Recent endeavors on molecular imaging for mapping metals in biology
Jing Gao,Yuncong Chen,2 ✉,Zijian Guo,2,Weijiang He ✉
1 State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China
2 Chemistry and Biomedicine Innovation Center, Nanjing University, Nanjing 210023, China
Abstract Transition metals such as zinc, copper and iron play vital roles in maintaining physiological functions and homeostasis of living systems. Molecular imaging including two-photon imaging (TPI), bioluminescence imaging (BLI) and photoacoustic imaging (PAI), could act as non-invasive toolkits for capturing dynamic events in living cells, tissues and whole animals. Herein, we review the recent progress in the development of molecular probes for essential transition metals and their biological applications.We emphasize the contributions of metallostasis to health and disease, and discuss the future research directions about how to harness the great potential of metal sensors.
Keywords Molecular imaging, Zinc, Copper, Iron
INTRODUCTION
Iron, zinc, and copper, the top three abundant transition metals in the human body, were wellrecognized for their essential roles in maintaining homeostasis and sustaining life. There were numerous examples of clinical diseases that were closely associated with metal misregulation, such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis(ALS) and Parkinson’s disease (PD) (Bleackley and MacGillivray 2011). In this regard, careful maintenance of transition metal homeostasis is required in most living systems.
Labile metal ions, part of the total metal pools, are weakly bound to ligands and can rapidly dissociate(Aron et al. 2015). Redox-active labile metals, such as copper and iron, can be harnessed to create new diagnostics and therapeutics (Chang 2015). Ferrous iron (Fe2+) is oxidized into ferric iron (Fe3+) through reaction with hydrogen peroxide (H2O2), leading to the formation of highly reactive hydroxyl radicals (OH·),referred to as the Fenton reaction. Fe3+is reduced back to Fe2+via reaction with superoxide radicals (O2·-). This redox cycle is called Harber-Weiss reaction (Hassannia et al. 2019). Like iron, copper (Cu+and Cu2+) can transform between two oxidation states under biological conditions. These metals can also undergo kinetically appreciable ligand exchange with sensors that respond to metal binding or reaction with an alteration in signal (light or sound) output (Ackerman et al. 2017).
Fluorescence imaging (FLI) has been widely used in identifying diversified living processes. Providing a potentially non-invasive set of tools with spatial and temporal resolution, molecular imaging drives scientists to peer into biological environments, and unravels the mysteries of metal ions in a sophisticated milieu. Small-molecule indicators have been assorted into two basic categories for achieving specificity:(1) chelation-based and (2) reaction-based (Ackerman et al. 2017). Fundamental mechanisms such as fluorescence resonance energy transfer (FRET), photoinduced electron transfer (PET), and intramolecular charge transfer (ICT) integrate the foundation for most sensor designs (Chang et al. 2020). Traditional FLI normally utilizes excitation light < 600 nm, which can lead to autofluorescence as well as cell damage, and can be vigorously scattered in deep tissues. To circumvent the high background and low sensitivity, many efforts have been made to develop probes under near infrared (NIR)light excitation and emission. Accordingly, other imaging modalities such as two-photon imaging (TPI),bioluminescence imaging (BLI) and photoacoustic imaging (PAI) have been developed and attracted much attention. Table 1 points to a contrastive analysis of the three imaging modalities.
Compared with FLI, BLI does not need an excitation light source, resulting in minimized autofluorescence and much higher signal to noise ratio (Li et al. 2013).Beetle luciferases oxidize D-luciferin with ATP and O2,generating primarily yellow-green light, whereas marine luciferases release blue photons via the oxidation of imidazopyrazinone analogs (Yao et al.2018). A growing field of “PAI”, which combines optical and ultrasound imaging, shows advantages of high resolution and high contrast at centimeter imaging depths. Theoretically, the design of molecular PAI sensors is primarily based on extending a large πconjugated backbone with strong PA signal, for example,porphyrin, boron-dipyrromethene (BODIPY), and cyanine derivatives. Excellent reviews of Chan and coworkers discussing the development of PA agents can be found elsewhere (Knox and Chan 2018; Reinhardt and Chan 2018), and fluorescent sensors for measuring metal ions in living systems are also recommended(Carter et al. 2014; Liu et al. 2013; Park et al. 2020;Trusso Sfrazzetto et al. 2016).
METALLOSTASIS
Zinc
In the cellular environment, zinc is redox inert and is present in the +2 oxidation state. As the second-most abundant metals in the human body following iron,Zn2+is a major cofactor in DNA replication, protein synthesis and cell differentiation (Szewczyk 2013).Some evidence demonstrates that zinc homeostasis inmammals is controlled by the two major families denoted as ZIP (SLC39A) (Eide 2004) and CDF/ZnT(SLC30A) (Palmiter and Huang 2004).
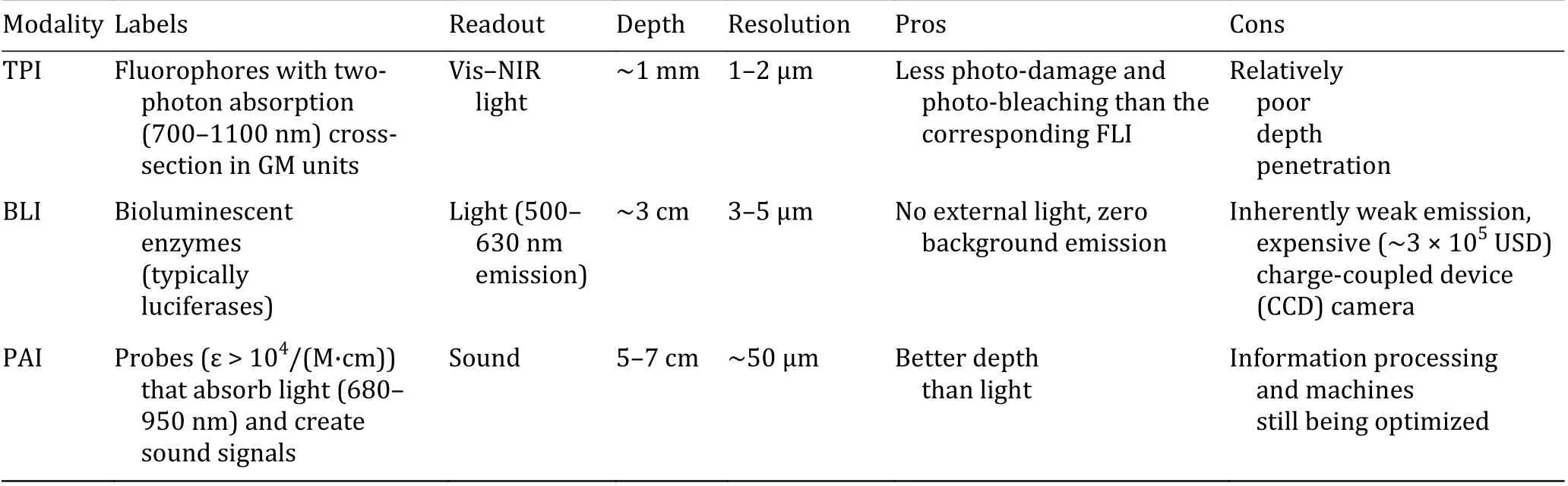
Table 1 Strengths and weaknesses of representative imaging modalities (Baker 2010)
ZIP transporters confer cytoplasmic Zn2+uptake(Huang and Tepaamorndech 2013). Zinc is first absorbed from the diet by ZIP4 (Fig.1), and is delivered to the basolateral membrane or bound to metallothioneins (MTs); the molecular mechanism behind this process has not yet been elucidated(Lazarczyk and Favre 2008; Nishito and Kambe 2018).An excess of unbound cytoplasmic Zn2+is exported outside cells by ZnTs, predominantly ZnT1, and transported to peripheral tissues. Zn2+possibly crosses the outer membrane of mitochondria through porin channels, and then bound to MTs in the intermembrane space or further shipped into the mitochondrial matrix via unknown proteins (Lazarczyk and Favre 2008;Nishito and Kambe 2018). Furthermore, a mutual exchange of Zn2+between the ER and Golgi apparatus through antero- and retrograde vesicular transport has been postulated. Zn2+is delivered in the opposite direction, from organelles to the cytosol by ZIPs, ZIP1 and ZIP7 (ER and Golgi apparatus) are closely related to this process. The ZIP and ZnT families are highly conserved, and their corresponding genes were found in zebrafish as well. The mRNA level of zinc exporter ZnT1 was upregulated in fish subjected to zinc overload and downregulated through zinc deprivation (Zheng et al. 2008).
Copper
As the third-most abundant trace metal in the human body, copper not only plays a critical role in physiological processes (Valko et al. 2016), but also associates with cell proliferation and major diseases(Brady et al. 2014). It is revealed that biological functions of copper are essentially dependent on Cu2+/Cu+transition, also associated with the production of reactive oxidative species (ROS).Abnormal Cu+/Cu2+equilibrium can lead to dyshomeostasis, causing many genetic disorders such as Menkes (MNK) disease (Andersson et al. 2014),Wilson disease (WD) (Bandmann et al. 2015),neurodegenerative diseases (Müller et al. 2017), and cancers (Wang et al. 2015).
Cu2+is reduced to Cu+by an elusive mechanism and can enter cells through copper transporter 1 (CTR1),the principal high-affinity copper importer (Cotruvo et al. 2015). Upon entry, copper interacts with cellular ligands and chaperones that adjust its shipping to specific proteins. Glutathione (GSH) can buffer Cu+pools and mediate Cu+transfer between CTR1 and metallochaperones. Carriage of copper to cytosolic superoxide dismutase 1 (SOD1) proceeds through copper chaperones for superoxide dismutase (CCS).This metallochaperone for Cu/Zn SOD is a known marker for alterations in copper metabolism that inversely correlates with intracellular copper bioavailability (Bertinato et al. 2003; Prohaska et al.2003). Metal regulatory transcription factor 1 (MTF1)transcriptionally activates MT genes and other targets.Cytosolic copper is delivered to mitochondria for loading onto cytochrome C oxidase (COX) (Festa and Thiele 2011). Atox1, homolog of Atx1, is responsible for copper delivery to two P-type ATPases, who also participate in copper efflux from cells. They are ATP7A and ATP7B in the trans-Golgi network, where most cuproproteins are metallated. MNK is an X-linked recessive disorder caused by mutations in genes coding for the copper-transport protein ATP7A, leading to copper deficiency. Conversely, WD is a rare genetic disorder characterized by excess copper stored in various body tissues (Bansagi et al. 2016).
Iron
As the most abundant transition metal in the human body, iron is essential and required for a variety of physiological and pathological processes (Theil and Goss 2009). Iron deficiency anemia (IDA) is imposed by a lack of iron, whereas hereditary hemochromatosis(HH) is an iron-overload disorder (Andrews 2000).Once iron is put into circulation, it is first bound to transferrin (TF) as Fe3+and can subsequently enter cells, which maintain iron homeostasis through a balance of import proteins including transferrin receptor (TFR), divalent metal transporter 1 (DMT1),storage proteins like ferritin (FTN), and export proteins like ferroportin (FPN) (Aron et al. 2018). It is worth noting that metalloreductase STEAP3 (sixtransmembrane epithelial antigen of prostate 3)converts Fe3+to Fe2+. In mitochondria, iron-sulfur(Fe/S) cluster proteins (ISCs) are generated from iron and cysteine and are indispensable cofactors for proteins in mitochondrial respiration and other cellular activities (Braymer and Lill 2017).
Aberrant iron levels are implicated in ailments such as pyroptosis and ferroptosis (Zhou et al. 2018).Ferroptosis is a newly recognized cell death modality marked by the oxidative modification of phospholipid membranes via an iron-dependent mechanism and that has drawn constantly increasing attention in chemical biology (Conrad et al. 2016; Friedmann Angeli et al.2019). Augmented levels of nuclear factor erythroid 2-related factor 2 (NRF2) protein after treatment with erastin and sorafenib (inducers of ferroptosis) render cells resistant to ferroptosis. This protection is traced to upregulation of NRF2 target genes like heme oxygenase 1 (HMOX1). Lysosomes can accumulate a large quantity of iron through degradation of FTN (coined ferritinophagy). Inhibition of lysosomal activity or silencing of nuclear receptor coactivator 4 (NCOA4), a cargo receptor recruiting FTN to autophagosomes for lysosomal degradation and iron release, suppresses ferroptosis (Hassannia et al. 2019). Remarkably, a novel quinolone-derived fluorescent turn-on probe IQ44 has been enabling the visualization of autophagosome-lysosome fusion in the process of autophagy (Lim et al. 2019).
MOLECULAR IMAGING
Zinc
Our laboratory reported a turn-on Zn2+sensor NBDTPEA, the first example for monitoring zinc ions in neuromasts of zebrafish via fluorescence (Qian et al.2009). By replacing −NO2moiety with −SO2NH2, we devised an ICT ratiometric fluorescent probe, SBDTPEA, for Zn2+imaging in biological systems (Liu et al.2014). In the presence of Zn2+(50 mmol/L HEPES/DMSO = 99.85/0.15, pH 7.2), SBD-TPEA exhibits an emission band centered at 585 nm, with an excitation maximum at 466 nm. However, addition of Zn2+to the probe leads to a distinct hypsochromic emission shift from 585 to 545 nm with a well-defined isoemission point at 585 nm. This change is attributed to ICT alteration when Zn2+binds to the probe. The dissociation constant (Kd) of the SBD-TPEA/Zn2+complex was estimated to be 2.1 nmol/L and its limit of detection (LOD) for Zn2+was 0.5 nmol/L. Because SBDTPEA responded selectively to Zn2+without interference from other metal ions, it was utilized to image Zn2+in HepG2 cells and zebrafish larvae (Fig. 2).
Through integrating a Zn2+ionophore N,N’-bis(pyridin-2-ylmethyl)ethane-1,2-diamine (BPEA) as the ICT donor of the ANaph fluorophore, we constructed Naph-BPEA with nuclear envelope penetrability (Zhang et al. 2013). Of note, this probe did not merely localize in the cell nucleus, it also fluoresced in the cytoplasm. More importantly, our group summarized photoluminescence Zn2+imaging in living subjects before 2015 (Chen et al. 2015). Apart from biological functions and sensing mechanisms of labile Zn2+, Zn2+roles within an array of subcellular compartments such as mitochondria, lysosome,endoplasmic reticulum (ER), and Golgi apparatus were illuminated in detail, with corresponding Zn2+sensors displayed. Localization is usually defined by comparing the colocalization of the sensor with a well-established organelle marker and quantifying the overlap using Pearson’s correlation coefficient (PCC) given by a imaging software (Carter et al. 2014). Organellespecific Zn2+probes have been developed to study Zn2+distribution in different subcellular compartments(Fig. 3), we will only introduce some representative works due to limited space of this review, readers are encouraged to refer to another review for more detailed information (Zhu et al. 2016).
Golgi-targeting
Golgi apparatus contain various metalloproteases and alkaline phosphatases, whose catalytic activities rely largely on the Zn2+ions. In 2015, the Kim research group reported a Golgi-localized two-photon ratiometric probe for zinc ions (Singh et al. 2015). The dipicolylamine moiety within the compound SZnC selectively makes a complex with Zn2+(30 mmol/L MOP/EtOH = 1:1, pH 7.2), with an emission enhancement peaked at 500 nm. The TP action crosssection values (Φδmax) of SZnC in the absence and presence of excess Zn2+were determined to be 16 and 92 GM, while the fluorescence quantum yield Φ were 0.12 and 0.93, respectively. Featuring low cytotoxicity and high photostability, this probe can be leveraged to monitor Zn2+fluctuations in real time and getting a 3D distribution picture in the Golgi apparatus.
ER-targeting
Metal ions, such as Cu+and Zn2+, are required for ER functions. Recently, Lippard and coworkers synthesized a new red-emitting fluorescent probe ZR1 to monitor mobile Zn2+in the ER, a benzoresorufin fluorophore functionalized with two 2,2’-dipicolylamine (DPA) arms(Loas et al. 2014). The free probe was non-fluorescent(50 mmol/L PIPES, pH 7.0) due to PET from the Zn2+chelators, while Zn2+-binding strongly elevated its emission maximum at 611 nm and an 18-nm Stokes shift. On average, the associated brightness values (εΦ)enhanced 44-fold to 2.43 × 104L/(mol·cm). In comparison with ZBR4, which anchors to the mitochondria, ZR1 spontaneously localizes to the endoplasmic reticulum of HeLa cells.
Mitochondria-targeting
Using fluorescein fluorophore instead, Lippard and coworkers presented a reaction-based fluorescent sensor DA-ZP1-TPP, which selectively localized at the mitochondria (Chyan et al. 2014). Coordination of Zn2+enhances fluorescence intensity by promoting ester hydrolysis and alleviating PET origination from the DPA motif. Adding nanomolar concentrations of free zinc ions resulted in large increases in both the absorption (λabs= 510 nm) and fluorescence (λem=529 nm) spectral bands of DA-ZP1-TPP (50 mmol/L PIPES, pH 7.0). These optical changes combined to yield a >140-fold increase in the fluorescence signal (ΦZn=0.75). With this probe, it is concluded that tumorigenic cancer cells lose the ability to accumulate Zn2+within their mitochondria in contrast to healthy epithelial prostate cells.
Indeed, close correlations between prostatic Zn2+levels and prostate cancer (PCa) have been reported(Costello et al. 2004). At least three ZIPs and six ZnTs are expressed in a lobe-dependent manner in the prostate (Kelleher et al. 2011). In malignant prostate tissues, there is a dramatic decline in Zn2+concentration (500 nmol/g vs. 3000 nmol/g for cancerous and healthy tissues, respectively), as well as a downregulation of ZIP1 (Kolenko et al. 2013).
Lysosome-targeting
Very recently, LysoDPP-C4 has been developed for the evaluation of low pH and Zn2+in an AND logic fashion to investigate lysosomal Zn2+in prostate cancer cells(Du et al. 2019). A morpholine unit is appended for targeting lysosomes, as confirmed by HeLa cell fluorescence imaging studies. LysoDPP-C4 demonstrated an excellent selectivity toward Zn2+and the resulting LysoDPP-C4/Zn2+complexes proved insensitive to pH 3.5-9. At low pH, Zn2+chelation contributes to a large increase in the fluorescence intensity at 515 nm (λex= 430 nm) ascribed to suppression of PET. The sensor formed a host-guest complex in 1:1 stoichiometry with Kd= 1.91 nmol/L.Besides, histological studies using a human sample revealed that LysoDPP-C4 could distinguish between cancerous prostate tissue and healthy one.
In view of the advances in Zn2+-specific chelators(TPEA, BPEA, DPA, etc.) and Zn2+-complexation-induced spirolactam ring-opening or hydrolysis (Jin et al. 2018),concurrent with phosphorescence lifetime imaging(PLIM) of labile Zn2+(Zhang et al. 2018), it is not surprising that Zn2+sensors constitute the largest family of fluorescent indicators for transition metals. Some of them are reversible and ratiometric, applied to appealing models. So far, the physiological participation of this ion is not completely clear in spite of the large arsenal of fluorescent Zn2+sensors.
Copper
Molecular sensors are intriguing tools to visualize the distribution and speciation of labile copper.Nevertheless, there are added challenges posed by aiming at labile copper over Zn2+due to the need for discrimination between different oxidation states, the quenching nature of Cu2+, and the fact that probes must have enough affinities to compete for copper within its biological window (10−21−10−17M) (Wegner et al.2011). In consequence, only a fraction of copper sensors has been utilized for biologically accessible copper. The Kim group shed light on the pathophysiological part of copper ions and sensors for the measurement thereof (Peter et al. 2015).
Cu+
Although Cu+is prone to disproportionation and acquires stabilization by specific ligands in aqueous media (Paredes and Das 2011), it is considered to be the dominant intracellular copper oxidation state of labile copper pools (Festa and Thiele 2011). Most of the probes designed for biological systems target Cu+,engaged in two common aspects as follows (Fig. 4).(A) NS4-based chelation
Thioether (NS4) receptors can be modified to develop fluorescent Cu+sensors. One notable example is CTAP-1, the first small-molecule sensor for mapping labile Cu+in cells (Yang et al. 2005). Soon after this study, the Chang group presented coppersensor 1 (CS1) with visible excitation and emission profiles (Zeng et al.2006). Tuning the BODIPY fluorophore of CS1 to a rhodol scaffold produced a more hydrophilic Cu+sensor called copper Fluor-3 (CF3) (Dodani et al. 2014). As described earlier, fluorescent sensors that excited at short wavelengths succumb to limited penetration depths, high autofluorescence, and inevitably induce photobleaching and photodamage. These flaws hinder their use for long-term imaging in tissues and organisms. One way to remove these limitations is by NIR single-photon excitation (Guo et al. 2014). Cao Cu-3 represents a novel NIR fluorescent turn-on probe suitable for imaging endogenous Cu+ions in living systems (Cao et al. 2012).
Another approach is to use two-photon microscopy(TPM), employing NIR photons of lower energy as the excitation source to possess the advantage of deepened penetration (>500 μm) (Kim et al. 2014). Our laboratory presented the first-generation ER-localized TP Cu+sensor CNSB (Guo et al. 2019). There is a distinct overlap between coumarin-A (donor) emission and 4-amino-7-sulfamoyl benzoxadiazole (ASBD,acceptor) absorption, implying these two compounds are qualified candidates as a FRET pair to compose ratiometric sensors. CNSB exhibits ~7.44 × 10−11M Kdvalue (10 mmol/L Tris-HCl/DMF = 6:4, pH 7.2) and the Cu+-enhanced emission ratio F470/F565is stable from pH 4.0 to 8.0. The sensor was applied to assessing Cu+fluctuation in MCF-7 cells pre-incubated with tunicamycin, proving the relationship between Cu+augmentation and ER stress. Meanwhile, the spatial distribution of Cu+in the heart slice of a 2-week rat was observed via TPI owing to the two-photon ability of coumarin fluorophore. Negligible enhancement of coumarin emission was observed during the fluorescent titration of CNSB.
(B) O2-dependent C−O bond cleavage
Inspired by the O2activation for copper-dependent enzymes, the Chang group adapted the Cu+chelator tris(2-pyridylmethyl)amine (TPA) to develop coppercaged luciferin-1 (CCL-1) (Heffern et al. 2016), a bioluminescent reporter for monitoring labile Cu+levels in a diet-induced mouse model of non-alcoholic fatty liver disease (NAFLD) that manifests as a hepatic copper deficiency, revealing altered expression levels of central copper trafficking proteins that accompany symptoms of glucose intolerance and weight gain(Fig. 5). As supported by Western blot, CCS levels were elevated in the high-fat diet (HFD) mice over control diet mice, and the major copper exporter proteins ATP7A and ATP7B were also up-regulated.
Not long ago, linking fluorescein donor and rhodamine acceptor through the TPA bridge, they reported reaction-based ratiometric FRET copper probe FCP-1. Together with its 2-color response (F526/F576),collective results indicate that oncogene-driven changes in the metabolism of GSH, a major cellular redox buffer,leads to a labile Cu+deficiency with differential expression of CTR1 (Chung et al. 2019). This work connects Cu+dysregulation and GSH stress in cancer,providing a roadmap for studying the crosstalk between metal and redox pathways in physiology and pathology with probes.
Cu2+
As synthetic fluorescent probes for monovalent copper have been thoroughly reviewed by others (Cotruvo et al. 2015; Fahrni 2013), we focus our discussion on Cu2+probes that compensate for these areas of copper sensing. A large library of luminescent chemodosimeters for Cu2+bioimaging has been collected by Fuyou Li et al. (Yang et al. 2013). Most of such probes recognize Cu2+by three principal categories: chelation, hydrolysis and oxidation (Fig. 6).Among them, the oxidation pattern draws in the fewest probes while the other two seem more attractive.
(A) Chelation
As mentioned previously, 2,2’-dipicolylamine (DPA) can be manipulated to design probes selective for Cu2+over Zn2+(Ballesteros et al. 2009). Modifying the FRET pair of CNSB with DPA unit forms a reversible ratiometric sensor CSBPA for intracellular Cu2+imaging (Chen et al.2013). Later we achieved in vivo fluorescence imaging for Cu2+in live mice for the first time by a BODIPYderived NIR fluorescent sensor BDPA (Xue et al. 2016),profiting from its large Stokes shift (~100 nm),excellent photostability and high quantum yield (Φ =0.85).
Wang et al. developed a series of activatable PA probes with low molecular weights (<438 Da), and could specifically chelate with Cu2+to form radicals displaying turn-on PA signals in the NIR region (Wang et al. 2019a).Introducing the electron-donating group N,Ndimethylaniline into the probe was found to significantly enhance the radical stability and PA intensity. The best probe in the series, RPS1, produced a fast response (within seconds) to Cu2+with a low LOD of 90.9 nmol/L (0.02 mol/L PBS, pH 7.4). Owing to the low molecular weight and amphiphilic structure, RPS1 could effectively cross the blood−brain barrier (BBB)and thus maps Cu2+in the brains of AD mice via PAI for the first time (Fig. 7).
(B) Hydrolysis
The group headed by Chan reported the synthesis and photoacoustic properties of APC-1 and APC-2, two ratiometric probes for mobile Cu2+(Li et al. 2015).Upon binding Cu2+, the sensors display 89- and 101-fold enhancements of normalized ratiometric turn-on responses, respectively. Both APCs are equipped with a 2-picolinic ester sensing module that is readily hydrolyzed in the presence of Cu2+but not by other divalent metal ions. Additionally, ratiometric PAI was realized by using an aza-BODIPY dye scaffold exhibiting two spectrally resolved NIR absorbance bands, which correspond to the 2-picolinic ester capped and uncapped phenoxide forms.
Combining Cu2+-promoted hydrolysis with spirolactam ring-opening generates an effective strategy to construct fluorescent Cu2+chemodosimeters.A ratiometric TP probe for Cu2+trafficking based on through-bond energy transfer (TBET), was designed by directly conjugating BODAN (donor, green emission)and rhodamine spirolactam (acceptor, red emission)(Fig. 8) (Zhou et al. 2014). Np-Rh elicits a ratiometric response (EtOH/H2O = 1:9) upon addition of Cu2+, with highly efficient energy transfer (93.7%) and two wellresolved emission peaks separated by 100 nm.Moreover, the Cu2+distribution in Np-Rh-labeled HeLa cells as well as rat liver frozen slices pretreated with Cu2+was visualized using TPM in the 450-530 and 540-650 nm collection windows.
Similarly, Fuyou Li and coworkers introduced a frequency upconversion luminescence (UCL) Cu2+chemodosimeter NRh (Liu et al. 2016), lighting up efficient single-photon upconversion emission at 730 nm under NIR excitation at 808 nm. This probe can serve as an ideal Cu2+sensor for ex vivo and in vivo assay in a WD mouse model. Impressively, the exploitation of UCL small-molecule probes and their bioimaging applications have been attracting much attention (Dong et al. 2018; Yang et al. 2016; Zhang et al. 2017).
(C) Oxidation
Subsequently, the same group developed an easy-touse probe CYDA based on UV-Vis-NIR absorption changes with excellent sensitivity and selectivity for Cu2+(Shi et al. 2018). The mechanism of CYDA oxidation by Cu2+was first explored (Fig. 9). In aqueous solution (EtOH/H2O = 1:1), addition of Cu2+to CYDA induced significant changes in the absorption spectra.When the solution was mildly acidic (pH 6.8), the absorbance at 670 nm went down, with the absorbance at 553 nm (ε = 1.09 × 105L/(mol·cm)) up, accompanied by the solution color changes from blue to red. In alkaline solutions (pH 8.0), the absorbance at 670 nm(ε = 2.14 × 105L/(mol·cm)) dropped gradually, whereas the absorbance at 823 nm (ε = 0.22 × 105L/(mol·cm))rose with an isosbestic point at λ = 793 nm. In the meantime, the solution color turned into greyish blue.These phenomena confirmed the formation of CY1 and CY2. They further demonstrated that the probe was able to quantify Cu2+in urine from WD patients.
Even a bit earlier than the above study, oxidative C−O bond cleavage turn-on probe FluHMPP has been prepared (Shi et al. 2013). On addition of 20 equiv of Cu(NO3)2(10 mmol/L Tris-HCl/CH3CN = 7:3, pH 7.2),there was a strong emission enhancement (30-fold)after 2 h. Confocal microscopy experiments have demonstrated that FluHMPP was membrane permeable and capable of tracing exogenous CuCl2in HeLa cells.Nevertheless, such C=N structures are unstable and sensitive to reactive oxygen species.
Iron
Despite great interest in labile iron pool (LIP), its biological function remains insufficiently understood,in part due to a relative lack of tools for directly assessing labile iron in living specimens (Dixon and Stockwell 2014). Molecular imaging using iron selective sensors opens up a broad avenue for better understanding the complex handling of iron in biology.Ideally, probes should be able to track biological iron status, selectively detecting either Fe2+or Fe3+or responding to iron species such as heme-iron or ISCs.Because Fe2+and Fe3+are potent emission quenchers,iron imaging within living cells encountered many challenges. Two early sensors, Calcein and Phen Green SK, exhibit turn-off responses to iron binding (Carter et al. 2014). However, both probes cannot adequately distinguish the two oxidation states of iron but can interact with other metal ions, leading to an interfering signal. In the past few years, a substantial body of efforts has been devoted toward construction of iron probes that may make a stunning breakthrough regarding cellular iron homeostasis.
Fe2+
The growing palette of chemodosimeters for Fe2+mapping exploits the potent redox activity of this metal ion, and the last several years have witnessed a tremendous number of novel Fe2+chemoprobes suitable for live-cell imaging experiments. Recent reviews by Hirayama, Aron and coworkers exhaustively profiled the development of molecular probes for Fe2+(Aron et al. 2018; Hirayama 2019). A palette of Fe2+-mediated principles have been reported, including Noxide or nitroxide reduction, cyclization, O2activation and endoperoxide cleavage (Fig. 10).
(A) N-oxide reduction
The deoxygenation of tertiary amine N-oxide is preferably mediated by Fe2+as it has a lower redox state than Fe3+. Utilizing the reaction, Hirayama and coworkers developed the first turn-on fluorescent Fe2+probe RhoNox-1 (Hirayama et al. 2013), which has been commercially available and applied to detecting Fe2+changes during ferroptosis (Wang et al. 2019b). To date, this Fe2+sensing strategy has emerged as the most widely used one, establishing a series of sensors. For instance, HMRhoNox for visualization of intracellular Fe2+delivered by TF (Niwa et al. 2014), CoNox-1 and FluNox-1 for scrutiny of intracellular redox equilibrium shift toward Fe2+in hypoxic HepG2 cells (Hirayama et al. 2017), and a membrane-anchoring probe Mem-RhoNox to scrutinize Fe2+release during endocytotic iron uptake (Niwa et al. 2018).
Recent research expanded the applicability to establish a series of organelle-specific fluorescent probes selective to Fe2+, i.e., MtFluNox, Lyso-RhoNox,ER-SiRhoNox and Gol-SiRhoNox. The former three chemosensors (Fig. 11) demonstrated similar off/on contrast and reaction rates (2.1 × 10−3s−1, 2.2 × 10−3s−1,and 1.7 × 10−3s−1), investigated Fe2+specifically at the targeted organelles (PCC = 0.81, 0.80, 0.80), and depicted fluorescence enhancement at 535 nm (green,100-fold), 575 nm (orange, 60-fold), and 660 nm(magenta, 60-fold), respectively. Furthermore, these probes indicated the aberrant elevation of labile Fe2+levels in the lysosomes and ER but no Fe2+fluctuation in the mitochondria prior to HT1080 cell death initiated by erastin (Hirayama et al. 2019b).
As depicts in Fig. 1, DMT1 serves as a primary transporter of Fe2+. Over the course of the endocytotic process, directing DMT1 toward the cellular membrane via the trans-Golgi network is partially regulated by a retromer-mediated protein-sorting machinery comprising vacuolar protein-sorting proteins (VPSs)(Burd and Cullen 2014). Therefore, the Golgi organelle functions as a node in this dynamic system for intracellular Fe2+species along with DMT1. Dysfunction of VPSs, particularly the mutation of VPS35, can activate the aberrant delivery of DMT1 to lysosomes concomitantly with Fe2+ions (Tabuchi et al. 2010). The last one Gol-SiRhoNox revealed an abnormal cellular Fe2+distribution induced by dysfunction of VPS35 with a molecular chaperone R55 (Hirayama et al. 2019a).
(B) Nitroxide reduction
In parallel studies, a related Fe2+-promoted nitroxide reduction reaction has been employed for reactionbased sensing of Fe2+(Maiti et al. 2015). A rhodaminelinked nitroxide probe, Rh-T, bears a pendant paramagnetic 2,2,6,6-tetramethylpiperidine-1-oxyl(TEMPO) group that quenches fluorescence. Fe2+reduces the receptor radical to a diamagnetic hydroxylamine, resulting in a significant fluorescence turn-on (~2.5-fold) with a LOD ~0.75 μmol/L. Fe2+-dependent reduction of the TEMPO radical of Rh-T triggers both a fluorescence turn-on and a change in electron paramagnetic resonance (EPR) signal.Following this example, Zhu group reported NT-Fe with the fastest response (<50 s), as well as the sensitive LOD of 89 nmol/L (Zhang et al. 2020). In zebrafish, NTFe responds to the addition of exogenous Fe2+, and its signal decreases when pretreated with Fe2+scavenger 2,2’-bipyridine (Bpy).
(C) Cyclization
By exploiting the Lewis acidity of Fe2+, a recent report highlighted the application of an Fe2+-specific cyclization of a phenolic unit adjacent to a C=N bond appended to a coumarin derivative (Long et al. 2018).Upon cyclization, generation of a benzoxazole ring handicaps C=N bond isomerization and rotation,turning on fluorescence in aqueous solution and HepG2 cells, which has also been confirmed by TD-DFT calculation. Significantly, the sensing reaction completed in 2 min with high sensitivity (LOD =45 nmol/L). However, more analogous probes should be rationally constructed to verify the validity of the proposed mechanism.
(D) O2activation
Prior work by Chang laboratory on creating Cu+indicators, presaged the general utility of biomimetic oxygen activation for Fe2+surveillance. Inspired by the O2chemistry of Fe2+, and in particular, the 2-His-1-carboxylate triad motif found in mononuclear nonheme Fe2+enzymes (cytochrome P450), they engineered iron probe 1 (IP1) (Au-Yeung et al. 2013).IP1 was capable of monitoring elevated intracellular labile Fe2+levels in HepG2/C3A cells caused by treatment with hepcidin or ascorbic acid.
Replacement of the fluorescein alcohol substituent with a cyanine dye (Cy7), the Tang group yielded another NIR fluorescent probe (LCy7) (Wu et al. 2020),comprising a tris(pyridine) carboxylate-type ligand cage as an iron recognition and activity site. Upon Fe2+coordination, O2activation triggers intramolecular C-O bond cleavage and oxidation to release the fluorophore,resulting in an increase (~3.7-fold) in the fluorescence emission peaked at 690 nm. Utilizing LCy7, dynamic Fe2+enhancement was distinctly monitored in HL-7702 cells under ER stress by acetaminophen (APAP) stimulation.In vivo FLI disclosed the conspicuous Fe2+ascent in the liver of mice during drug-induced liver injury (DILI).
(E) Endoperoxide cleavage
Motivated by Fe2+-reactive endoperoxide activation observed in antimalarial drugs (e.g., Artemisinin),Renslo and coworkers designed Trx-puro, a histochemical stain for labile Fe2+comprised of a trioxalane-caged puromycin (Spangler et al. 2016).Upon reaction of the probe with Fe2+, free puromycin is released, which can be translated onto nascent peptides and quantified via a conventional immunostaining method by a puromycin antibody after fixation.Application of Trx-puro to a panel of cancer cells (PC-3,U-2 OS, MCF10A and RKO) reveals FTN/FPN overexpression and declined Fe2+stores. Linking fluorescein and Cy3 through this bioinspired endoperoxide trigger, Chang et al. envisioned FRET iron probe 1 (FIP-1) for ratiometric fluorescence Fe2+imaging upon 35MEW28-induced ferroptosis in MDAMB-231 cells (Aron et al. 2016).
Expanding the scope of oxidative cleavage to BLI, the same group then reported the first bioluminescent indicator for Fe2+(Aron et al. 2017), iron-caged luciferin-1 (ICL-1), to surveil labile Fe2+accumulation in a luciferase-expressing murine (FVB-Luc+) model of Acinetobacter (A.) baumannii infection (Fig. 12). The probe also retains high selectivity and sensitivity for Fe2+over a variety of biologically-relevant metal ions,oxidants, and reductants and is feasible for detecting both Fe2+rise and fall in PC3M-luc cells and other Fe2+-supplemented/depleted models.
Despite the exquisite selectivity toward Fe2+for bioimaging, these reaction-based probes were not shown to have a reversible monitoring mode,hampering the capability to probe Fe2+fluxes. If a probe is able to synchronously measure Fe2+and Fe3+without interference from other metal ions, the privileged part of iron taking in ferroptosis will no longer remain a mystery.
Fe3+
In contrast to the growing usage of chemical probes for bioimaging of Fe2+, measurement of Fe3+in biological environments is rarer. Sahoo et al. reviewed advances on Fe3+-specific fluorescent probes (Sahoo and Crisponi 2019; Sahoo et al. 2012). Due to the paramagnetic nature of ferric iron, its previous recognition by fluorescent probes involves different quenching mechanisms, which is improper for bioimaging applications. A new class of turn-on Fe3+sensors can be classified into two major categories, chelation and spirolactam ring-opening (Fig. 13). Furthermore, some ratiometric sensors recognizing the metal with an appropriate ligand group have been proposed to interrogate intracellular Fe3+ions.
(A) Turn-on Fe3+probes
(a) Chelation
The modularity of the PET platform has been applied to developing Fe3+-triggered fluorescent turn-on probes with enhanced properties. A novel PET-based reversible fluorescence turn-on probe L2 for selective assay of Fe3+was introduced (Sui et al. 2014). The probe was comprised of BODIPY fluorophore with a 1,10-diaza-18-crown-6-based cryptand that acted as the analyte binding unit. Upon addition of Fe3+in aqueous media (H2O/CH3CN = 9:1), the weakly fluorescent L2 (PET process is active) displayed 23-fold fluorescence enhancement at 512 nm (λex= 480 nm).The significant fluorescence enhancement occurred due to inhibition of PET from cryptand to BODIPY fluorophore upon complexation with Fe3+. The probe displayed a LOD of 0.13 μmol/L and was applied to sensing intracellular Fe3+ions in HCT-116 cells.
Our group introduced a simple anthracene-based fluorescent turn-on probe L3 containing N2-hydroxyethyldiethylenetriamine (HEDTA) for inferring Fe3+concentrations (Qiu et al. 2014). When excited at 373 nm, the [2+2] macrocycle fluorescent sensor(20 mmol/L Tris-HCl/MeOH = 1:1, pH 7.2) exhibits weak emission at 398, 421, and 447 nm. In the presence of Fe3+, the formation of a L3-Fe3+complex in 1:2 ratio restricts the PET process, causing significant fluorescence enhancement. Probe L3 shows a linear response ranging from 1 to 10 μmol/L with the LOD of 0.58 μmol/L Fe3+. Confocal imaging disclosed that the probe possesses the ability of tracing cytosolic Fe3+in SKOV-3 cells.
(b) Spirolactam ring-opening
Subsequently, we took advantage of the HEDTA ligand to develop another turn-on Fe3+probe Mito-RhFe by appending the HEDTA receptor onto rhodamine B as a cationic, lipophilic tag to localize the probe to mitochondria (Zhu et al. 2018). In UV−Vis Fe3+titration of this probe (20 mmol/L Tris-HCl/MeOH = 1:1, pH 7.2), the solution color changes from colorless to magenta, thereby offering Mito-RhFe as an Fe3+-induced ‘naked-eye’ chemosensor. With this probe, the mitochondrial labile Fe3+fluctuation in adherent HeLa cells (PCC = 0.90) upon ferric ammonium citrate (FAC)incubation was visualized via confocal imaging, and the flow cytometric assay for mitochondrial Fe3+in suspension MEL cells, which is difficult to be monitored in an imaging manner, was also developed. Erythroid differentiation is normally accompanied by hemoglobin biosynthesis and upregulation of the mitochondrial iron importing protein mitoferrin-1 (MFRN1)containing ISC (Shaw et al. 2006). Interestingly, the labile Fe3+drop in mitochondria of human K562 erythroleukemia cells undergoing the DMSO-stimulated erythroid differentiation was observed for the first time(Fig. 14).
In addition to Zn2+and Cu2+, Fe3+can trigger the hydrolysis and spirolactam ring-opening process of rhodamine derivatives, which has provided a valuable method to design Fe3+-selective luminescent sensors with a turn-on response. Lee et al. have presented six rhodamine-derived Schiff base probes' selectivity of the Fe3+ion in biological systems (Lee et al. 2016). After exposure to Fe3+, the probes appeared a new absorption peak at 526 nm, with a concomitant emission maximum at 551 nm. Confocal microscopic analysis showcased that these probes underwent turn-on changes caused by cellular Fe3+ions, and demonstrated a largely enhanced fluorescence upon iron overloading of the HepG2 cell line. They also found that the Fe3+-mediated fluorescence augmentation was mainly localized in the ER. Among them, probe L5 exhibited the highest selectivity for ER localization (PCC = 0.7854) over other organelles.
(B) Ratiometric sensors for Fe3+
Ratiometric probes are highly valued because their intrinsic internal standard can correct potential variations in light intensity, dye localization, and other experimental imaging conditions (Ackerman et al.2017). The aforementioned two turn-on platforms can be adapted to quantify labile Fe3+concentrations in a ratiometric readout, such as a bithiazole derivative(Geng et al. 2016), and a spirolactam analog RQBTE(Das et al. 2016)
It has been reported that ftn-1 (encoding the iron sequestering protein FTN) is upregulated in the presence of exogenous FAC supplementation, and downregulated upon treatment with an iron chelator deferoxamine (DFO) in the nematode Caenorhabditis(C.) elegans (Valentini et al. 2012). Goel et al. created the first dual colorimetric and ratiometric fluorescent Fe3+probe NAP-3 (Goel et al. 2015), consisting of a naphtho[2,1-b]-[1,10]-phenanthroline (NAP). After exposure to increasing concentrations of Fe3+ions(DMSO/H2O = 1:9, pH 7.4), a new peak with maximum at 605 nm emerged and the peak at 544 nm decreased in the emission spectrum, with a final enhancement factor(I605/I544) over 8.5-fold. It can approximately measure as low as 9.1 nmol/L Fe3+. Possibly due to steric hindrance, Fe3+chelator DFO failed to remove Fe3+from the complex (NAP-3/Fe3+). Common ions do not imply any significant interference in the determination of Fe3+ion. The indicator enabled direct visualization of exogenous Fe3+in HepG2 cells, and endogenous Fe3+in a multicellular organism, ftn-1 silenced C. elegans (Fig. 15).
In fact, most conventional organic dyes exhibit aggregation-caused quenching (ACQ) when aggregated.Aggregation-induced emission (AIE) phenomena,originating from the restriction of intramolecular motions (RIM), is thus a unique turn-on approach for addressing the ACQ issue (Chen et al. 2019a). AIE luminogens (AIEgens) tend to behave in a non-emissive manner when well dissolved in some solvents, but emit intense fluorescence when poorly dissolved by means of forming aggregates (Mei et al. 2015). AIEgens have emerged as a powerful methodology for the assay of transition metal ions such as Zn2+(Jung et al. 2015), Cu2+(Feng et al. 2014), and Hg2+(Chen et al. 2016).
By making use of the position-dependent substituent effects, an AIE featured Fe3+probe (TPE-o-Py)from ortho-substituted pyridinyl-functionalized tetraphenylethylene is synthesized by Tang’s research group (Feng et al. 2018). From weak blue to strong red emission (peak 472 nm to 573 nm) under 365 nm UV irradiation, TPE-o-Py displays high metal selectivity and oxidation-state specificity (THF/H2O = 3:7) arising from the position isomer of ortho-substitution. And it has a low acid dissociation constant (pKa~3.27) that is close to that of hydrolyzed Fe3+. More importantly, the lightup fluorescent probe TPE-o-Py can be applied to sensing Fe3+in HeLa cells with a pronounced red shift in fluorescence emission.
It should be noted that the reduction potential in the cytosol favors Fe2+over Fe3+(Aron et al. 2018), and Fe3+is poorly soluble at neutral pH in aqueous media. On the other hand, the vast majority of Fe3+probes have not been well suited for endogenous investigations due to a lack of high sensitivity. In this context, exploring improved motifs to decode Fe3+roles in biology is of tremendous significance.
SUMMARY AND PERSPECTIVES
In this review, we summarized endeavors made in molecular imaging for transition metal cations.Specifically, we highlighted synthetic sensors that are utilized to image zinc, copper, and iron in biological systems (Table 2). Great challenges still remain in the field to be confronted with:
(1) Expanding metal probes bioavailable. It is the quality of probes, including solubility, sensitivity and selectivity (3S), not the quantity that matters.Deliberate design of metal sensors with improved bioavailability is of paramount importance. In addition,other transition metals like manganese (Mn) also take active part in health and disease (Avila et al. 2013). Yet
Mn sensors are still on an early stage, and there have been far fewer small-molecule probes prepared for Mn2+detection (Bakthavatsalam et al. 2015; Liang and Canary 2010).
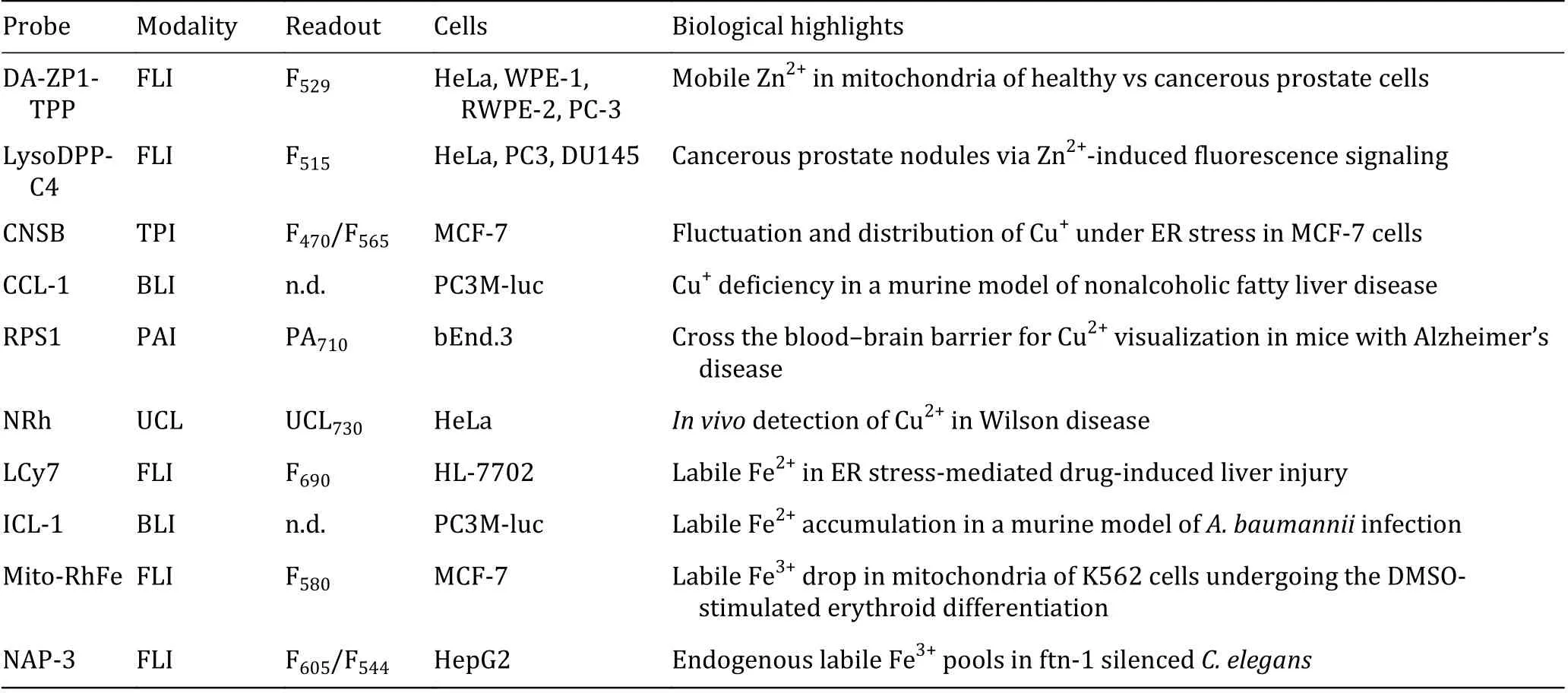
Table 2 Summary of selected probes for metal detection
(2) Combining super-resolution techniques. Superresolution techniques, such as stimulated emission depletion (STED), structured illumination microscopy(SIM), and stochastic optical reconstruction microscopy(STORM), provide a valuable starting point to study analytes in nano-dimension (Kozma and Kele 2018).Application of this technique to molecular probes for mapping metals is an important and arduous task.
(3) Designing dual-responsive probes. Using two selective probes simultaneously to monitor multiple species is troublesome, and it is therefore preferable to pursue dual-responsive single probes, operating as a logic gate in response to two elements (Kolanowski et al.2018). In this case, interrelations between multiple elements in biology can be deciphered. For instance, if a probe is able to synchronously measure Cu+and GSH without any interference from other analytes, more straightforward data would be offered with less work in NAFLD mentioned above.
(4) Mining multi-mode imaging. Given that diverse imaging modes can be complementary, our laboratory recently reported a FLI/PAI dual-modality probe HSCyBz (Chen et al. 2019b), realizing ratiometric in/ex vivo tracking for stimulated H2S fluctuations in mice.Integrating several imaging modes into a single metal probe, should be appropriately achieved in the longer term.
To sum up, roles for labile metals in cells and tissues,have been elucidated to a certain extent using these molecular probes. Continued innovations in exploiting chemical reagents, in cooperation with physicists and biologists, presage further opportunities for uncovering how metal cations work in biology.
AcknowledgementsThe work was under financial supports from the National Natural Science Foundation of China(21977044, 21907050, 21731004, 91953201), the Natural Science Foundation of Jiangsu Province (BK20190282) and the Excellent Research Program of Nanjing University (ZYJH004).We thank Dr. Chengcheng Zhu, Dr. Lin Qiu and Ms. Jungu Guo for offering the original images shown in abstract. We also thank sincere friends Miss Xiaotong Li (NJUST) and Mr. Dong Han (USTC) for polishing up the writing.
Compliance with Ethical Standards
Conflict of interestJing Gao, Yuncong Chen, Zijian Guo and Weijiang He declare that they have no conflict of interest.
Human and animal rights and informed consentThis article
does not contain any studies with human or animal subjects performed by any of the authors.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use,sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
- Biophysics Reports的其它文章
- Direct structural evidence supporting a revolving mechanism in DNA packaging motors
- An efficient autometallography approach to localize lead at ultra-structural levels of cultured cells
- Biofriendly micro/nanomotors operating on biocatalysis:from natural to biological environments
- Stimuli-responsive polymeric nanomaterials for rheumatoid arthritis therapy

