [PFS or overall survival (OS)] based on the Gleason score, although the course of disease was worse in patients in the placebo group with Gleason scores ≥7[10,11].
In recent years, a growing proportion of high-risk patients undergo radical prostatectomy, mainly as part of the multimodal treatment approach supported by urologists. However, the risk of BCR in these patients is high, ranging from 50%-70%in most series[32]. Gandaglia et al[33]found that, together with nodal involvement and stage pT3-T4 disease, the presence of GS 8-10 was the third least favourable factor in patients treated with adjuvant RT. Indeed, patients who presented all three of these unfavourable factors had the worst prognosis, with 10-year OS rates of 62% when no adjuvant RT was performed.
There is no evidence to suggest that the Gleason score or the initial risk group are contraindications for SRT in patients who develop BCR after surgery. However, from a radiation oncology perspective, the presence of these factors creates uncertainties regarding: (1) The optimal target volume (especially in patients who did not undergo initial lymphadenectomy); (2) The indication and duration of concomitant ADT; and especially (3) Whether SRT should be performed in the absence of data from imaging tests ruling out distant disease.
DOSIMETRIC FACTORS
Table 1 shows the recommended dose constraints for the organs at risk used in most studies of SRT. The difficulty of bladder filling in previously-operated patients increases the risk of both acute and chronic urinary toxicity. Numerous publications have recommended limiting the radiation dose and/or treatment volume to avoid an exponential increase in treatment-related complications and long-term sequelae[34-36].Although the use of rectal spacers has been proven to reduce rectal toxicity in brachytherapy, their efficacy has not been validated in SRT. In patients with unfavourable dose-volume histograms (DVH), no other local measures are available to reduce the dose to the rectum and bladder. Consequently, image-guided RT is imperative in these cases to ensure accuracy and to optimize the dosimetric parameters. In addition, the treating radiation oncologist should discuss with the patient the risks of radiation-induced toxicity (based on the DVH values) and the expected benefits of the radiotherapy treatment. If the patient’s comorbidities are likely to increase the risk of developing toxicity > grade 3 in patients with unfavourable DVH values, then it is reasonable to rule out SRT, just as surgery is often ruled out in high-risk (ASA III-IV) patients.
EXCLUSION CRITERIA IN PHASE 3 TRIALS OF SALVAGE RT
Given the lack of universally-accepted criteria regarding the contraindications of SRT,in Table 3 we provide a summary of the exclusion criteria used in the phase 3 RCTs that have evaluated SRT with and without ADT. That table also includes the exclusion criteria in currently ongoing studies comparing adjuvant RT to SRT. Based on those data, we have developed a decision algorithm to identify patients considered "unfit"for SRT (Figure 1). As with most therapeutic indications, it is important not only to define the patients who are likely to benefit from a given treatment, but also to identify those patients in whom treatment could reduce life expectancy and/or lead to complications without providing a clear clinical benefit. Patients considered “unfit”for SRT would therefore include those who meet several of the following criteria: (1) >75 years of age; (2) Significant comorbidities; (3) Poor baseline urinary function; (4)Low risk of developing BCR; and (5) Unfavourable DVH values. These patients should be offered alternative approaches, which may include surveillance or hormonal therapy depending on the patient’s individual characteristics, life expectancy, and the "aggressiveness" of the recurrent disease. Finally, in patients with PSA > 1 ng/mL and/or PSADT < 6 mo, SRT should not be performed until the recurrence has been localized on imaging tests or at least until distant metastasis has been ruled out.
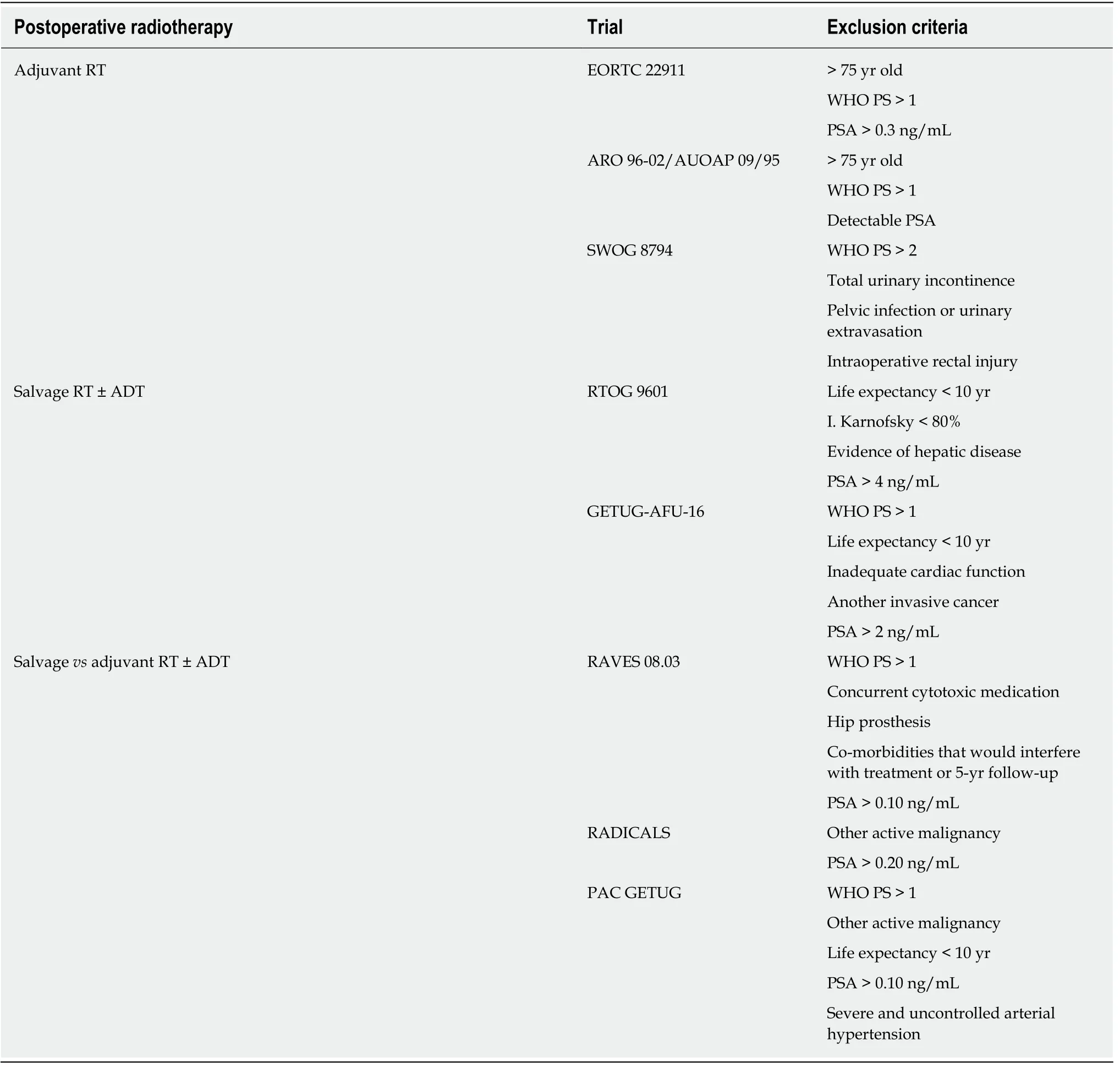
Table 3 Exclusion criteria in postoperative radiotherapy phase 3 trials

Figure 1 Algorithm to identify patients considered "unfit" for salvage radiotherapy. Risk group stratification based on reference 25. One asterisk:Chemotherapy addition according multidisciplinary board decision; two asterisks: Salvage radiotherapy if patients assume the risks. SRT: Salvage radiotherapy; ADT:Androgen deprivation therapy; CT: Computed tomography; PET: Positron emission tomography; RM: Resonance magnetic.
 World Journal of Clinical Oncology2020年1期
World Journal of Clinical Oncology2020年1期
- World Journal of Clinical Oncology的其它文章
- Predictors of distant metastasis in acinic cell carcinoma of the parotid gland
- Ipsilateral breast tumor recurrence in early stage breast cancer patients treated with breast conserving surgery and adjuvant radiation therapy: Concordance of biomarkers and tumor location from primary tumor to in-breast tumor recurrence
- Current concepts in ameloblastoma-targeted therapies in B-raf proto-oncogene serine/threonine kinase V600E mutation: Systematic review
